-
PDF
- Split View
-
Views
-
Cite
Cite
Hideyuki Shimizu, Atsuo Mori, Akihiro Yoshitake, Tatsuya Yamada, Hiroshi Morisaki, Hideyuki Okano, Ryohei Yozu, Thoracic and thoracoabdominal aortic repair under regional spinal cord hypothermia, European Journal of Cardio-Thoracic Surgery, Volume 46, Issue 1, July 2014, Pages 40–43, https://doi.org/10.1093/ejcts/ezt574
Close - Share Icon Share
Abstract
Spinal cord deficits are devastating complications after surgery for thoracic and thoracoabdominal aortic aneurysms. We developed a regional spinal cord cooling system using an epidural catheter containing cold saline within an isolated counter-current lumen to prevent such complications and reviewed the clinical results.
We enrolled 37 patients with thoracic (n = 13) and thoracoabdominal (n = 24) aortic aneurysms that were repaired using the regional spinal cord cooling system under mild hypothermia with a partial femoro-femoral bypass.
Although 2 patients died before hospital discharge (hospital mortality, 5.4%), none developed neurological deficits such as paraplegia or paraparesis.
The outcomes of surgery for thoracic and thoracoabdominal aortic aneurysms under regional spinal cord hypothermia using a custom-designed epidural catheter were excellent. Although our patient cohort was small, the results indicate that our technique might help to improve the outcomes of thoracic and thoracoabdominal aortic repair.
INTRODUCTION
Paraplegia remains a devastating complication associated with surgery for thoracic and thoracoabdominal aortic aneurysms. The causes of spinal cord insufficiency are multifactorial and preventive measures considerably vary. Among these, hypothermia can reliably protect the spinal cord from ischaemic damage, but it can lead to the adverse systemic effects of prolonged cardiopulmonary bypass and profound hypothermia (coagulopathy, endothelial dysfunction, systemic inflammation, etc.) when applied systemically. Thus, the concept of providing regional hypothermia only to the spinal cord is considered of value if it could provide the same degree of spinal cord protection as deep hypothermic circulatory arrest. We experimentally demonstrated the effects of regional spinal cord cooling using a custom-designed epidural catheter to prevent ischaemic spinal cord injury [1–3] and introduced its application to the clinical setting [4]. Here, we describe the results of thoracic and thoracoabdominal aortic repair using this innovative method.
PATIENTS AND METHODS
Patients
Thirty-seven patients (male, n = 27; female, n = 10) with a mean age of 63.8 (range, 23–82) years with aneurysms of the descending thoracic (n = 13) or thoracoabdominal (n = 24; Extents I, II, III and IV: n = 2, 10, 9 and 3) aorta underwent elective surgery using our novel cooling method between September 2008 and February 2012 (Table 1). The aetiology of aortic disease was atherosclerotic aneurysm (n = 13), aortic dissection (n = 23) and pseudoaneurysm after infection (n = 1). Two patients had Marfan syndrome and 16 had undergone prior operations on the aortic root (n = 1), the ascending (n = 3), transverse (n = 5), descending (n = 3) and abdominal (n = 7) aorta. Preoperative morbidity comprised a history of myocardial infarction (n = 2), percutaneous coronary intervention (n = 3), pacemaker implantation (n = 1), chronic obstructive pulmonary disease (n = 4), pleuritis from asbestos (n = 1) and a history of tracheotomy (n = 1), cerebral infarction (n = 2) and diabetes mellitus (n = 3).
