-
PDF
- Split View
-
Views
-
Cite
Cite
Yutaka Iba, Kenji Minatoya, Hitoshi Matsuda, Hiroaki Sasaki, Hiroshi Tanaka, Tatsuya Oda, Junjiro Kobayashi, How should aortic arch aneurysms be treated in the endovascular aortic repair era? A risk-adjusted comparison between open and hybrid arch repair using propensity score-matching analysis, European Journal of Cardio-Thoracic Surgery, Volume 46, Issue 1, July 2014, Pages 32–39, https://doi.org/10.1093/ejcts/ezt615
Close - Share Icon Share
Abstract
Recent advances in endovascular aortic repair have changed the treatment of aortic arch aneurysms. The purpose of this study was to compare the early and mid-term outcomes of open repair and hybrid arch repair for aortic arch aneurysms.
This study included 143 and 50 patients who underwent open aortic repair and hybrid thoracic endovascular aortic repair (TEVAR), respectively, for non-dissecting aortic arch aneurysms from 2008 to 2013. The European System for Cardiac Operative Risk Evaluation II scores were 4.35 ± 3.65% and 7.78 ± 5.49% for the open and hybrid TEVAR groups, respectively (P < 0.001). Furthermore, 35 patients from each group were matched using propensity scores to adjust for differences in patient characteristics.
There was no significant difference in early mortality between the open and hybrid groups (3 vs 2%, P = 0.76). Early morbidity was equivalent in both groups, but intensive care unit (ICU) lengths of stay were shorter in members of the hybrid group (4.7 vs 1.6 days, P = 0.018). During the follow-up, survival rates were not significantly different (87 vs 81% at 3 years, P = 0.13), but reinterventions for the aortic arch were required in 1 patient (pseudoaneurysm) in the open group and 5 (endoleak in 4, brachiocephalic artery stenosis in 1) in the hybrid group. The rates of freedom from reintervention at 3 years were 99% in the open group and 80% in the hybrid group (P < 0.001). Propensity score matching yielded similar results for shorter ICU and hospital lengths of stay and more frequent reintervention in the hybrid group.
Surgical outcomes in both groups were satisfactory. Hybrid TEVAR was superior in terms of early recovery from surgery; however, open arch repair showed more reliable long-term outcomes. When properly selected according to patient risk, these two strategies improve the surgical results in all patients with aortic arch aneurysms.
INTRODUCTION
Surgical treatment for aortic arch aneurysms has been considered challenging because of its significantly high mortality and morbidity. However, recent advances in surgical techniques and management have improved outcomes during the past two decades. In particular, the widespread use of selective cerebral perfusion (SCP) to prevent brain damage has contributed to lower mortality and stroke rate for open arch repair [1–4]. Endovascular aortic repair has been implemented as an alternative option for the treatment of aortic aneurysms. This new technology was used initially for descending thoracic and abdominal aortic aneurysms. However, with improvements in devices and techniques, the application of this endovascular repair modality has been extended to aortic arch lesions. Thoracic endovascular aortic repair (TEVAR) of the arch lesion often requires a supra-aortic bypass to debranch the arch vessels; therefore, this less-invasive alternative technology is regarded as hybrid TEVAR [5, 6]. Ever since a commercially available TEVAR device was introduced in Japan in 2008 [7], this new surgical technique has been used at our institution mainly for high-risk patients such as elderly patients, those with severe comorbidities and those with a history of cardiac surgery.
Therefore, the surgical technique used for repairing aortic arch aneurysms must be reconsidered given the widespread application of hybrid TEVAR and the improved outcomes of open aortic arch repair. However, to our knowledge, few reports comparing the surgical outcomes of these two therapeutic strategies exist [8]. We assumed that this is perhaps related to the differences of preoperative patient characteristics because hybrid TEVAR is usually indicated for high-risk patients.
In the present study, we assessed the early and mid-term results of open repair and hybrid arch repair for aortic arch aneurysms, and compared propensity score-matched groups for adjusting the differences in patient characteristics.
MATERIALS AND METHODS
From 2008 to 2013, 143 patients underwent total arch replacement (TAR) and 50 patients also underwent hybrid TEVAR for non-dissecting aortic arch aneurysms at our institution. The exclusion criteria included thoracic aneurysms extending below the level of the carina, acute or chronic aortic dissection, and those in patients requiring a concomitant cardiac procedure. The patient characteristics of both groups are listed in Table 1. The mean ages were 72.1 ± 9.2 years in the TAR group and 78.6 ± 9.3 years in the hybrid group (P < 0.001). Six patients each in the TAR group (4%) and in the hybrid group (12%) had a past history of cardiac surgery (P = 0.049). There were 3 (2%) patients with poor cardiac function in the TAR group and 5 (10%) in the hybrid group (P = 0.016). European System for Cardiac Operative Risk Evaluation (EuroSCORE) II is a new model for calculating the risk of death after heart surgery [9]. EuroSCORE II was 4.35 ± 3.65% for the TAR group and 7.78 ± 5.49% for the hybrid group (P < 0.001). Characteristics of a matched population according to propensity scores are given in Table 1, and the procedural details of TAR and hybrid TEVAR are given in Table 2.
