-
PDF
- Split View
-
Views
-
Cite
Cite
Prashant N. Mohite, Bartlomiej Zych, Aron F. Popov, Anton Sabashnikov, Diana G. Sáez, Nikhil P. Patil, Mohamed Amrani, Toufan Bahrami, Fabio DeRobertis, Olaf Maunz, Nandor Marczin, Nicholas R. Banner, Andre R. Simon, CentriMag® short-term ventricular assist as a bridge to solution in patients with advanced heart failure: use beyond 30 days, European Journal of Cardio-Thoracic Surgery, Volume 44, Issue 5, November 2013, Pages e310–e315, https://doi.org/10.1093/ejcts/ezt415
Close - Share Icon Share
Abstract
Left ventricular assist devices (LVADs) offer very valuable therapeutic options for patients with advanced heart failure. CentriMag® (Thoratec, Pleasanton, CA, USA) is an extracorporeal short-term circulatory assist device Conformité Européenne-marked in Europe for use up to 30 days.
Retrospective analysis of 41 patients with advanced heart failure who, from 2003 to 2011, were supported with CentriMag for >30 days as a bridge to recovery, long-term VAD or transplantation.
Forty-one adult patients were supported with 46 CentriMag devices for a total of 2695 days with a mean support time of 59 (range: 31–167) days. Indications were post-cardiotomy cardiogenic shock (PC = 4), primary graft failure (PGF = 7) and refractory heart failure (RHF = 35). Six devices were used to support the left ventricle, 19 to support the right ventricle and 21 to support both the ventricles (biventricular support considered as single device unit). In the PC cohort, 3 (75%) patients were weaned from support, while 4 (57%) were weaned from support in the PGF cohort. In the RHF cohort, 8 patients were bridged to long-term VAD and 5 were bridged to transplantation; heart function recovered and device explanted in 14, while 8 patients died on support. There were no device failures. Overall, 34 (74%) patients were recovered or bridged, with a 1-year survival of 54%.
CentriMag proved to be a versatile, safe and effective short-term circulatory support for patients with advanced heart failure as a bridge to solution. Its use over 30 days is associated with acceptable survival and does not increase device-related complications.
INTRODUCTION
Heart failure remains a major cause of morbidity and mortality in the patient population, with a dismal prognosis in advanced cases [1]. CentriMag® (Thoratec, Pleasanton, CA, USA) is an extracorporeal temporary (short-term) ventricular assist device specifically designed for treatment of patients with acute cardiogenic shock of any aetiology including acute myocardial infarction, myocarditis, cardiotomy and complications that may arise in the cardiac catheterization laboratory [2]. The CentriMag is licensed for 30 days of support in Europe. In the USA, Food and Drug Administration has approved its clinical use for up to 6 h, and a clinical trial is currently underway to investigate CentriMag use as a VAD for support for up to 30 days (http://www.thoratec.com/medical-professionals/vad-product-information/thoratec-centrimag.aspx). Many patients who are successfully rescued from a moribund state due to advanced heart failure do not become optimal candidates for weaning, upgrade to a long-term device or transplantation within 30 days. Therefore, physicians may become committed to short-term VAD therapy beyond the licensed period. The aim of this study was to assess the feasibility and outcome of extending the CentriMag ventricular support beyond 30 days and to assess its safety and effectiveness in the extended period in terms of improvement in physiological and biochemical markers as well as incidence of complications.
MATERIALS AND METHODS
CentriMag short-term VAD has been described in detail in various case series and clinical trials [4, 5]. Its main component is rotor in the pump head, which is levitated magnetically, so that frictionless rotation may be achieved without regions of stasis or wear and tear. This study is a retrospective analysis of data prospectively collected and maintained in our mechanical circulatory support registry. First CentriMag device was implanted in August 2003 and 154 devices were implanted till June 2011, of which 46 devices were used for >30 days.