| Age (years) | |
| Mean (SD) | 63.8 (12.8) |
| Range | 23–82 |
| Sex (n) | |
| Male | 27 |
| Female | 10 |
| Marfan syndrome (n) | 2 |
| Aetiology (n) | |
| Atherosclerotic aneurysm | 13 |
| Aortic dissection | 23 |
| Pseudoaneurysm after infection | 1 |
| Extent of aneurysm (n) | |
| Descending thoracic aorta | 13 |
| Thoracoabdominal aorta | 24 |
| Extent I, II, III, IV (n) | 2, 10, 9, 3 |
| Preoperative morbidity (n) | |
| Cerebrovascular disease | 2 |
| Coronary artery disease | 5 |
| Chronic obstructive pulmonary diseasea | 4 |
| Pleuritis from asbestos | 1 |
| Diabetes mellitus | 3 |
| History of pacemaker implantation | 1 |
| History of tracheotomy | 1 |
| Age (years) | |
| Mean (SD) | 63.8 (12.8) |
| Range | 23–82 |
| Sex (n) | |
| Male | 27 |
| Female | 10 |
| Marfan syndrome (n) | 2 |
| Aetiology (n) | |
| Atherosclerotic aneurysm | 13 |
| Aortic dissection | 23 |
| Pseudoaneurysm after infection | 1 |
| Extent of aneurysm (n) | |
| Descending thoracic aorta | 13 |
| Thoracoabdominal aorta | 24 |
| Extent I, II, III, IV (n) | 2, 10, 9, 3 |
| Preoperative morbidity (n) | |
| Cerebrovascular disease | 2 |
| Coronary artery disease | 5 |
| Chronic obstructive pulmonary diseasea | 4 |
| Pleuritis from asbestos | 1 |
| Diabetes mellitus | 3 |
| History of pacemaker implantation | 1 |
| History of tracheotomy | 1 |
aFEV1/FVC ratio <70%, and/or on chronic inhaled or oral bronchodilator therapy.
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
| Age (years) | |
| Mean (SD) | 63.8 (12.8) |
| Range | 23–82 |
| Sex (n) | |
| Male | 27 |
| Female | 10 |
| Marfan syndrome (n) | 2 |
| Aetiology (n) | |
| Atherosclerotic aneurysm | 13 |
| Aortic dissection | 23 |
| Pseudoaneurysm after infection | 1 |
| Extent of aneurysm (n) | |
| Descending thoracic aorta | 13 |
| Thoracoabdominal aorta | 24 |
| Extent I, II, III, IV (n) | 2, 10, 9, 3 |
| Preoperative morbidity (n) | |
| Cerebrovascular disease | 2 |
| Coronary artery disease | 5 |
| Chronic obstructive pulmonary diseasea | 4 |
| Pleuritis from asbestos | 1 |
| Diabetes mellitus | 3 |
| History of pacemaker implantation | 1 |
| History of tracheotomy | 1 |
| Age (years) | |
| Mean (SD) | 63.8 (12.8) |
| Range | 23–82 |
| Sex (n) | |
| Male | 27 |
| Female | 10 |
| Marfan syndrome (n) | 2 |
| Aetiology (n) | |
| Atherosclerotic aneurysm | 13 |
| Aortic dissection | 23 |
| Pseudoaneurysm after infection | 1 |
| Extent of aneurysm (n) | |
| Descending thoracic aorta | 13 |
| Thoracoabdominal aorta | 24 |
| Extent I, II, III, IV (n) | 2, 10, 9, 3 |
| Preoperative morbidity (n) | |
| Cerebrovascular disease | 2 |
| Coronary artery disease | 5 |
| Chronic obstructive pulmonary diseasea | 4 |
| Pleuritis from asbestos | 1 |
| Diabetes mellitus | 3 |
| History of pacemaker implantation | 1 |
| History of tracheotomy | 1 |
aFEV1/FVC ratio <70%, and/or on chronic inhaled or oral bronchodilator therapy.
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
The Institutional Review Board of Keio University Hospital approved the study protocol, and all patients provided written, informed consent to participate in all procedures associated with the study.
Continuous spinal cord cooling using a custom-designed epidural catheter
A custom-designed polyurethane epidural catheter (Unitika, Tokyo, Japan), an external circuit tube and a pump with a hollow fibre heat exchanger (Senko-Ika Co., Ltd., Tokyo, Japan) comprised the circuit for the continuous cooling system as described [4]. The catheter (16-gauge outer diameter; length, 30 cm) has two ends that form the inlet and outlet of a single U-shaped closed lumen inside it. The direction of the flow of saline infused from the inlet reverses at the tip of the catheter and flows out of the outlet (Fig. 1).