| . | Overall . | Matched cohorts . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAR . | . | Hybrid TEVAR . | . | P-value . | TAR . | . | Hybrid TEVAR . | . | P-value . | |
| Number of patients | 143 | 50 | 35 | 35 | ||||||
| Age (year) | 72.1 ± 9.2 | 78.6 ± 9.3 | <0.001 | 76.3 ± 8.5 | 75.7 ± 9.3 | 0.85 | ||||
| Median | 73 | 80 | 77 | 77 | ||||||
| Range | 17–87 | 44–95 | 37–87 | 44–89 | ||||||
| Male gender | 117 | 82% | 40 | 80% | 0.78 | 25 | 71% | 29 | 83% | 0.26 |
| Hypertension | 127 | 89% | 48 | 96% | 0.13 | 33 | 94% | 33 | 94% | >0.99 |
| Diabetes | 23 | 16% | 9 | 18% | 0.75 | 5 | 14% | 5 | 14% | >0.99 |
| Hypelipidaemia | 64 | 45% | 18 | 36% | 0.28 | 15 | 43% | 16 | 46% | 0.81 |
| Prior cardiotomy | 6 | 4% | 6 | 12% | 0.049 | 4 | 11% | 5 | 14% | 0.72 |
| CVD | 25 | 18% | 12 | 24% | 0.31 | 12 | 34% | 9 | 26% | 0.43 |
| Coronary artery disease | 28 | 20% | 14 | 28% | 0.21 | 8 | 23% | 10 | 29% | 0.58 |
| Low ejection fraction (<40%) | 3 | 2% | 5 | 10% | 0.016 | 2 | 6% | 3 | 9% | 0.64 |
| COPD | 20 | 14% | 10 | 20% | 0.31 | 5 | 14% | 7 | 20% | 0.53 |
| Arteriopathy | 63 | 44% | 25 | 50% | 0.47 | 13 | 37% | 15 | 43% | 0.63 |
| CKD (creatinine >1.5) | 18 | 13% | 8 | 16% | 0.54 | 4 | 11% | 6 | 17% | 0.50 |
| Emergency | 12 | 8% | 1 | 2% | 0.12 | 2 | 6% | 1 | 3% | 0.56 |
| EuroSCORE II (%) | 4.35 ± 3.65 | 7.78 ± 5.49 | <0.001 | 5.81 ± 4.39 | 7.03 ± 5.19 | 0.46 | ||||
| . | Overall . | Matched cohorts . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAR . | . | Hybrid TEVAR . | . | P-value . | TAR . | . | Hybrid TEVAR . | . | P-value . | |
| Number of patients | 143 | 50 | 35 | 35 | ||||||
| Age (year) | 72.1 ± 9.2 | 78.6 ± 9.3 | <0.001 | 76.3 ± 8.5 | 75.7 ± 9.3 | 0.85 | ||||
| Median | 73 | 80 | 77 | 77 | ||||||
| Range | 17–87 | 44–95 | 37–87 | 44–89 | ||||||
| Male gender | 117 | 82% | 40 | 80% | 0.78 | 25 | 71% | 29 | 83% | 0.26 |
| Hypertension | 127 | 89% | 48 | 96% | 0.13 | 33 | 94% | 33 | 94% | >0.99 |
| Diabetes | 23 | 16% | 9 | 18% | 0.75 | 5 | 14% | 5 | 14% | >0.99 |
| Hypelipidaemia | 64 | 45% | 18 | 36% | 0.28 | 15 | 43% | 16 | 46% | 0.81 |
| Prior cardiotomy | 6 | 4% | 6 | 12% | 0.049 | 4 | 11% | 5 | 14% | 0.72 |
| CVD | 25 | 18% | 12 | 24% | 0.31 | 12 | 34% | 9 | 26% | 0.43 |
| Coronary artery disease | 28 | 20% | 14 | 28% | 0.21 | 8 | 23% | 10 | 29% | 0.58 |
| Low ejection fraction (<40%) | 3 | 2% | 5 | 10% | 0.016 | 2 | 6% | 3 | 9% | 0.64 |
| COPD | 20 | 14% | 10 | 20% | 0.31 | 5 | 14% | 7 | 20% | 0.53 |
| Arteriopathy | 63 | 44% | 25 | 50% | 0.47 | 13 | 37% | 15 | 43% | 0.63 |
| CKD (creatinine >1.5) | 18 | 13% | 8 | 16% | 0.54 | 4 | 11% | 6 | 17% | 0.50 |
| Emergency | 12 | 8% | 1 | 2% | 0.12 | 2 | 6% | 1 | 3% | 0.56 |
| EuroSCORE II (%) | 4.35 ± 3.65 | 7.78 ± 5.49 | <0.001 | 5.81 ± 4.39 | 7.03 ± 5.19 | 0.46 | ||||
COPD: chronic obstructive pulmonary disease, CKD: chronic kidney disease, EuroSCORE: European System for Cardiac Operative Risk Evaluation; TAR: total arch replacement; TEVAR: thoracic endovascular aortic repair.
| . | Overall . | Matched cohorts . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAR . | . | Hybrid TEVAR . | . | P-value . | TAR . | . | Hybrid TEVAR . | . | P-value . | |
| Number of patients | 143 | 50 | 35 | 35 | ||||||
| Age (year) | 72.1 ± 9.2 | 78.6 ± 9.3 | <0.001 | 76.3 ± 8.5 | 75.7 ± 9.3 | 0.85 | ||||
| Median | 73 | 80 | 77 | 77 | ||||||
| Range | 17–87 | 44–95 | 37–87 | 44–89 | ||||||
| Male gender | 117 | 82% | 40 | 80% | 0.78 | 25 | 71% | 29 | 83% | 0.26 |
| Hypertension | 127 | 89% | 48 | 96% | 0.13 | 33 | 94% | 33 | 94% | >0.99 |
| Diabetes | 23 | 16% | 9 | 18% | 0.75 | 5 | 14% | 5 | 14% | >0.99 |
| Hypelipidaemia | 64 | 45% | 18 | 36% | 0.28 | 15 | 43% | 16 | 46% | 0.81 |
| Prior cardiotomy | 6 | 4% | 6 | 12% | 0.049 | 4 | 11% | 5 | 14% | 0.72 |
| CVD | 25 | 18% | 12 | 24% | 0.31 | 12 | 34% | 9 | 26% | 0.43 |
| Coronary artery disease | 28 | 20% | 14 | 28% | 0.21 | 8 | 23% | 10 | 29% | 0.58 |
| Low ejection fraction (<40%) | 3 | 2% | 5 | 10% | 0.016 | 2 | 6% | 3 | 9% | 0.64 |
| COPD | 20 | 14% | 10 | 20% | 0.31 | 5 | 14% | 7 | 20% | 0.53 |
| Arteriopathy | 63 | 44% | 25 | 50% | 0.47 | 13 | 37% | 15 | 43% | 0.63 |
| CKD (creatinine >1.5) | 18 | 13% | 8 | 16% | 0.54 | 4 | 11% | 6 | 17% | 0.50 |
| Emergency | 12 | 8% | 1 | 2% | 0.12 | 2 | 6% | 1 | 3% | 0.56 |
| EuroSCORE II (%) | 4.35 ± 3.65 | 7.78 ± 5.49 | <0.001 | 5.81 ± 4.39 | 7.03 ± 5.19 | 0.46 | ||||
| . | Overall . | Matched cohorts . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAR . | . | Hybrid TEVAR . | . | P-value . | TAR . | . | Hybrid TEVAR . | . | P-value . | |
| Number of patients | 143 | 50 | 35 | 35 | ||||||
| Age (year) | 72.1 ± 9.2 | 78.6 ± 9.3 | <0.001 | 76.3 ± 8.5 | 75.7 ± 9.3 | 0.85 | ||||
| Median | 73 | 80 | 77 | 77 | ||||||
| Range | 17–87 | 44–95 | 37–87 | 44–89 | ||||||
| Male gender | 117 | 82% | 40 | 80% | 0.78 | 25 | 71% | 29 | 83% | 0.26 |
| Hypertension | 127 | 89% | 48 | 96% | 0.13 | 33 | 94% | 33 | 94% | >0.99 |
| Diabetes | 23 | 16% | 9 | 18% | 0.75 | 5 | 14% | 5 | 14% | >0.99 |
| Hypelipidaemia | 64 | 45% | 18 | 36% | 0.28 | 15 | 43% | 16 | 46% | 0.81 |
| Prior cardiotomy | 6 | 4% | 6 | 12% | 0.049 | 4 | 11% | 5 | 14% | 0.72 |
| CVD | 25 | 18% | 12 | 24% | 0.31 | 12 | 34% | 9 | 26% | 0.43 |
| Coronary artery disease | 28 | 20% | 14 | 28% | 0.21 | 8 | 23% | 10 | 29% | 0.58 |
| Low ejection fraction (<40%) | 3 | 2% | 5 | 10% | 0.016 | 2 | 6% | 3 | 9% | 0.64 |
| COPD | 20 | 14% | 10 | 20% | 0.31 | 5 | 14% | 7 | 20% | 0.53 |
| Arteriopathy | 63 | 44% | 25 | 50% | 0.47 | 13 | 37% | 15 | 43% | 0.63 |
| CKD (creatinine >1.5) | 18 | 13% | 8 | 16% | 0.54 | 4 | 11% | 6 | 17% | 0.50 |
| Emergency | 12 | 8% | 1 | 2% | 0.12 | 2 | 6% | 1 | 3% | 0.56 |
| EuroSCORE II (%) | 4.35 ± 3.65 | 7.78 ± 5.49 | <0.001 | 5.81 ± 4.39 | 7.03 ± 5.19 | 0.46 | ||||
COPD: chronic obstructive pulmonary disease, CKD: chronic kidney disease, EuroSCORE: European System for Cardiac Operative Risk Evaluation; TAR: total arch replacement; TEVAR: thoracic endovascular aortic repair.