The cannulas are supplied by the manufacturer. The inflow is a 32-French (F) angled cannula that is wire-reinforced and malleable, whereas the outflow is a 22-F straight cannula. In some cases, 22-F elongated one-piece arterial Medtronic (Medtronic, Inc., Minneapolis, MN, USA) arterial and 32-F Edwards Lifesciences (Edwards Lifesciences, Irvine, CA, USA) or 32-F Sarns malleable (Terumo, Somerset, NJ, USA) venous cannulas were also used. In the left ventricular assist device (LVAD) configuration the inflow cannula was inserted in the left atrium at the level of the junction between the right superior pulmonary vein and the left atrium. In the right ventricular assist device (RVAD), the inflow cannula was placed in the right atrium and the outflow cannula was inserted in the main pulmonary artery. Intraoperative transoesophageal echocardiography (TOE) was used to confirm cannula positioning, absence of a patent foramen ovale and adequate volume for support. All cannulas were secured with dual pledgeted purse-string sutures, exteriorized through separate stab incisions and connected to the circuit after deairing. Target flows were 60 ml/kg of body weight. When the flows were not achieved and the central venous pressure was low (<10 mmHg), fluid challenge for 250–500 ml was given. If no response to extra volume or central venous pressure was normal/high, another TOE was performed to assess the cannula position.
The pump head tubing was replaced at the bedside once fibrin threads were noticed in the circuit (Fig. 1). The patients were transferred to the ward once they were stabilized haemodynamically and were partially mobilized. Anticoagulation was with intravenous unfractionated Heparin started initially at 5 U/kg/h following chest-drainage <50 ml/h, and later titrated aiming for activated partial thromboplastin time 60–80 s or activated clotting time 160–180 s. Aspirin 75 mg od was given to the patients with a history of hypercholesterolaemia (post-cardiotomy and heart failure due to ischaemic heart disease). It is our observation that these patients have a tendency to coat the circuit with fibrin and antiplatelet drugs may prevent this to a certain extent. Baseline haemodynamic status, laboratory measurements targeting end-organ function and neurological status were noted before CentriMag implantation. After implantation, daily assessments were made in terms of these parameters till the device was weaned, upgraded or patient died on support. Survival after device explantation was calculated at 30 days, 6 months, 1 and 2 years.
Follow-up: no patient was lost to follow-up. The follow-up time ranged from 0 to 2482 days (mean 608 days) and patients were followed up till 2 years at regular intervals.
Statistical analysis
All data were analysed using the Statistical Package for Social Sciences, version 21.0 (SPSS, Inc., Chicago, IL, USA), and are presented as continuous or categorical variables. Continuous variables are presented as means ± standard deviation and categorical variables as percentages. 95% confidence interval (CI) for continuous variables was calculated. Kaplan–Meier survival estimation was applied for patient's survival analysis and mean estimates for survival time, standard error and 95% CI were calculated.
RESULTS
Forty-one adults (30 males) were supported with 46 CentriMag devices for a total of 2695 days with a mean support time of 58.9 ± 29 days (range: 31–167 days), 95% CI 49.9–67.3. Five patients needed CentriMag support twice during their course of illness; before and after upgradation to long-term VAD (n = 3) or heart transplantation (n = 2). Six devices were used to support the LVAD, 19 to support the RVAD, while 21 were used to support both ventricles (BiVAD). Although two individual devices were used for BiVADs, it was functionally considered a single unit. Indications were post-cardiotomy cardiogenic shock (PC = 4), primary graft failure (PGF = 7) and refractory heart failure (RHF = 35). In the RHF group, aetiology was ischaemic cardiomyopathy in 6 patients, while it was dilated cardiomyopathy in the rest (n = 29).
Eighteen patients were on intra-aortic balloon pump (IABP) at the time of CentriMag implantation. IABP was removed in 4 cases at the time of CentriMag implantation, while it was weaned off in 10 cases within 7 days. None of the patients were on IABP by the 15th day of CentriMag support. At the time of CentriMag implantation, all the patients were supported by at least one inotrope. On the 7th day of the support, only 8 patients needed inotropes. The average levels of blood urea, serum creatinine and serum bilirubin before implantation and at 24 h, 7 days, 30 days and at the time of explantation are shown in Fig. 2A–C. The numbers of patients with renal failure, hepatic dysfunction and requiring ventilator support at the time of implantation at Day 1, Day 7, Day 30 and at the time of explantation are shown in Fig. 3.