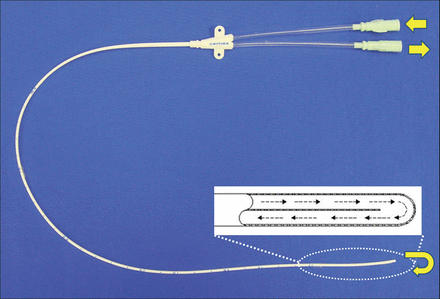
Custom-designed polyurethane epidural catheter. The catheter (16-gauge outer diameter; length, 30 cm) has two ends that form the inlet and outlet of a single U-shaped closed lumen inside it. Cold saline circulates in the closed lumen of the catheter, reversing at tip without leakage.
The catheter was positioned percutaneously in the epidural space on the day before surgery. The patients were placed in the prone position, and the skin on the back was punctured at Level 1–2 of the lumbar spine. The catheter was introduced into the epidural space and advanced in the direction of the head under fluoroscopic guidance (Supplementary Video 1). Another catheter was concurrently placed intrathecally to drain cerebrospinal fluid in 31 patients. In the operation room, the indwelling epidural catheter was connected to an external circuit tube and a pump with a hollow fibre heat exchanger (Senko-Ika Co., Ltd) (Supplementary Video 2).
Regional spinal cord cooling was started 30 min beforehand, continued during aortic cross-clamping and terminated 30 min after unclamping. The temperature of the saline was reduced to 12–13°C by a cooling unit and the saline was infused into the inlet of the counter-current catheter and then circulated by an external roller pump at an approximate flow rate of 60 ml/min. Cerebrospinal fluid (CSF) pressure was maintained at 13 cmH2O or less throughout the procedure.
Surgical procedure
Patients under general anaesthesia underwent selective bronchial intubation using a double-lumen endotracheal tube for selective lung ventilation and separation. Surgery proceeded with patients in the right lateral position (Table 2).
| Approach | |
| Spiral incision | 24 |
| Lateral thoracotomy | 13 |
| Type of prosthetic graft | |
| Branched graft | 24 |
| +Y-graft | +3 |
| Straight graft | 13 |
| Site of proximal clamp | |
| Between LCA and LSA | 7 |
| Distal to LSA | 30 |
| Number of reconstructed renal/visceral vessels | |
| 4 | 22 |
| 2 | 2 |
| 0 | 13 |
| Reconstruction of intercostal arteries | |
| Yes | 28 |
| No | 9 |
| Pump run-time (min; means ± SD) | |
| Partial cardiopulmonary bypass | 115 ± 56 |
| Aortic cross-clamp | 102 ± 69 |
| Approach | |
| Spiral incision | 24 |
| Lateral thoracotomy | 13 |
| Type of prosthetic graft | |
| Branched graft | 24 |
| +Y-graft | +3 |
| Straight graft | 13 |
| Site of proximal clamp | |
| Between LCA and LSA | 7 |
| Distal to LSA | 30 |
| Number of reconstructed renal/visceral vessels | |
| 4 | 22 |
| 2 | 2 |
| 0 | 13 |
| Reconstruction of intercostal arteries | |
| Yes | 28 |
| No | 9 |
| Pump run-time (min; means ± SD) | |
| Partial cardiopulmonary bypass | 115 ± 56 |
| Aortic cross-clamp | 102 ± 69 |
LCA: left carotid artery; LSA: left subclavian artery.
| Approach | |
| Spiral incision | 24 |
| Lateral thoracotomy | 13 |
| Type of prosthetic graft | |
| Branched graft | 24 |
| +Y-graft | +3 |
| Straight graft | 13 |
| Site of proximal clamp | |
| Between LCA and LSA | 7 |
| Distal to LSA | 30 |
| Number of reconstructed renal/visceral vessels | |
| 4 | 22 |
| 2 | 2 |
| 0 | 13 |
| Reconstruction of intercostal arteries | |
| Yes | 28 |
| No | 9 |
| Pump run-time (min; means ± SD) | |
| Partial cardiopulmonary bypass | 115 ± 56 |
| Aortic cross-clamp | 102 ± 69 |
| Approach | |
| Spiral incision | 24 |
| Lateral thoracotomy | 13 |
| Type of prosthetic graft | |
| Branched graft | 24 |
| +Y-graft | +3 |
| Straight graft | 13 |
| Site of proximal clamp | |
| Between LCA and LSA | 7 |
| Distal to LSA | 30 |
| Number of reconstructed renal/visceral vessels | |
| 4 | 22 |
| 2 | 2 |
| 0 | 13 |
| Reconstruction of intercostal arteries | |
| Yes | 28 |
| No | 9 |
| Pump run-time (min; means ± SD) | |
| Partial cardiopulmonary bypass | 115 ± 56 |
| Aortic cross-clamp | 102 ± 69 |
LCA: left carotid artery; LSA: left subclavian artery.