| Variables . | . | . |
|---|---|---|
| Open arch repair (TAR) | ||
| Operation time (min) | 393 ± 103 | |
| Cardiopulmonary bypass time (min) | 220 ± 49 | |
| Myocardial ischaemic time (min) | 128 ± 35 | |
| Selective cerebral perfusion time (min) | 148 ± 42 | |
| Lower body circulatory arrest time (min) | 63 ± 13 | |
| Bleeding (ml) | 2326 ± 1762 | |
| Hybrid arch TEVAR | ||
| Proximal landing zone of stent-graft | Design of bypass | |
| Zone 0 | 14 (28%) | |
| Total debranching bypass through sternotomy | 8 | Asc Ao to BCA, LCCA and LSCA: 4 |
| Asc Ao to BCA, LCCA and LAxA: 4 | ||
| Chimney graft technique and supra-aortic bypass | 5 | RCCA to LCCA and LAxA: 4 |
| RAxA to LCCA and LAxA: 1 | ||
| Bypass from abdominal aorta | 1 | Abd Ao to RAxA, LCCA and LAxA: 1 |
| Zone 1 | 30 (60%) | RAxA to LCCA and LAxA: 20 |
| RAxA to LCCA and LSCA: 5 | ||
| RCCA to LCCA and LSCA: 3 | ||
| RCCA to LCCA and LAxA: 2 | ||
| Zone 2 | 6 (12%) | RAxA to LAxA: 5 |
| LCCA to LSCA: 1 | ||
| Device | ||
| TAG | 41 (82%) | |
| Valiant | 7 (14%) | |
| Talent | 2 (4%) | |
| Variables . | . | . |
|---|---|---|
| Open arch repair (TAR) | ||
| Operation time (min) | 393 ± 103 | |
| Cardiopulmonary bypass time (min) | 220 ± 49 | |
| Myocardial ischaemic time (min) | 128 ± 35 | |
| Selective cerebral perfusion time (min) | 148 ± 42 | |
| Lower body circulatory arrest time (min) | 63 ± 13 | |
| Bleeding (ml) | 2326 ± 1762 | |
| Hybrid arch TEVAR | ||
| Proximal landing zone of stent-graft | Design of bypass | |
| Zone 0 | 14 (28%) | |
| Total debranching bypass through sternotomy | 8 | Asc Ao to BCA, LCCA and LSCA: 4 |
| Asc Ao to BCA, LCCA and LAxA: 4 | ||
| Chimney graft technique and supra-aortic bypass | 5 | RCCA to LCCA and LAxA: 4 |
| RAxA to LCCA and LAxA: 1 | ||
| Bypass from abdominal aorta | 1 | Abd Ao to RAxA, LCCA and LAxA: 1 |
| Zone 1 | 30 (60%) | RAxA to LCCA and LAxA: 20 |
| RAxA to LCCA and LSCA: 5 | ||
| RCCA to LCCA and LSCA: 3 | ||
| RCCA to LCCA and LAxA: 2 | ||
| Zone 2 | 6 (12%) | RAxA to LAxA: 5 |
| LCCA to LSCA: 1 | ||
| Device | ||
| TAG | 41 (82%) | |
| Valiant | 7 (14%) | |
| Talent | 2 (4%) | |
TEVAR: thoracic endovascular aortic repair; Asc Ao: ascending aorta; BCA: brachiocephalic artery; LCCA: left common carotid artery; LSCA: left subclavian artery; LAxA: left axillary artery; RCCA: right common carotid artery; RAxA: right axillary artery.
| Variables . | . | . |
|---|---|---|
| Open arch repair (TAR) | ||
| Operation time (min) | 393 ± 103 | |
| Cardiopulmonary bypass time (min) | 220 ± 49 | |
| Myocardial ischaemic time (min) | 128 ± 35 | |
| Selective cerebral perfusion time (min) | 148 ± 42 | |
| Lower body circulatory arrest time (min) | 63 ± 13 | |
| Bleeding (ml) | 2326 ± 1762 | |
| Hybrid arch TEVAR | ||
| Proximal landing zone of stent-graft | Design of bypass | |
| Zone 0 | 14 (28%) | |
| Total debranching bypass through sternotomy | 8 | Asc Ao to BCA, LCCA and LSCA: 4 |
| Asc Ao to BCA, LCCA and LAxA: 4 | ||
| Chimney graft technique and supra-aortic bypass | 5 | RCCA to LCCA and LAxA: 4 |
| RAxA to LCCA and LAxA: 1 | ||
| Bypass from abdominal aorta | 1 | Abd Ao to RAxA, LCCA and LAxA: 1 |
| Zone 1 | 30 (60%) | RAxA to LCCA and LAxA: 20 |
| RAxA to LCCA and LSCA: 5 | ||
| RCCA to LCCA and LSCA: 3 | ||
| RCCA to LCCA and LAxA: 2 | ||
| Zone 2 | 6 (12%) | RAxA to LAxA: 5 |
| LCCA to LSCA: 1 | ||
| Device | ||
| TAG | 41 (82%) | |
| Valiant | 7 (14%) | |
| Talent | 2 (4%) | |
| Variables . | . | . |
|---|---|---|
| Open arch repair (TAR) | ||
| Operation time (min) | 393 ± 103 | |
| Cardiopulmonary bypass time (min) | 220 ± 49 | |
| Myocardial ischaemic time (min) | 128 ± 35 | |
| Selective cerebral perfusion time (min) | 148 ± 42 | |
| Lower body circulatory arrest time (min) | 63 ± 13 | |
| Bleeding (ml) | 2326 ± 1762 | |
| Hybrid arch TEVAR | ||
| Proximal landing zone of stent-graft | Design of bypass | |
| Zone 0 | 14 (28%) | |
| Total debranching bypass through sternotomy | 8 | Asc Ao to BCA, LCCA and LSCA: 4 |
| Asc Ao to BCA, LCCA and LAxA: 4 | ||
| Chimney graft technique and supra-aortic bypass | 5 | RCCA to LCCA and LAxA: 4 |
| RAxA to LCCA and LAxA: 1 | ||
| Bypass from abdominal aorta | 1 | Abd Ao to RAxA, LCCA and LAxA: 1 |
| Zone 1 | 30 (60%) | RAxA to LCCA and LAxA: 20 |
| RAxA to LCCA and LSCA: 5 | ||
| RCCA to LCCA and LSCA: 3 | ||
| RCCA to LCCA and LAxA: 2 | ||
| Zone 2 | 6 (12%) | RAxA to LAxA: 5 |
| LCCA to LSCA: 1 | ||
| Device | ||
| TAG | 41 (82%) | |
| Valiant | 7 (14%) | |
| Talent | 2 (4%) | |
TEVAR: thoracic endovascular aortic repair; Asc Ao: ascending aorta; BCA: brachiocephalic artery; LCCA: left common carotid artery; LSCA: left subclavian artery; LAxA: left axillary artery; RCCA: right common carotid artery; RAxA: right axillary artery.