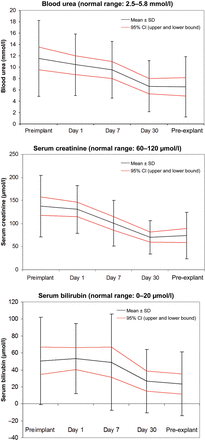
Trends in levels of blood urea, serum creatinine and bilirubin during CentriMag therapy.
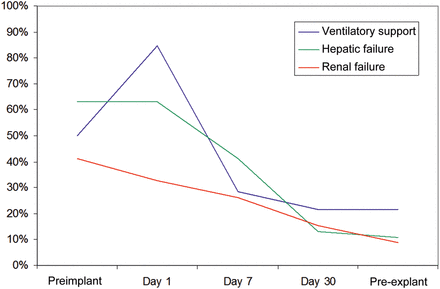
Number of patients with end-organ failure during CentriMag therapy.
Table 1 provides distribution and the outcome of devices in different groups. In the PC cohort, 3 (75%) patients were weaned off from the support and were discharged home. Two patients could not survive till 1 year, while one is still surviving. Four (57.14%) patients were weaned from support in the PGF cohort; however, only 2 could be discharged home and none could survive for a year. The RHF was the biggest cohort in the study population with 35 devices. Eight devices in RHF cohort (BiVAD = 6, LVAD = 2) were bridged to the long-term VAD. All six BiVADs and one LVAD were bridged to HeartMate II® (Thoratec Corp., Pleasanton, CA, USA), while one LVAD was bridged to HVAD® (HeartWare International, Inc., Framingham, MA, USA). Of these 8 patients who were bridged to the long-term device, the long-term device was explanted following recovery of heart function in 1, 1 received heart transplant, 5 died on the support while 1 is still on support waiting for heart transplantation. In this subgroup of bridge to long-term device, 6 of 8 patients survived >1 year. In a subgroup with CentriMag bridged to heart transplant, all the 5 patients survived discharge home and lived >1 year. Kaplan–Meier survival estimation was applied for patient's survival analysis and mean estimates for survival time, standard error and 95% CI were calculated. In that, patients who were alive at the cut-off of the study were censored. The mean estimate for survival time was 1037.8 days with standard error 711.3 (95% CI 711.3–1364.2).
| Post-cardiotomy (n = 4) | |
| Bridge to recovery | 3 |
| Death on support | 1 |
| Primary graft failure (n = 7) | |
| Bridge to recovery | 4 |
| Death on support | 3 |
| Refractory heart failure (n = 35) | |
| Bridge to long-term device | 8 |
| Bridge to heart transplant | 5 |
| Bridge to recovery | 14 |
| Death on support | 8 |
| Post-cardiotomy (n = 4) | |
| Bridge to recovery | 3 |
| Death on support | 1 |
| Primary graft failure (n = 7) | |
| Bridge to recovery | 4 |
| Death on support | 3 |
| Refractory heart failure (n = 35) | |
| Bridge to long-term device | 8 |
| Bridge to heart transplant | 5 |
| Bridge to recovery | 14 |
| Death on support | 8 |
| Post-cardiotomy (n = 4) | |
| Bridge to recovery | 3 |
| Death on support | 1 |
| Primary graft failure (n = 7) | |
| Bridge to recovery | 4 |
| Death on support | 3 |
| Refractory heart failure (n = 35) | |
| Bridge to long-term device | 8 |
| Bridge to heart transplant | 5 |
| Bridge to recovery | 14 |
| Death on support | 8 |
| Post-cardiotomy (n = 4) | |
| Bridge to recovery | 3 |
| Death on support | 1 |
| Primary graft failure (n = 7) | |
| Bridge to recovery | 4 |
| Death on support | 3 |
| Refractory heart failure (n = 35) | |
| Bridge to long-term device | 8 |
| Bridge to heart transplant | 5 |
| Bridge to recovery | 14 |
| Death on support | 8 |
Incidence of complications is summarized in Table 2. Bleeding was the most common complication, encountered in 24 (52%) cases and 17 (37%) of them required re-exploration of chest. No specific source of bleeding was found in ∼50% of the re-explored cases, whereas cannula site bleeding was found in 10% and generalized ooze was the cause of bleeding in the remaining of the cases. Infection was the second common complication, found in 17 (37%) of the cases and was more common in re-explored cases. Ten patients developed frank septicaemia and it was proved fatal in 5 of them. Five patients had a stroke and 3 of these had Long-term LAVD and CentriMag RVAD, so stroke could not be directly attributed to the CentriMag device. The remaining 2 patients with stroke were on BiVAD and made full neurological recovery and the device was also explanted. Nineteen patients were already on haemodiafiltration when CentriMag was implanted in whom renal function was gradually improved. Four (8.7%) patients (2 in PC and 2 in RHF group) developed renal failure needing haemofiltration after CentriMag implant. Twenty-nine patients had hepatic dysfunction preimplant while 3 (6.5%) developed it after CentriMag implantation. Twenty-three patients were on ventilatory support at the time of implant while 10 (21.7%) needed reintubation or tracheostomy during CentriMag support. Haemolysis was diagnosed by laboratory tests in 4 (8.7%), but was resolved in all.