A left thoracotomy was carried out through an appropriate intercostal space in 13 patients with a descending thoracic aortic aneurysm. After full heparinization, the femoral artery and vein were cannulated for arterial return and venous drainage followed by a femoro-femoral bypass with a centrifugal pump, and a membrane oxygenator was established for distal perfusion during aortic cross-clamping. The blood was not cooled actively with the external heat exchanger, although the rectal temperature spontaneously dropped to 34–36°C. The aorta was cross-clamped using the serial shift technique if necessary (n = 10), and replaced with a prosthetic graft. The proximal clamp was applied between the left carotid and left subclavian arteries in 4 patients. The intercostal arteries were reconstructed in a bevelled fashion in 9 patients.
The skin incision was extended towards the umbilicus, and the diaphragm was divided in 24 patients with thoracoabdominal aortic aneurysms. The abdominal aorta was exposed via the retroperitoneal approach. A 4-branched prosthesis (Gelweave, Vascutek, UK) was applied, and an additional bifurcated graft (Gelsoft plus, Vascutek, UK) was anastomosed beforehand with a branched prosthesis for iliac reconstruction in 4 patients. All but one patient underwent aortic cross-clamping using a retrograde serial shift technique. A distal anastomosis was created between clamps placed in the infra-renal aorta or the iliac artery before starting the cardiopulmonary bypass, then the proximal clamp was shifted to the supra-celiac aorta and the visceral and renal arteries were selectively perfused and reconstructed. The celiac, superior mesenteric and bilateral renal arteries were selectively perfused with blood and reconstructed in 22 patients, and only the celiac and the superior mesenteric arteries were perfused and reconstructed in the other two. The proximal clamp was shifted more proximally, the segmental arteries were reconstructed, the proximal clamp was finally shifted proximal to the aneurysm and a proximal anastomosis was created. The segmental arteries were reconstructed in an island fashion (n = 11), a bevelled fashion (n = 4) and using an interposition graft (n = 4). The left subclavian artery was proximally clamped in 3 patients.
RESULTS
The mean durations of aortic cross-clamping and external corporeal bypass were 142 ± 69 and 150 ± 56 min, respectively. At 30 min after starting the external corporeal bypass, the temperatures of the rectum and the inlet and outlet of the cooling catheter were 35.2 (SD, 0.6) , 13.2 (SD, 1.1) and 15.1 (SD, 1.7)°C, respectively.
Two patients died in the hospital due to respiratory failure (n = 1) and multiple embolization (n = 1). All other patients recovered full use of the lower limbs with no paraplegia/paraparesis and were discharged from the hospital. The tracheal tube was removed from 32 of the 37 patients within 24 h of surgery. Postoperative complications were chylothorax (n = 1), tracheostomy (n = 1) and temporary haemodialysis (n = 3; 2 of whom died in the hospital). Complications including coagulopathy and cardiac events did not arise.
During follow-up, 2 patients died of old age and alcoholic liver cirrhosis at 1 and 2 years after surgery, respectively. The other patients remain alive and well.