Data were collected from the medical records for patients who visited our outpatient department for follow-up, and the data for the others were acquired by telephone or mail. The follow-up rate was 94% and the mean follow-up period was 25 ± 16 months (1–63 months: median, 24 months). Our institution approved this retrospective study and patient consent was waived on the condition that the patients were not identified.
Operative techniques
Open arch repair (TAR)
The details of our surgical technique for open arch repair (TAR) have been reported previously [10–15]. Median sternotomy was performed as the approach to repair aortic arch aneurysms. To establish a cardiopulmonary bypass, perfusion using the distal part of the right axillary artery (RAxA) in the axilla was routinely used together with ascending aortic or femoral artery cannulation. Our strategy for brain protection employed SCP with perfusion through the RAxA and two other arch vessels using hypothermic circulatory arrest (HCA). During the term of this study, we have preferably used moderate HCA of 25°C–28°C at the lowest bladder and nasopharyngeal temperatures, except in high-risk patients with cerebrovascular disease (CVD) or chronic kidney disease (CKD). After the induction of HCA, RAxA perfusion permitted rapid conversion to SCP by clamping the brachio-cephalic artery (BCA). After the ascending aorta and aortic arch were opened, balloon-tipped SCP cannulas were inserted into the left common carotid artery (LCCA) and left subclavian artery (LSCA). Open distal anastomosis was performed during HCA of the lower body. A stepwise distal anastomosis was frequently used to perform an easy and secure anastomosis. An invaginated tube graft was inserted into the descending aorta. The proximal end was anastomosed to the descending aorta and the distal end of the inserted graft was extracted proximally. Debris was flushed from the descending aorta using femoral artery perfusion. The multibranched aortic arch graft was connected to this interposed graft. Systemic circulation was resumed through a side branch of the arch graft. The LSCA was reconstructed using a branch of the arch graft and the patient was rewarmed to 30–32°C. The proximal aortic stump was anastomosed to the main graft above the sinotubular junction. Finally, the coronary circulation was initiated by unclamping the main graft. The other two arch vessels were reconstructed individually with the branch of the arch graft and the patient was fully rewarmed.
Hybrid thoracic endovascular repair
Zone 2 (n = 6)
In cases in which the RAxA was used as the inflow artery, both axillary arteries were exposed under the subclavicular incision. An 8 mm expanded polytetrafluoroethylene (ePTFE) graft was sutured onto both axillary arteries in a side-to-end fashion. In patients for whom the LCCA was used as the inflow, the LCCA and the LSCA were exposed through the same incision, and an ePTFE graft connected the LCCA to the LSCA in an end-to-side fashion.
Zone 1 (n = 30)
Either the right common carotid artery (RCCA) or the RAxA was chosen as the inflow artery. The RCCA and the LCCA were exposed through a middle cervical incision, and the RAxA was exposed at the subclavicular incision. The LSCA was often exposed at the supraclavicular incision when the RCCA was the inflow artery for the bypass, and the left axillary artery (LAxA) was exposed through the subclavicular incision when the RAxA was the inflow artery. After systemic heparinization, a T-shaped branched-type 8-mm ePTFE graft was anastomosed.
Zone 0 (n = 14)
In cases involving total debranching of the arch vessels, median sternotomy was performed. After systemic heparinization, the ascending aorta was partially clamped and a Dacron prosthetic graft was sutured in a side-to-end fashion. The BCA was clamped and divided while the mean systemic blood pressure was increased to >80 mmHg, reconstructed in an end-to-end fashion. Then, the LCCA was anastomosed in the same fashion. Next, the LSCA was reconstructed in an end-to-end fashion near its origin, or the LAxA was reconstructed in a side-to-end fashion at the axillary segment. The origin of the LSCA was closed by coil embolization after TEVAR. In cases using the chimney graft technique, supra-aortic bypass was established in the same fashion as for Zone 1 landing, and a sheath was inserted through the RAxA as a chimney graft access. The main device was advanced into the ascending aorta from the femoral artery to provide a sufficient proximal landing zone. Furthermore, a self-expandable stent graft (Excluder Iliac Extender; W.L. Gore & Associates, Inc., Flagstaff, AZ, USA) was introduced into the ascending aorta from the RAxA and positioned at the proximal edge of the main stent graft. Next, the stent graft in the BCA was deployed first, followed by the main stent graft.
The transfemoral approach was selected as the access route for main stent-graft placement, if possible. If the femoral artery was unsuitable as the access route, the external iliac artery was selected. The Gore TAG (W.L. Gore & Associates, Inc.) was used in 41 patients (84%), the Valiant (Medtronic, Inc., Minneapolis, MN, USA) was used in 7 patients (14%) and the Talent (Medtronic, Inc.) was used in 2 patients (2%). For preventing supra-aortic bypass graft occlusion, all patients were given low-dose aspirin. Warfarin was concomitantly administered to patients who had severely diseased arteries or whose left vertebral artery was reconstructed.