| Complications . | n . | % . |
|---|---|---|
| Bleeding requiring transfusion | 24 | 52 |
| Bleeding requiring re-exploration | 17 | 37 |
| Infection | 17 | 37 |
| Septicaemia | 10 | 21.7 |
| Stroke | 5 | 10.8 |
| Renal failure | 4 | 8.7 |
| Liver dysfunction | 3 | 6.5 |
| Respiratory failure | 10 | 21.7 |
| Haemolysis | 4 | 8.7 |
| Complications . | n . | % . |
|---|---|---|
| Bleeding requiring transfusion | 24 | 52 |
| Bleeding requiring re-exploration | 17 | 37 |
| Infection | 17 | 37 |
| Septicaemia | 10 | 21.7 |
| Stroke | 5 | 10.8 |
| Renal failure | 4 | 8.7 |
| Liver dysfunction | 3 | 6.5 |
| Respiratory failure | 10 | 21.7 |
| Haemolysis | 4 | 8.7 |
| Complications . | n . | % . |
|---|---|---|
| Bleeding requiring transfusion | 24 | 52 |
| Bleeding requiring re-exploration | 17 | 37 |
| Infection | 17 | 37 |
| Septicaemia | 10 | 21.7 |
| Stroke | 5 | 10.8 |
| Renal failure | 4 | 8.7 |
| Liver dysfunction | 3 | 6.5 |
| Respiratory failure | 10 | 21.7 |
| Haemolysis | 4 | 8.7 |
| Complications . | n . | % . |
|---|---|---|
| Bleeding requiring transfusion | 24 | 52 |
| Bleeding requiring re-exploration | 17 | 37 |
| Infection | 17 | 37 |
| Septicaemia | 10 | 21.7 |
| Stroke | 5 | 10.8 |
| Renal failure | 4 | 8.7 |
| Liver dysfunction | 3 | 6.5 |
| Respiratory failure | 10 | 21.7 |
| Haemolysis | 4 | 8.7 |
Thirteen patients (31.7%) died on CentriMag support (8 BiVADs and 5 RVADs). Multiorgan failure was the most common cause of death (found in 9 patients, 22%), which was present in 8 (19.5%) of them before CentriMag implantation. One patient on BiVAD required multiple laparotomies due to bleeding following percutaneous endoscopic gastrotomy tube insertion. Three patients on CentriMag RVAD with long-term LVAD died due to intracranial haemorrhage, LVAD pump invasion of stomach and dislodgement of pulmonary cannula leading to catastrophic bleeding. Thirty-four (74%) devices were explanted due to recovery, bridge to long-term device or bridge to transplant. Patient survival was estimated using Kaplan–Meier analysis and is shown in Fig. 4.
