-
PDF
- Split View
-
Views
-
Cite
Cite
Paul P. Urbanski, Mahli Raad, Aristidis Lenos, Petros Bougioukakis, Michael Zacher, Anno Diegeler, Open aortic arch replacement in the era of endovascular techniques, European Journal of Cardio-Thoracic Surgery, Volume 44, Issue 3, September 2013, Pages 431–437, https://doi.org/10.1093/ejcts/ezt030
Close - Share Icon Share
Abstract
Despite the progress in protection and surgical techniques, the proponents of endovascular techniques for aortic arch repair still consider conventional arch replacement to be high risk, mostly due to deep hypothermia, which in the past was generally used for cerebral and organ protection. The aim of the study was to evaluate the operative results of open aortic arch replacement using current perfusion and surgical techniques in which deep hypothermia is avoided.
Between October 2004 and February 2012, 131 consecutive patients with non-acute-dissected aortic arch pathology (mean age: 66 ± 11 years) were referred for surgery. All patients were operated on conventionally using circular aortic arch replacement with repair of one (10), two (58) or all arch branches (63). The adjacent aorta was replaced in all cases (ascending—115, descending—2 and both—14). Nine (6.9%) patients had previous neurological defects with residual symptoms and 17 (13.0%) had previous cardiac surgery.
Either unilateral (130) or bilateral (1) cerebral perfusion at a blood temperature of 28°C (mean duration 36 ± 14, range: 16–80 min) was performed for brain protection during circulatory arrest under mild-to-moderate hypothermia (mean rectal temperature 30.0 ± 1.6°C). Concomitant cardiac procedures, mostly on the aortic valve, were necessary in 121 (92%) patients. Among 114 patients needing aortic valve/root surgery, there were 70 aortic valve-preserving procedures. Permanent neurological deficit or temporary dysfunctions occurred in 1 (0.8%) and 6 patients (4.6%), respectively. No patient suffered from paraplegia. The postoperative 30-day mortality was 2.3% (3 patients). A total of 17 patients died during the follow-up time of up to 97 months (mean 37 ± 27 months), resulting in an actuarial survival of 81.9 ± 4.3% at 5 years. No patient needed any reoperation or new intervention on the repaired aorta.
Conventional arch surgery offers definitive repair and can be safely performed using current perfusion and operative techniques. Open procedures ensure simultaneous aortic valve repair, which is frequently necessary, and can be performed by reconstruction in more than half of the cases. The use of refined surgical and cerebral perfusion techniques allows the avoidance of deep hypothermia with all its negative side effects and leads to excellent outcomes against which the results of alternative approaches should be compared.
INTRODUCTION
Changes in operative techniques and technical progress during the last decades have led to a significant improvement in survival and morbidity after open aortic arch surgery [1–3]. On the other hand, there is also a rapid growth of alternative techniques and evolving technologies that should be benchmarked adequately. The aim of the study was to evaluate the operative results of open aortic arch replacement using current perfusion and surgical techniques in which deep hypothermia is avoided.
PATIENTS AND METHODS
Between October 2004 and February 2012, 131 consecutive patients (median age: 69, range: 16–82 years) with non-acute-dissected aortic arch pathology necessitating repair of the arch and at least one supra-aortic artery were referred for surgery. Patients with isolated replacement of the ascending or descending aorta using open anastomosis with the arch (hemiarch replacement) were not included in this evaluation. All patients were operated on conventionally using circular aortic arch replacement with repair of one (10), two (58) or all arch branches (63). The adjacent aorta was replaced in all cases (ascending—115, descending—2 and both—14). Nine (6.9%) patients had previous neurological defects with residual symptoms and 17 (13.0%) had previous cardiac surgery.
Surgical technique
According to the extent of surgery, the thoracic aorta was exposed via median sternotomy in 123 patients, including a left lateral extension in 4. Anterior bilateral (clamshell) thoracotomy was performed in 8 patients.
The arterial return for cardiopulmonary bypass (CPB) was achieved by cannulation of the right or left common carotid artery in 94 (71.8%) and 29 (22.1%) patients, respectively. The innominate artery was used for cannulation in 8 (6.1%) cases, one of which was combined with a cannulation of the left common carotid artery because of an occlusion of the right internal carotid artery, hypoplasia of the left vertebral artery and multiple pathologies of the intracranial cerebral arteries. This was the only patient in the series in whom bilateral cerebral perfusion was performed during circulatory arrest (CA). The decision for choosing the particular cannulation technique was made preoperatively on the basis of CT angiography, which was performed on all patients.