Definitions
Early mortality was defined as death during hospitalization or within 30 days after surgery. Permanent neurological dysfunction (PND) was defined as the presence of either new permanent focal or global neurological dysfunction persisting at discharge. CVD included a history of cerebrovascular events or severe carotid artery lesions with >75% stenosis or multiple plaques revealed using ultrasound examination. Chronic obstructive pulmonary disease (COPD) was defined as a forced expiratory volume <70% of the normal value or daily use of a bronchodilator. Arteriopathy was defined as previous or planned intervention on the abdominal aorta or coexistence of peripheral artery disease. CKD was defined as a serum creatinine level >1.5 mg/dl or a requirement for haemodialysis.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation and compared using Student's t-test or the Mann–Whitney U-test. Category variables were compared using χ2 tests or Fisher's exact tests. Survival and aortic reintervention-free rates were estimated using the Kaplan–Meier method, and differences between each group were determined using log-rank analysis. P-values < 0.05 were considered significant. When we noted marked differences in a patient's preoperative characteristics, patient matching was employed to compare the results between both groups. Therefore, propensity score-matching analysis was performed to compensate for this difference. The propensity scores were estimated using multivariable logistic regression analyses for each patient, and the covariables included age, gender, hypertension, diabetes, hyperlipidemia, prior cardiotomy, CVD, coronary artery disease, poor cardiac function, COPD, arteriopathy, CKD and emergencies. A patient in the hybrid group was then matched with a patient in the TAR group with the closest propensity score, and the maximum difference of the propensity score was <0.02. Statistical analysis was performed using JMP version 9.0 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
All patients
Early mortalities were 3 and 2% in the TAR and hybrid groups, respectively (P = 0.76). The causes of early death in the TAR groups were sepsis in 2 patients and respiratory failure in 2 patients, and 1 patient in the hybrid group died because of cerebral infarction. PND developed in 3 patients each in the TAR (2%) and hybrid (6%) groups (P = 0.17). No spinal cord injury occurred in either group. Renal failure requiring haemodialysis occurred in 2 patients (1%) in the TAR group, but not in the hybrid group (P = 0.40). Re-entry for bleeding was required necessary for 8 (6%) patients in the TAR group and for 4 patients (8%) in the hybrid group (P = 0.54). Prolonged ventilation for >72 h was required for 8 (6%) and 2 (4%) in the TAR and hybrid groups, respectively (P = 0.66). The lengths of stay in the intensive care unit (ICU) were 4.7 ± 9.0 and 1.6 ± 2.2 days for the TAR and hybrid groups, respectively (P < 0.001). The duration of postoperative hospitalization was 32.9 ± 35.7 and 25.9 ± 29.5 days in the TAR and the hybrid groups, respectively (P = 0.001) (Table 3).
| . | Overall . | Matched cohorts . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAR . | . | Hybrid TEVAR . | . | P-value . | TAR . | . | Hybrid TEVAR . | . | P-value . | |
| Number of patients | 143 | 50 | 35 | 35 | ||||||
| In-hospital death | 4 | 3% | 1 | 2% | 0.76 | 1 | 3% | 1 | 3% | >0.99 |
| PND | 3 | 2% | 3 | 6% | 0.17 | 0 | 0% | 3 | 9% | 0.077 |
| Renal failure | 2 | 1% | 0 | 0% | 0.40 | 1 | 3% | 0 | 0.31 | |
| Re-entry | 8 | 6% | 4 | 8% | 0.54 | 2 | 6% | 3 | 9% | 0.64 |
| Prolonged ventilation | 8 | 6% | 2 | 4% | 0.66 | 2 | 6% | 2 | 6% | >0.99 |
| ICU length of stay | 4.7 ± 9.0 | 1.6 ± 2.2 | <0.001 | 4.8 ± 5.1 | 1.8 ± 2.5 | <0.001 | ||||
| Median (IQR) | 3 (2–4) | 1 (1–1) | 3 (2–5) | 1 (1–1) | ||||||
| Hospitalization (day) | 32.9 ± 35.7 | 25.9 ± 29.5 | 0.001 | 32.8 ± 21.3 | 27.7 ± 31.4 | 0.015 | ||||
| Median (IQR) | 23 (16–30) | 15 (11–29) | 28 (18–38) | 17 (11–31) | ||||||
| . | Overall . | Matched cohorts . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAR . | . | Hybrid TEVAR . | . | P-value . | TAR . | . | Hybrid TEVAR . | . | P-value . | |
| Number of patients | 143 | 50 | 35 | 35 | ||||||
| In-hospital death | 4 | 3% | 1 | 2% | 0.76 | 1 | 3% | 1 | 3% | >0.99 |
| PND | 3 | 2% | 3 | 6% | 0.17 | 0 | 0% | 3 | 9% | 0.077 |
| Renal failure | 2 | 1% | 0 | 0% | 0.40 | 1 | 3% | 0 | 0.31 | |
| Re-entry | 8 | 6% | 4 | 8% | 0.54 | 2 | 6% | 3 | 9% | 0.64 |
| Prolonged ventilation | 8 | 6% | 2 | 4% | 0.66 | 2 | 6% | 2 | 6% | >0.99 |
| ICU length of stay | 4.7 ± 9.0 | 1.6 ± 2.2 | <0.001 | 4.8 ± 5.1 | 1.8 ± 2.5 | <0.001 | ||||
| Median (IQR) | 3 (2–4) | 1 (1–1) | 3 (2–5) | 1 (1–1) | ||||||
| Hospitalization (day) | 32.9 ± 35.7 | 25.9 ± 29.5 | 0.001 | 32.8 ± 21.3 | 27.7 ± 31.4 | 0.015 | ||||
| Median (IQR) | 23 (16–30) | 15 (11–29) | 28 (18–38) | 17 (11–31) | ||||||
PND: permanent neurological dysfunction; ICU: intensive care unit; IQR: interquartile range; TAR: total arch replacement; TEVAR: thoracic endovascular aortic repair.
