-
PDF
- Split View
-
Views
-
Cite
Cite
Arda Ozyuksel, Ozgur Yildirim, Mustafa Avsar, Mehmet Hayirlioglu, Sener Demiroluk, Osman Kucukosmanoglu, Mehmet Salih Bilal, Surgical correction of cor triatriatum sinister in the paediatric population: mid-term results in 15 cases, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 1, January 2015, Pages e25–e28, https://doi.org/10.1093/ejcts/ezu390
Close - Share Icon Share
Abstract
Cor triatriatum sinister (CTS) is a rare developmental cardiac abnormality resulting in left ventricular inflow obstruction. In this report, we aimed to present our mid-term results of early childhood patients operated for CTS and associated cardiac abnormalities.
We enrolled 15 patients with CTS who were operated by a single surgeon between 2001 and 2013. A retrospective analysis was performed in order to determine the demographics, operative and postoperative results of the patients. The median age was 14 months and the median body weight was 8.2 kg at the time of operation.
Fourteen patients had concomitant cardiac pathology. Three of the patients had atrial septal defect and 1 of the patients had partial abnormal pulmonary venous connection, whereas 4 of the patients had both. In 2 cases of complete atrioventricular septal defect, 1 case with ventricular septal defect, 1 case with patent ductus arteriosus, 1 case with double outlet right ventricle and another case with tetralogy of Fallot, complete repair was performed together with membrane resection in the left atrium (LA). The mean preoperative left atrial gradient was 13.3 mmHg, whereas the mean LA pressure at the first postoperative year was 4.2 mmHg. There was 1 case with early mortality due to septic multiorgan failure secondary to pneumonia.
CTS is a rare congenital cardiac anomaly in which the results of the corrective surgery are encouraging. Early and long-term outcomes may be variable according to the associated congenital heart defects.
INTRODUCTION
Congenital obstructions of the left ventricle inflow can be classified into four subtypes: (i) congenital mitral stenosis, (ii) cor triatriatum sinister (CTS), (iii) pulmonary venous (PV) obstruction and (iv) supramitral ring [1]. CTS is encountered with an incidence of 0.1–0.4%; in congenital heart diseases (CHDs) [2].
The first post-mortem case of an abnormal membrane in the left atrium (LA) was described by Church in 1868 [3]. CTS is characterized by the presence of a fibromuscular membrane that subdivides the LA [4]. The postero-superior proximal cavity receives the pulmonary veins, whereas the anteroinferior chamber (true LA) contains mitral valve and left atrial appendage (LAA) [5]. Rarely, this membrane may divide the LA into multiple chambers leading to cor polyatriatum [6].
Clinical presentation and symptoms of patients with CTS are mainly due to obstruction at the PV–LA junction, which leads to a pressure overload at the right side of the heart [7]. The severity of the clinical symptoms depends on the size and the number of fenestrations on the obstructing membrane as well as the presence and location of the atrial septal defect (ASD) and associated CHD [8]. The blood flow between the proximal chamber and the distal left atrial cavity can be through three paths: from the proximal chamber to the right atrium (RA), from the RA to the distal LA or through the fenestrations on the membrane. Figure 1 schematically demonstrates the fibromuscular membrane dividing the atrial cavity into two separate spaces and possible paths of interchamber communications.
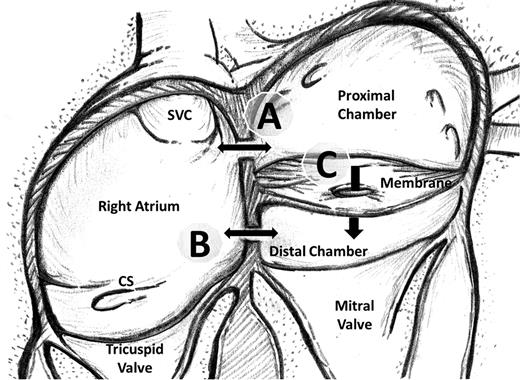
Schematic demonstration of the fibromuscular membrane dividing the left atrial cavity into two chambers. Possible ways of communication between the proximal left atrial chamber, right atrium and the distal chamber (true left atrium) are demonstrated. The communication may lie between the proximal chamber and the right atrium (A) and/or between the right atrium and the distal chamber (B) and/or through the fenestrations on the membrane (C). SVC: superior vena cava; CS: coronary sinus.
Most of the cases with CTS are diagnosed in infancy, whereas in cases with a wide orifice and non-significant pressure drop through the membrane, the diagnosis of CTS may be challenging until late adulthood due to missing symptoms [9–11]. Since calcification at the fenestrations may hinder the blood flow between the proximal and distal chambers, CTS could be detected in adulthood with atrial fibrillation and symptoms of mitral stenosis [12, 13].
MATERIALS AND METHODS
A retrospective analysis was performed in order to determine the patients with a history of surgical intervention for CTS following the approval of the ethical committee. Clinical presentations, preoperative echocardiographic findings, perioperative course and follow-up records were evaluated.