The arterial cannulation and unilateral cerebral perfusion (UCP) techniques have been described previously [4, 5]. In short, the innominate artery was isolated within the chest, or a common carotid artery was prepared through a separate approach in the neck along the medial margin of the sternocleidomastoid muscle. In all patients, after heparinization, the exposed segment of the artery was cross-clamped, a longitudinal incision was made and an 8- or 10-mm vascular-sealed polyester graft was anastomosed to the artery with a continuous 5.0 polypropylene suture. After connecting the arterial line and cannulating the right atrium, CPB was started with a mean flow of 4.6 ± 0.5, range 3.0–6.0 l/min (2.2–2.4 l/min/m² of body surface). During CA under mild-to-moderate hypothermia, UCP was used for cerebral protection. The arch arteries were cross-clamped, and UCP was set up at a constant blood temperature of 28°C by simply reducing the arterial flow to about 1.5 l/min for right-sided or 0.9 l/min for left-sided UCP [5]. Cerebral monitoring tools included arterial pressure lines in radial arteries, electroencephalography and assessment of cerebral saturation measured by near-infrared spectroscopy. In the early phase of the study, additional measurement of somatosensory-evoked potentials and transcranial Doppler ultrasonography of the middle cerebral arteries were performed and later abandoned [5].
| Characteristics . | n (%) or mean ± SD (range) . |
|---|---|
| Sex male | 67 (51) |
| Age (years) | 66 ± 11 (16–82) |
| NYHA functional class | |
| III/IV | 51 (39) |
| Concomitant disease | |
| Hypertension | 123 (94) |
| Coronary heart diseasea | 33 (25) |
| Diabetes | 12 (9) |
| COPD | 11 (8) |
| Previous neurological events | 15 (12) |
| with residuals | 9 (7) |
| without residuals | 6 (5) |
| Previous cardiac surgery | 17 (13) |
| One | 14 (11) |
| Two or more | 3 (2) |
| Logistic EuroSCORE (%) | 15 ± 11 (4.4–49.6) |
| Characteristics . | n (%) or mean ± SD (range) . |
|---|---|
| Sex male | 67 (51) |
| Age (years) | 66 ± 11 (16–82) |
| NYHA functional class | |
| III/IV | 51 (39) |
| Concomitant disease | |
| Hypertension | 123 (94) |
| Coronary heart diseasea | 33 (25) |
| Diabetes | 12 (9) |
| COPD | 11 (8) |
| Previous neurological events | 15 (12) |
| with residuals | 9 (7) |
| without residuals | 6 (5) |
| Previous cardiac surgery | 17 (13) |
| One | 14 (11) |
| Two or more | 3 (2) |
| Logistic EuroSCORE (%) | 15 ± 11 (4.4–49.6) |
aWith surgical relevant stenosis.
NYHA: New York Heart Association; COPD: chronic obstructive pulmonary disease.
| Characteristics . | n (%) or mean ± SD (range) . |
|---|---|
| Sex male | 67 (51) |
| Age (years) | 66 ± 11 (16–82) |
| NYHA functional class | |
| III/IV | 51 (39) |
| Concomitant disease | |
| Hypertension | 123 (94) |
| Coronary heart diseasea | 33 (25) |
| Diabetes | 12 (9) |
| COPD | 11 (8) |
| Previous neurological events | 15 (12) |
| with residuals | 9 (7) |
| without residuals | 6 (5) |
| Previous cardiac surgery | 17 (13) |
| One | 14 (11) |
| Two or more | 3 (2) |
| Logistic EuroSCORE (%) | 15 ± 11 (4.4–49.6) |
| Characteristics . | n (%) or mean ± SD (range) . |
|---|---|
| Sex male | 67 (51) |
| Age (years) | 66 ± 11 (16–82) |
| NYHA functional class | |
| III/IV | 51 (39) |
| Concomitant disease | |
| Hypertension | 123 (94) |
| Coronary heart diseasea | 33 (25) |
| Diabetes | 12 (9) |
| COPD | 11 (8) |
| Previous neurological events | 15 (12) |
| with residuals | 9 (7) |
| without residuals | 6 (5) |
| Previous cardiac surgery | 17 (13) |
| One | 14 (11) |
| Two or more | 3 (2) |
| Logistic EuroSCORE (%) | 15 ± 11 (4.4–49.6) |
aWith surgical relevant stenosis.