| . | Overall . | Matched cohorts . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAR . | . | Hybrid TEVAR . | . | P-value . | TAR . | . | Hybrid TEVAR . | . | P-value . | |
| Number of patients | 143 | 50 | 35 | 35 | ||||||
| In-hospital death | 4 | 3% | 1 | 2% | 0.76 | 1 | 3% | 1 | 3% | >0.99 |
| PND | 3 | 2% | 3 | 6% | 0.17 | 0 | 0% | 3 | 9% | 0.077 |
| Renal failure | 2 | 1% | 0 | 0% | 0.40 | 1 | 3% | 0 | 0.31 | |
| Re-entry | 8 | 6% | 4 | 8% | 0.54 | 2 | 6% | 3 | 9% | 0.64 |
| Prolonged ventilation | 8 | 6% | 2 | 4% | 0.66 | 2 | 6% | 2 | 6% | >0.99 |
| ICU length of stay | 4.7 ± 9.0 | 1.6 ± 2.2 | <0.001 | 4.8 ± 5.1 | 1.8 ± 2.5 | <0.001 | ||||
| Median (IQR) | 3 (2–4) | 1 (1–1) | 3 (2–5) | 1 (1–1) | ||||||
| Hospitalization (day) | 32.9 ± 35.7 | 25.9 ± 29.5 | 0.001 | 32.8 ± 21.3 | 27.7 ± 31.4 | 0.015 | ||||
| Median (IQR) | 23 (16–30) | 15 (11–29) | 28 (18–38) | 17 (11–31) | ||||||
| . | Overall . | Matched cohorts . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAR . | . | Hybrid TEVAR . | . | P-value . | TAR . | . | Hybrid TEVAR . | . | P-value . | |
| Number of patients | 143 | 50 | 35 | 35 | ||||||
| In-hospital death | 4 | 3% | 1 | 2% | 0.76 | 1 | 3% | 1 | 3% | >0.99 |
| PND | 3 | 2% | 3 | 6% | 0.17 | 0 | 0% | 3 | 9% | 0.077 |
| Renal failure | 2 | 1% | 0 | 0% | 0.40 | 1 | 3% | 0 | 0.31 | |
| Re-entry | 8 | 6% | 4 | 8% | 0.54 | 2 | 6% | 3 | 9% | 0.64 |
| Prolonged ventilation | 8 | 6% | 2 | 4% | 0.66 | 2 | 6% | 2 | 6% | >0.99 |
| ICU length of stay | 4.7 ± 9.0 | 1.6 ± 2.2 | <0.001 | 4.8 ± 5.1 | 1.8 ± 2.5 | <0.001 | ||||
| Median (IQR) | 3 (2–4) | 1 (1–1) | 3 (2–5) | 1 (1–1) | ||||||
| Hospitalization (day) | 32.9 ± 35.7 | 25.9 ± 29.5 | 0.001 | 32.8 ± 21.3 | 27.7 ± 31.4 | 0.015 | ||||
| Median (IQR) | 23 (16–30) | 15 (11–29) | 28 (18–38) | 17 (11–31) | ||||||
PND: permanent neurological dysfunction; ICU: intensive care unit; IQR: interquartile range; TAR: total arch replacement; TEVAR: thoracic endovascular aortic repair.
During the follow-up period, there were 9 deaths in the TAR group; 2 were cardiovascular related, including 1 due to ventricular arrhythmia and the other due to residual thoracoabdominal aortic aneurysm rupture. The patient with thoracoabdominal aortic aneurysm rupture had been observed medically because of her compromised condition. The other causes of late death were sepsis in 2, cancer in 2, gastrointestinal complication in 2 and pneumonia in 1. In contrast, there were 6 late deaths in the hybrid group, including respiratory failure in 3, a cerebrovascular event in 2 and due to cancer in 1. The cumulative survival rates at 1, 2 and 3 years were 96, 91 and 87%, respectively, in the TAR group and 91, 88 and 81%, respectively, in the hybrid group (P = 0.13) (Fig. 1A).

Cumulative survival curve. (A) All patients. (B) Propensity score-matched cohorts of the TAR and hybrid groups.
Late reintervention for the previously repaired arch segment was required for 1 patient in the TAR group and 5 patients in the hybrid group. The patient in the TAR group received TEVAR for a pseudoaneurysm at a distal anastomotic site 1 month after TAR. In the hybrid group, the causes of late reintervention for the aortic arch were type Ia endoleak in 4 patients including one case of aneurysmal rupture, and BCA stent-graft stenosis after the chimney graft technique in 1 patient. In recurring cases of type Ia endoleak, 3 patients had endoleaks after the Zone 1 landing endografting and 1 had an endoleak at the Zone 2 landing. All reintervention cases of type Ia endoleak underwent another endografting, which was performed at a more proximal site. Of 3 cases of type Ia endoleak after TEVAR with Zone 1 landing, 1 patient underwent a total debranching bypass from the ascending aorta through a median sternotomy for Zone 0 landing TEVAR, and the other case received Zone 0 landing TEVAR using the chimney graft technique. The last case involved an additional left external iliac artery to supra-aortic bypass and followed Zone 0 landing endografting. The patient with endoleak after Zone 2 landing TEVAR underwent an additional LCCA bypass from the previous RAxA-to-LAxA bypass, and a Zone 1 landing endografting was performed. The remaining case of the BCA stenosis after the chimney graft technique received stenting using a bare stent. The rates of freedom from reintervention for previously repaired arch segment at 1, 2 and 3 years were 99, 99 and 99%, respectively, in the TAR group, and 97, 86 and 80%, respectively, in the hybrid groups (P < 0.001) (Fig. 2A).
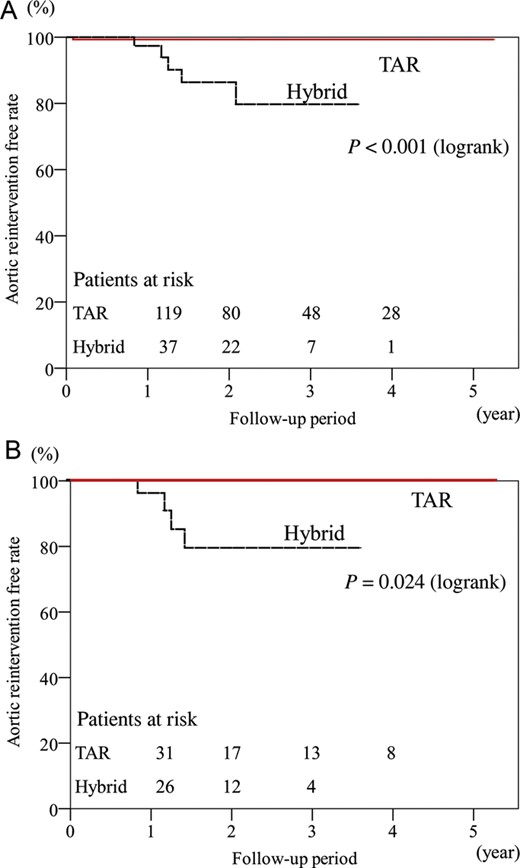
Freedom from late aortic reintervention for previous arch repair. (A) All patients. (B) Propensity score-matched cohorts of the TAR and hybrid groups.
Analysis of matched cohorts by propensity score matching
There was no significant difference in baseline patient characteristics of matched pairs (Table 1). The early mortality rate was identical (3%) in the TAR and hybrid groups. There was no significant difference in early morbidity in both groups, and the incidence of PND (P = 0.077), postoperative renal failure (P = 0.31), re-entry for bleeding (P = 0.64) and prolonged ventilation (P > 0.99). The ICU lengths of stay (P < 0.001) and the duration of postoperative hospitalization (P = 0.015) were significantly shorter in the hybrid group (Table 3). There was no significant difference in cumulative survival (P = 0.098) (Fig. 1B); however, freedom from aortic reintervention for a previously repaired arch segment was higher in the hybrid group (P = 0.024) (Fig. 2B).