Fifteen patients (7 male and 8 female) with CTS were operated by a single surgeon between 2001 and 2013. Median age was 14 months (range 1 month to 7 years) and median body weight was 8.2 (range 3–33 kg). Eleven of the patients (73%) were infants.
RESULTS
We encountered 14 cases with classical CTS (Type A) where the proximal chamber was receiving the pulmonary venous return. Seven of these patients had ASD, all of which were located between the proximal chamber and RA. One patient had pulmonary venous connection of left-sided pulmonary veins into coronary sinus (CTS, Type B).
In our patient population, 14 of the patients (93%) had concomitant cardiac pathology. Three of the patients had ASD and 1 of the patients had partial abnormal pulmonary venous connection (PAPVC), whereas 4 of the patients had both ASD and PAPVC. In cases with PAPVC, the abnormal pulmonary veins were draining into the RA (right superior PV) or coronary sinus (left superior and inferior PV). All the ASDs were between the proximal chamber and RA in our patients. There were 2 patients with concomitant complete atrioventricular septal defect (AVSD—Rastelli type C), 1 with ventricular septal defect, 1 with patent ductus arteriosus (PDA), 1 with double outlet right ventricle (DORV) and another patient with tetralogy of Fallot (TOF).
The mean preoperative LA gradient that was 13.3 mmHg (range 6–21), which was calculated by either cardiac catheterization or transthoracic echocardiography. Since cardiac catheterization was reserved for detection of the associated CHD, not all patients went through it. For cases where both cardiac catheterization and transthoracic echocardiography were implemented, we took the transmembrane pull-back gradient results of the cardiac catheterization into account regardless of the echocardiographic calculations. None of the cases with CTS had mitral valve apparatus involvement.
The preoperative symptoms ranged from mild respiratory distress to critical congestive heart failure. The surgical indication for intervention was predominantly determined by the accompanying symptoms and the concomitant CHD. The only case where the surgical intervention was deemed necessary based merely on symptoms (exertional dyspnoea and fatigue) was a 7-year-old child with isolated CTS, whose pressure gradient across the membrane was 21 mmHg at echocardiographic evaluation.
Eight of the patients (53%) had preoperative pulmonary hypertension, which was either detected by echocardiography or cardiac catheterization. The pulmonary hypertension was defined as follows: where (i) the mean pulmonary artery pressure (PAP) was over 25 mmHg, if measured by cardiac catheterization, or (ii) the ratio of systolic PAP to systolic systemic arterial pressure was more than 0.4 as a result of transthoracic echocardiographic evaluation. The PAPs were within normal limits in the follow-up period for all patients, except for a 4-year-old patient with PDA and CTS, who had moderate pulmonary hypertension that was medically managed.
Cardiopulmonary bypass (CPB) with mild-to-moderate hypothermia was implemented in all cases. Mean aortic cross-clamping time and total CPB time were 55 ± 24 and 79 ± 41 min, respectively. Following the cross-clamping of the aorta, cold antegrade blood cardioplegia was administered where intermittent doses were applied every 15 min.
Our surgical procedure incorporated resection of the membrane at the left atrial cavity and a complete correction of the accompanying CHD. There was one exception to this approach, in which the membrane at the LA was resected with palliative pulmonary artery banding in a newborn with AVSD. All patients presented with ASD and/or PAPVC underwent atrial reseptation. In all cases with PAPVC, the pulmonary venous return was rerouted to the LA. A patient with DORV and another with TOF underwent complete repair together with membrane resection at the LA. Two patients with AVSD (both Rastelli type C) were successfully operated. One patient underwent complete repair with a modified single patch along with LA membrane resection, whereas LA membrane resection with pulmonary artery banding was the choice of treatment modality for a newborn patient with unbalanced ventricles. We operated 1 patient with ventricular septal defect and another with a PDA with concomitant membrane resection in the LA.
A right atrial approach was preferred in 13 cases, all of which included concomitant CHD. In a young patient with isolated CTS, the membrane was excised with a left atriotomy, whereas in another case with a concomitant persistent left superior vena cava (PLSVC), a combined biatrial approach was performed in order to facilitate the surgical exposure.
The early postoperative results were evaluated using transthoracic echocardiography at the first postoperative day, before discharge and at the third postoperative month. Afterwards, annual transthoracic echocardiographic evaluations were recorded. The mean postoperative first year LA pressure was 4.2 mmHg (range 2–6). The preoperative LA gradients and follow-up LA pressures are shown in Figure 2. None of the patients needed postoperative reintervention following the initial repair.
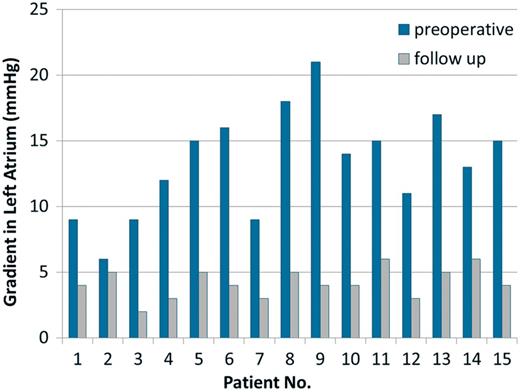
The preoperative left atrium (LA) gradients and follow-up LA pressures (mmHg) are shown. (For the case with mortality, postoperative fourth week gradient is represented).