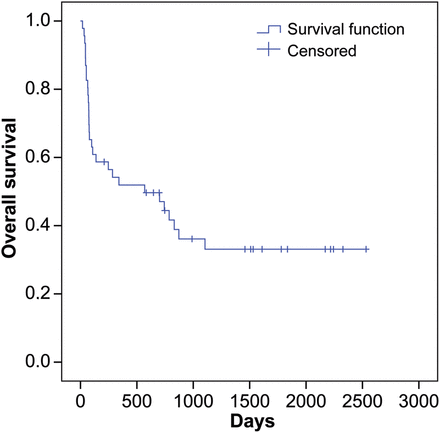
Kaplan–Meier survival estimation for overall postexplantation survival.
There was no device (pump) failure in any case. Pump and the tubing were exchanged once fibrin was observed in the circuit. Twenty-one pumps did not require pump exchange. Three pumps required exchange thrice, 4 required it twice and 18 required it once. The average number of days to pump exchange was 37.75 ± 10. The longest tenure spent before pump exchange in the series was 51 days and the shortest at 21 days.
DISCUSSION
The earliest reports of the clinical use of the CentriMag short-term VAD came from our hospital in 2006 [4, 6]. Present study focuses on the devices used beyond 30 days. The longest support was 104 days as BiVAD and 167 days as RVAD in our series; however, support of 183 days as LVAD and 304 days as RVAD has recently been reported in individual cases [7, 8]. The short-term VADs are used as salvage therapy with the goal of restoring haemodynamic stability and end-organ function in view of subsequent recovery of heart function, heart transplantation or bridge to long-term VAD. This allows salvage of some patients who otherwise would have died from advanced heart failure despite conventional medical management with inotropes and IABP. The CentriMag is a magnetically levitated rotor system which consists of polycarbonate pump head, a motor drive unit, cannulas and a bedside console and it can deliver flow up to 9.9 l/min [4]. In Europe and the USA, CentriMag is licensed for use up to 30 days [3]. Usually in the clinical practice, the fate of most of the patients supported on short-term VADs is decided in the first 10 days, either due to poor prognosis and withdrawal of support or due to recovery and weaning. Many patients can be optimized within 30 days to a condition from where they can either receive heart transplant or be upgraded to the long-term device. In some cases, however, use of the CentriMag needs to be extended beyond 30 days. Among 154 devices put in our institute, 46 were used for more than 30 days with a mean of 59 days in this particular cohort.
For cases in which weaning from short-term VAD is difficult, bridge to heart transplantation remains an attractive option. Unfortunately, long waiting time for heart transplantation is a major limiting factor for this. In the UK, there is a provision of ‘Urgent waiting list for heart transplantation’ and patient on short-term VAD can be listed in it. The average waiting time for patients on this ‘Urgent waiting list’ at our hospital in the financial year 2011–12 was 37 days. Therefore, to bridge the patient supported with short-term VADs to heart transplantation, it was necessary to prolong the short-term VAD support. In our study population, 5 patients were successfully bridged to heart transplantation directly from CentriMag short-term VAD with an average waiting time of 76 days and the longest being 104 days. This shows the efficiency of CentriMag short-term VAD to optimize the patient from initial heart failure, maintain the physiology and maintain the patient on waiting list by supporting the failing heart for a longer duration in bid to bridge the patient directly to heart transplantation.
The mortality in heart failure patients remains high with the use of short-term VADs mainly due to the underlying severe heart disease and subsequent multiorgan failure, in the setting of which they are mostly implanted. Also, the postoperative complications such as bleeding, multiple blood transfusions, sepsis and multiorgan failure play a major role in the poor outcomes. The mortality and morbidity of this cohort ‘in which the CentriMag was used for >30 days’ cannot be compared fairly with the contemporary reports in which the majority of the patients were on the CentriMag support for <30 days, simply because our cohort consists of patients who survived early (first 30 days) mortality. But then, mortality and complications inherent with the prolonged use of the short-term VAD were bound to be greater in our series. In our series, 74% of the devices were weaned off or upgraded to long-term VADs and a 1-month postexplant survival of 67.4% was achieved, which dropped to 54% at 1 year. The 30-day survival after explantation in contemporary studies ranges between 75 and 30% with the mean support time stretching between 9.4 and 15 days [4, 5, 9, 10]. This shows that the survival with the CentriMag used for >30 days is comparable with or rather better than most of the study groups with mean support time <15 days. As stated earlier, even though these series cannot be compared fairly with ours, at least it is evident that the use of CentriMag short-term VAD over 30 days does not have inferior outcome compared with the series in which it was used for a lesser duration. And, therefore, it is safe to argue that its use beyond 30 days is possible and safe, with a low impact on complications and mortality.