DISCUSSION
Because of the recent widespread application of endovascular aortic repair, the surgical strategy for aortic arch aneurysms needs to be reconsidered on the basis of recent outcomes of open arch repair and hybrid arch repair.
In open arch repair, recent advances in brain protection, surgical techniques, prosthetic grafts and critical care have improved surgical outcomes. Of these, antegrade SCP with HCA is widely accepted as a reliable technique for brain protection. Subsequent to the widespread use of SCP, some reports indicate that the core temperature at circulatory arrest should be increased from deep hypothermia to moderate or mild hypothermia to avoid deep hypothermia-associated coagulopathy and to decrease the level of inflammatory substances associated with prolonged cardiopulmonary bypass [4, 16]. Moreover, recent reports demonstrated that some refinement of technical aspects and modification of SCP resulted in lower mortality rates of 3.4–5.2% and lower incidence rates of PND of 2.5–6.7% [15–20]. Kazui et al. [2] reported actuarial survival rates at 5 and 7 years of 79 and 77%, respectively, and Patel et al. [17] reported a 12-year survival rate of 51.2%. Furthermore, we reported that the rate of freedom from aortic reintervention related to initial arch repair was 96.9% at 8 years [15].
The results of the present study indicate that our surgical technique of open arch repair, which included routine use of SCP, preferable application of moderate HCA and stepwise distal anastomosis, contributed to the low mortality rate of 3% and the lower incidence rate of PND of 2%. The data show that open arch repair was reliable with a remarkably lower incidence of aortic reintervention for previous aortic repair during follow-up.
Endovascular aortic repair has recently been recognized as an alternative therapy for thoracic aortic aneurysms because of its minimal invasiveness. This new technique requires a supra-aortic bypass or debranching of arch vessels with or without sternotomy to repair aortic arch lesions. Milewski et al. [6] also published a comparative study of open arch debranching with endovascular stent placement and conventional total and distal aortic arch reconstruction, which concluded that the hybrid arch approach had a lower mortality for high-risk patients aged more than 75 years. In our institution, this less-invasive alternative was applied predominantly to high-risk patients such as the elderly (>75 years of age), those with severe comorbidities (e.g. impaired cardiac, pulmonary, liver or renal function) and those with a history of cardiac surgery [21]. Some reports of hybrid arch procedures reveal an early mortality rate of 7.4–23.7% and incidence rate of stroke of 0–13.1% [5, 6, 22, 23]. Furthermore, Koullias et al. [24] reported a meta-analysis of 463 patients who underwent hybrid arch surgery that reveals a 30-day mortality rate of 8.3% and incidence rates of stroke and paraplegia of 4.4 and 3.9%, respectively. Compared with these findings, our results for hybrid TEVAR are favourable with a low mortality rate of 2%, lower incidence rate of PND of 6% and no spinal cord injury.
Therefore, the optimal therapeutic strategy for aortic arch aneurysms should be considered on the basis of these recent data for both procedures. The results of a meta-analysis of open TAR vs hybrid TEVAR for aortic arch aneurysms showed that hybrid TEVAR did not significantly improve operative mortality, whereas it was associated with a slight, insignificant increase in PND. The authors concluded that no definitive evidence supports the superiority of the hybrid TEVAR relative to open arch repair [8]. However, most cases of employing hybrid TEVAR have included high-risk patients unsuitable for conventional open aortic repair. Thus, these results cannot be easily compared. Prospective randomized control trials seem to be the most desirable to compare the outcomes of both surgical strategies; however, this was difficult. To overcome this issue, we performed propensity score-matching analysis to compensate for the patient selection bias. This analysis shows that there were no significant differences in early mortality and PND incidence; however, the hybrid group experienced shorter ICU lengths of stay and lesser in-hospital days. In the mid-term follow-up, late survival rates were similar in both groups; however, more frequent late aortic reintervention occurred significantly in the hybrid group. Most of the reinterventions for previous aortic repair were performed for type Ia endoleaks, which caused further dilatation of the aneurysm with persistent risk of rupture. Hence, the superiority of hybrid TEVAR compared with open arch repair was not evident except for shorter ICU lengths of stay and lesser in-hospital days even in this matched analysis. Recently, Cao et al. [25] published a systematic review of the clinical outcomes for the hybrid arch procedure and concluded that the hybrid repair of the aortic arch carries not negligible risks of perioperative mortality and neurological morbidity, and also described that no reliable long-term data exist to ascertain the durability of the hybrid arch procedure. As we previously reported, we agree that hybrid TEVAR is a beneficial alternative for high-risk patients in whom high mortality and morbidity rates are expected by conventional open arch repair [21]. Hybrid arch TEVAR is still in a developing stage and new techniques such as the chimney stent graft technique, or new fenestrated or branched devices are under trial; however, we now advocate that the extended application of this new technology to patients with a reasonable risk should be reconsidered according to the results of the present risk-adjusted study. In any case, we should reconsider the classification criteria for regarding patients as high risk for aortic repair. Furthermore, we suggest that the establishment of a ‘risk-oriented strategy’ based on a proper risk evaluation for aortic repair is an important issue to be addressed in the future.
In conclusion, the recent outcomes of open arch repair and hybrid TEVAR demonstrate acceptable results, particularly early after the procedure; however, open arch repair provides more reliable outcomes in follow-up. These two surgical strategies when properly selected according to each patient's risk improve the surgical outcomes in all patients with aortic arch aneurysms. The limitations of this study include the size of each cohort and the small number of matched pairs between both groups. Therefore, further investigations and follow-up are required to support decision-making in choosing a surgical strategy for aortic arch pathologies.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr V.X. Mosquera Rodríguez(A. Coruna, Spain): Dr Iba and coworkers have presented an interesting study comparing early and mid-term outcomes of open repair and hybrid endovascular procedures for treating aortic arch aneurysms. There is a lack of prospective randomized or large nonrandomized studies addressing this issue, which is why this sort of study is so important.
The proportion of patients with previous cardiac surgery or low ejection fraction was significantly higher in the hybrid group. Thus, as expected, hybrid patients presented a higher surgical risk, and their predicted in-hospital mortality was almost two-fold. The authors concluded with an important issue suggesting that the establishment of a "risk-oriented strategy" based on proper preoperative risk evaluation of patients with aortic arch disease is of paramount importance in deciding from which approach patients will derive most benefit.
Dr Iba, I would like to draw your attention to some shortcomings present in your study as well as to ask you a couple of technical questions. My first technical question is whether or not you used any neuroprotective measures such as carotid filters to prevent atheroembolic events, especially when performing the chimney technique. In case of an affirmative answer, do you use them on a routine basis when stenting the aortic arch? Secondly, it is quite striking that the rate of reoperation for bleeding was so high at 8% in the hybrid group. Please, could you comment more on this fact?