Early postoperative mortality (8%) was encountered in a patient with VSD and CTS due to septic multiorgan failure secondary to pneumonia 32 days after surgery. The postoperative transthoracic echocardiography evaluation of this patient revealed an intact ventricular septal patch without any residual LA gradient. We did not encounter any late mortality after a mean follow-up of 64 months (range 1–125 months).
DISCUSSION
CTS is a rare morphological cardiac abnormality. The embryological basis of CTS remains controversial along with three possible explanations. The first one is the idea of malincorporation that suggests that the common pulmonary vein fails to incorporate into the LA, which results in the development of an obstructing fibromuscular membrane at the junction of these structures during the fifth week of gestation [14, 15]. Although this theory is widely accepted, it fails to explain the presence of fossa ovalis and atrial muscular fibres in the proximal chamber [16]. The malseptation theory proposes that the abnormal membrane that is located at the LA is a result of septum primum overgrowth. The theory of entrapment considers that the left horn of the sinus venosus entraps the common pulmonary vein, which prevents its incorporation into the LA [17]. As a variation of the entrapment theory, the impingement of the PLSVC on the LA is suggested to result in the production of the obstructing membrane in the LA [13, 18].
Two basic classifications are favoured in CTS. Loeffler [19] first classified CTS into three groups in 1949, depending on the size and number of fenestrations at the membrane: (i) no communication between proximal and distal chambers, (ii) communication through small perforations at the membrane and (iii) incomplete membranous subdivision of the LA. Lam et al. [20] suggested another classification in 1962. Type A (also named as the classical form) consists of a proximal chamber that receives all the pulmonary veins and a distal chamber that receives the LAA and the mitral valve. There is no ASD and the proximal and distal chambers connect through the small holes on the membrane in the classical form. The subtype A1 defines an ASD between the RA and the proximal chamber, whereas subtype A2 defines an ASD between the RA and the distal chamber. Type B defines hearts in which the pulmonary veins drain into the coronary sinus, which is also accepted as a variant of total anomalous pulmonary connection. Type C defines hearts in which the proximal chamber receives no pulmonary veins (the rarest variant) [20]. In our patient population, all the patients were classified as Type A, with an exception of 1 case classified as Type B.
The CTS may be associated with other CHD in 24–80% of the cases [21]. Partial or total anomalous pulmonary venous connection is the most commonly associated cardiac pathology, which has been reported in nearly one-third of the patients with CTS [21]. We encountered PAPVC and ASD as the leading associated CHDs in our patient population.
Surgical correction is indicated in patients with obstructive symptoms regardless of age [21]. The urgency of operation is primarily determined by the severity of the presenting symptoms, but CTS can remain asymptomatic until late adulthood. Therefore, it is reasonable to observe patients with an incidental diagnosis, provided the opening in the diaphragm is non-restrictive and regular follow-up is possible [21]. The specific surgical approach is chosen primarily based on the presence of associated lesions and the size of the atria. In our surgical experience, we predominantly preferred a right atrial approach for the resection of the membrane as well as the correction of the associated intracardiac pathologies. In elder children with isolated CTS and an enlarged LA, a left atriotomy may be preferred. A combined biatrial approach could be necessary in order to facilitate the surgical exposure in selected patients.
In cases of CTS, the timing of surgical intervention primarily depends on the severity of stenosis at the left atrial venous return and the coexistence of other CHD. We operated two critically ill patients during the newborn period. In cases where the membrane at the LA does not lead to severe stenosis, the coexisting CHDs predominate the timing of surgery. The increased utilization of the transthoracic echocardiography in the infancy led us to encounter low age and body weights at the time of diagnosis and surgical repair. Eleven of our cases were operated at the infancy period. The follow-up echocardiography measurements revealed encouraging results with a mean left atrial pressure below 5 mmHg.
Although percutaneous balloon dilatation of the obstructing membrane in simple CTS may be performed in selected cases at older ages [22], most of the patients have co-existing CHDs, which will eventually need to be treated surgically.
CONCLUSION
CTS is a rare cardiac anomaly that can be detected in any age. Correct preoperative diagnosis of the membrane separating the LA and the associated cardiac anomalies is important in the management of these patients. Early and long-term results may be variable according to the associated CHD; otherwise, the mid-term results are excellent.
Conflict of interest: none declared.
REFERENCES
- tetralogy of fallot
- double outlet right ventricle
- divided left atrium
- left atrium
- lung
- atrial septal defect
- atrioventricular septal defect
- congenital heart defects
- patent ductus arteriosus
- ventricular septal defect
- child
- demography
- objective (goal)
- tissue membrane
- pediatrics
- pneumonia
- preoperative care
- surgical procedures, operative
- heart
- mortality
- pathology
- surgery specialty
- multiple organ dysfunction syndrome
- inflow tract of left ventricle