DISCUSSION
Paraplegia remains the most serious complication associated with surgery for thoracic and thoracoabdominal aortic aneurysms. The causes of spinal cord insufficiency are multifactorial and preventive measures considerably vary. Among them, hypothermia is a reliable way to protect the spinal cord and viscera from ischaemic damage, as it reduces metabolism and increases tolerance to ischaemia. Thus, some surgeons preferentially apply deep hypothermia to most patients who require extensive operations on the descending thoracic and thoracoabdominal aorta [5–7]. However, the duration of CPB is longer and inflammatory consequences are associated. A study of >5000 patients who underwent cardiac surgery has shown that prolonged CPB duration is an independent risk factor for postoperative death, pulmonary, renal and neurological complications, multiorgan failure, reoperation for bleeding and multiple blood transfusions [8]. The advantages of mild over deep hypothermia include maintaining a stable intrinsic cardiac rhythm, a decreased risk of coagulopathy and a shorter period of cardiopulmonary bypass. Because the incidence of serious complications in deep hypothermia has increased, some surgeons recommend judicious application of this method in limited specific situations when cross-clamping the aorta is unfeasible or catastrophic intraoperative bleeding leaves the surgeon with no other option [9–11]. So far, the outcomes of surgery to repair the descending thoracic and thoracoabdominal aorta with and without deep hypothermic arrest have not been compared in a large-scale randomized trial. A dilemma remains because although hypothermia optimally protects various organs, including the spinal cord, it has major drawbacks when applied systemically.
Vacanti and Ames [12] found in an experimental study of rabbits that a temperature reduction of only 3°C during the period of circulatory impairment caused a doubling of the duration of ischaemia that could be reversibly sustained. Strauch et al. [13] also found that mild hypothermia (32°C) obviously increased spinal cord tolerance to ischaemia in an experimental study using pigs. Paraplegia rarely occurs during repair of aortic arch aneurysms in the clinical setting if body temperature is lowered to 28°C, despite a mean duration of circulatory arrest with antegrade cerebral perfusion of 46 min [14]. Ideally, spinal cord temperature should be aggressively lowered to a level that cannot be conventionally achieved. We speculate that regional hypothermia, in which the spinal cord temperature is aggressively lowered while that of the body is maintained at mildly hypothermic levels, will achieve the target without the drawbacks of deep hypothermia. Several experiments have shown that regional cooling by infusing cold saline into the intrathecal or epidural space protects against ischaemic spinal cord insult [15–17]. Cambria et al. [18] and Tabayashi et al. [19] described favourable clinical results of this procedure. However, it might cause a detrimental increase in CSF pressure. To avoid such potential adverse effects, we developed a method of regional spinal cord cooling using an epidural catheter containing cold saline within its isolated counter-current lumen, which enabled effective regional cooling without elevating CSF pressure [1–3]. We described our initial experience of the first 6 patients treated using this method during surgery mainly for descending thoracic aortic aneurysms [4]. As the combination of epidural cooling and CSF drainage might be a promising strategy, we have recently started to apply it to patients upon request. In our series, no paraplegia/paraparesis developed in 37 patients, although it developed in 4 of 120 (3.3%) patients who underwent thoracic (n = 75)/thoracoabdominal (n = 45) aortic aneurysm repair using CSF drainage, but not using regional cord cooling system. The volume of CSF drainage during aortic clamping was less in our series compared with patients without the regional cord cooling system (8.3 ± 9.4 vs 13.1 ± 12.3 ml), although the difference was not statistically significant.
Thoracic and thoracoabdominal repair with a regional spinal cord cooling system using a custom-designed counter-current epidural catheter achieved excellent outcomes. Although our patient cohort was small, the technology seems promising and worthy of a randomized trial.
SUPPLEMENTARY MATERIAL
Supplementary material (Videos 1 and 2) is available at EJCTS online.
Video 1. Insertion of an epidural catheter. The special designed catheter is positioned percutaneously in the epidural space on the day before surgery. Patients are placed in the prone position and the skin on the back was punctured at level 1–2 of the lumbar spine. The catheter is introduced into the epidural space and advanced in the direction of the head under fluoroscopic guidance.
Video 2. Continuous cord cooling system. In the operation room, the indwelling catheter is connected to an external circuit tube and a pump with a hollow fibre heat exchanger.
Funding
This work was supported in part by a grant-in-aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology.
Conflict of interest: none declared.
REFERENCES
- aortic aneurysm
- hypothermia, natural
- objective (goal)
- hospital mortality
- paraparesis
- paraplegia
- patient discharge
- surgical procedures, operative
- spinal cord
- surgery specialty
- bypass
- thoracoabdominal aortic aneurysm
- normal saline
- aortic surgery
- epidural catheters
- neurologic deficits
- saline solutions
- surgical outcome