Another point which is one of the major drawbacks of this study is that the authors resorted to the use of many different techniques for hybrid procedures. Therefore such heterogeneity in the hybrid techniques may have jeopardized the comparability of the outcomes. Although the authors claim that there were no significant differences in the rate of PND between both groups, the matched cohort reveals a marked trend to a higher incidence of PND in the hybrid group: it was indeed 8.6% versus 0%. Furthermore, I think it would be very interesting to underline which type of hybrid procedure the patients who suffered a stroke had undergone. Please, can you specify which proximal landing zone was used in the hybrid patients suffering a preoperative stroke?
And finally, although in my opinion the patient population in the present study reflects the wide anatomical spectrum normally seen in clinical practice, this study may lack sufficient statistical power to determine with confidence clinically relevant differences between both approaches. Future studies should incorporate data on outcome variables acquired during a longer follow-up period and use objective assessment of outcomes to make definitive conclusions on the effectiveness and indications of the hybrid arch repair.
Dr Iba: I will first answer your question about the detail of the chimney graft technique. The indication for the chimney graft technique is in high-risk patients where sternotomy is not feasible for those with prior cardiac surgery or severely compromised respiratory function. With regard to the chimney graft procedure, supra-aortic bypass was established at first. Recently, we performed right carotid to left carotid and left axillary artery bypass as a supra-aortic bypass, because a sheath was inserted through the right axillary artery as a chimney graft access. The main device was advanced into the ascending aorta from the femoral artery. Furthermore, an iliac extender device was introduced into the ascending aorta. Next, the stent-graft in the brachiocephalic artery was deployed first, followed by the main stent graft. There have been no stroke events in the chimney group up to the present; however, one patient experienced type Ia endoleak two years after the hybrid procedure with the chimney graft technique.
Turning to your question about stroke events, there were three strokes in our series in the hybrid group. Two of them received hybrid TEVAR with zone 1 landing. The remaining patient underwent zone 2 landing TEVAR. The patients who received hybrid TEVAR with zone 0 landing had no stroke events. In three stroke cases, one patient had a shaggy aorta and developed multiple cerebral emboli. The other two cases sustained cerebral embolism in the occipital lobe and the cerebellum, perhaps through the vertebrobasilar artery system.
So in our recent hybrid arch TEVAR series, a left subclavian artery balloon occlusion is routinely carried out at the time of the deployment of the stent graft for protection from embolism. We think it may reduce stroke.
About bleeding complications, postoperative bleeding occurred in four patients in our hybrid series. Three of them were due to retroperitoneal haematoma after iliac artery exposure as an access for the stent graft. The remaining patient experienced postoperative bleeding at a subclavian incision for supra-aortic bypass. As you say, there were rather frequent bleeding events in our series, though all cases were not fatal.
Regarding comparability in the study, as you say, TAR is a uniform and established procedure for arch pathologies. On the other hand, a hybrid procedure is now developing. And the outcomes of that hybrid arch procedure may depend on the concept or the method. The patients' backgrounds are somewhat different. So a simple comparison of the results of both procedures may be difficult. Therefore, we performed propensity score-matched analyses of both procedures.
Even in this risk-adjusted study, early mortality and morbidity were similar except for shorter ICU stay in the hybrid group. And the mid-term outcomes showed that TAR is more reliable. The indication may change depending on future technical advancement or more long-term follow-up data. However, we think hybrid arch TEVAR should be indicated for limited high-risk patients at the moment.
Dr J. Bachet(Paris, France): Your experience confirms what I thought for years: I don't understand the superiority of debranching and the hybrid procedures. Indeed, you showed us statistics demonstrating that conventional surgery had less important mortality, that the late survival was much better, and that the rate of reinterventions was much less. So why do you still propose debranching?
In my opinion, it is not comparing a very simple procedure to a very difficult one. If the hybrid procedure was very simple, I would understand, but you have to open the chest exactly as in conventional surgery. You have to do a lot of anastomosis, et cetera, so it is heavy surgery. So how do you explain your choice? And in particular, how do you explain that you prefer to do this in what you call "high-risk patients"?
This is indeed another important issue. Nobody can tell what a high-risk patient is. I've seen on your slides, for instance, that in both groups, the EuroSCORE, which is a bad score for aortic surgery by the way, was 5 and 7. 5 and 7 are not high-risk scores. So what are your criteria for deciding that the patient is a high-risk patient who should have this kind of surgery?
Dr Iba: What constitutes a high-risk group for aortic repair is a very important issue. Our previously reported experience showed that some organ failures such as COPD, renal failure, and liver dysfunction are risk factors for early mortality following open arch repair, and age is not necessarily a risk factor of early mortality and stroke. However, the reliability of long-term outcome is more important, more especially for young patients than elderly patients. So the determinants of our surgical strategy for arch lesions include the patient's age. Anyway, what represents a high-risk group patient for aortic repair is a very important issue, we think. So proper methods of risk evaluation should also be established in the near future.
Dr Bachet: Yes, but for instance, look at the mid-term outcome of your patients, and the rate of reinterventions, which are not a promenade in the park from the patient's point of view, as being called again to have a new stent graft, et cetera, is a real psychological and physical stress. Do you really think that this is better than having a conventional surgery with maybe a slightly increased risk than to come back to have one, two, three reinterventions? I don't think so, really. I'm sorry for this disagreement, but I think we should stop saying that a hybrid procedure, which represents for me a real heavy surgery, is a very simple thing as compared to conventional surgery because this is not true and, obviously, your data show that this is not true.
Dr E. Weigang(Berlin, Germany): That is a very important comment from Dr Bachet. However, I also agree with the authors' conclusion. It makes good sense to establish a risk score for hybrid procedures in the arch and to have available a genuine tool to help in deciding which patient is most likely to benefit from the procedure.
Dr K. Minatoya(Osaka, Japan): This was a great comment from Dr. Bachet. But the main message from this presentation is that open surgery is still sort of mainstream, in my understanding, and reintervention is sometimes okay for a really old, high-risk patient. So, therefore, we have the same problem using this terminology "high-risk". In really high-risk patients who sometimes had dementia, who could not have major surgery, we applied this hybrid TEVAR operation for the pathology. So both of them are applied properly, as Professor Harringer said. That's my comment.
Dr G. D'Ancona(Palermo, Italy): I have a comment. I am not sure that you are proposing the correct stent for the arch. There are better stents tailor-made, so to speak: for example, the Najuta precurved fenestrated graft that's been proposed in over 400 patients by a group from Tokyo, with excellent results in arch hybrid, fully percutaneously with a precurved stent, without even debranching, just with fenestration. The results are superior to what you are presenting, in over 400 patients presented last year at the European meeting. So I'm not sure you can really come and say something about hybrid arch if you're using the standard stents. Those are not done for the arch.
Dr Iba: That Najuta fenestrated graft has not been used in our centre because we don't know its durability.
Author notes
Presented at the 27th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 5–9 October 2013.