The vast difference in survival among different groups is mainly due to differences in the patient population. In addition, most of these studies report findings in the first half of last decade when the surgical experience with CentriMag was relatively new. The 30-day survival with the short-term VADs used in post-cardiotomy scenario is reported as poor (33–48%) from different parts of the world [4, 10–12]. In the present study, the survival in post-cardiotomy patients was 75%. This high survival rate could be because of the fact that most of the patients supported on the short-term VADs succumb early due to the assault from long cardiopulmonary bypass time and poor heart function and those who survive this initial period have better prognosis. CentriMag short-term VAD is now widely accepted to support the transplanted heart in case of PGF [13]. Usually, transplanted heart supported on the short-term VAD recovers or fails completely in the first few days of support making the decision process of explantation of short-term VAD easy. But, in some cases the heart recovery is slow or inadequate for explantation of VAD which prolongs the support duration.
Currently, Abiomed BVS, extracorporeal membrane oxygenator (ECMO), Impella, TandemHeart, CentriMag etc. are the available options of short-term support in heart failure due to various aetiologies. Reported survival with Abiomed BVS in the management of acute cardiac failure is around 30% [14, 15]. However, disadvantages include the need for performing anastomoses for aortic and pulmonary artery cannulation, with resulting bleeding complications, and the need for pump exchanges at ∼1-week intervals. ECMO is being used as an emergency procedure in cases of post-cardiotomy heart failure and PGF, and to support failing heart in acute myocarditis since at least a decade before introduction of CentriMag. It has shown comparable survival when used for PGF, whereas survival remains poor when used in post-cardiotomy settings [16, 17]. The biggest disadvantage with these options (Abiomed BVS and ECMO) lies with their limited duration of support.
Percutanously implantable short-term mechanical circulatory assist devices is an important development and these devices are useful in emergency situations like post-cardiotomy cardiac failure. Early institution of haemodynamic support with an easy-to-insert catheter-based device, Impella Recover, in post-cardiotomy and post-resuscitation shock is safe, feasible and might help bridge patients to recovery or to the next therapy, and improve the outcomes [18, 19]. The TandemHeart is a percutaneously implanted left atrial-to-femoral artery bypass system off-loading the left atrium through a trans-septal cannula and pumping it into the femoral artery with a centrifugal blood pump. It has been found to improve end-organ function and overall condition when implanted in end-stage heart failure patients, thus decreasing the preoperative risk factors for heart transplantation or implantation of the long-term device [20]. However, partial support and limited duration is an Achilles' heel for percutaneously implanted short-term MCS devices like Impella and TandemHeart.
Often patients are not adequately optimized for weaning, transplant or transfer to long-term VAD in this limited time frame. In addition, another surgery for the exchange of device or transfer to long-term VAD could be fatal in patients with abnormal coagulation profile, poor heart function and brittle haemodynamics. Some patients succumb to the complications related to prolonged used of mechanical support and device failure which is still common in ECMO mostly due to clotting or leaking of the oxygenator. CentriMag support can be extended safely with 3–4 weekly pump exchanges while keeping the cannulas in situ. This gives the surgeon sufficient time for evaluation and to reach a decision about explantation or transfer to long-term VAD.
CONCLUSIONS
CentriMag is a versatile and reliable ventricular assist device. It can provide uni- and biventricular support for bridging patients to recovery, heart transplantation or long-term VAD. Its use beyond 30 days is possible, safe and associated with a low impact on complications and mortality. This allows sufficient time for evaluation and decision in cases that could not be optimized adequately in a limited time frame.
Conflict of interest: none declared.
REFERENCES
Author notes
Paper presented as a poster at the Annual Meeting of International Society of Heart & Lung Transplantation, Prague, Czech Republic, 19 April 2012.