NYHA: New York Heart Association; COPD: chronic obstructive pulmonary disease.
Aortic arch replacement was performed with a branched aortic arch prosthesis (InterGard Hemabridge or InterGard Aortic Arch; InterVascular, MAQUET Cardiovascular, La Ciotat, France) to enable shortening of the lower body ischaemia in complex aortic procedures by gradual re-perfusion as described elsewhere in detail [1]. Separate vascular grafts were mostly used for the repair of adjacent aortic segments, particularly when an aortic root and/or a descending aorta replacement was performed (Fig. 1). The latter was carried out by conventional replacement in 13, and elephant trunk technique in 3 patients, respectively. In 5 patients, a complete thoracic aorta replacement through clamshell thoracotomy was performed (Fig. 1). Coronary revascularizations due to coronary heart disease were carried out in 33 patients. The surgical procedures and the operative data are shown in Table 2.
| Variables . | n (%) or mean ± SD (range) . |
|---|---|
| Extension of arch repair | |
| Repair 1 arch artery | 10 (8) |
| Repair 2 arch arteries | 58 (44) |
| Repair 3 arch arteries | 63 (48) |
| Ascending aorta replacement | 129 (98) |
| Supracoronary | 39 (29) |
| Root repair | 60 (46) |
| Valve conduit | 30 (23) |
| Descending aorta repair | 16 (12) |
| Replacement | 13 (10) |
| Elephant trunk | 3 (2) |
| Aortic valve surgery | 114 (87) |
| Repair (valve and/or root) | 70 (53) |
| Replacement | 44 (34) |
| Mitral valve surgery | 7 (5) |
| Repair | 6 (5) |
| Replacement | 1 (1) |
| CABG due to coronary heart disease | 33 (25) |
| Others | 6 (5) |
| CPB duration (min) | 177 ± 48 |
| Aortic cross-clamp time (min)a | 111 ± 34 |
| Circulatory arrest time (min) | 31 ± 11 |
| UCP time (min) | 36 ± 14 |
| Lowest rectal temperature (°C) | 30 ± 1.6 |
| Variables . | n (%) or mean ± SD (range) . |
|---|---|
| Extension of arch repair | |
| Repair 1 arch artery | 10 (8) |
| Repair 2 arch arteries | 58 (44) |
| Repair 3 arch arteries | 63 (48) |
| Ascending aorta replacement | 129 (98) |
| Supracoronary | 39 (29) |
| Root repair | 60 (46) |
| Valve conduit | 30 (23) |
| Descending aorta repair | 16 (12) |
| Replacement | 13 (10) |
| Elephant trunk | 3 (2) |
| Aortic valve surgery | 114 (87) |
| Repair (valve and/or root) | 70 (53) |
| Replacement | 44 (34) |
| Mitral valve surgery | 7 (5) |
| Repair | 6 (5) |
| Replacement | 1 (1) |
| CABG due to coronary heart disease | 33 (25) |
| Others | 6 (5) |
| CPB duration (min) | 177 ± 48 |
| Aortic cross-clamp time (min)a | 111 ± 34 |
| Circulatory arrest time (min) | 31 ± 11 |
| UCP time (min) | 36 ± 14 |
| Lowest rectal temperature (°C) | 30 ± 1.6 |
aIncluding circulatory arrest.
CABG: coronary artery bypass.
| Variables . | n (%) or mean ± SD (range) . |
|---|---|
| Extension of arch repair | |
| Repair 1 arch artery | 10 (8) |
| Repair 2 arch arteries | 58 (44) |
| Repair 3 arch arteries | 63 (48) |
| Ascending aorta replacement | 129 (98) |
| Supracoronary | 39 (29) |
| Root repair | 60 (46) |
| Valve conduit | 30 (23) |
| Descending aorta repair | 16 (12) |
| Replacement | 13 (10) |
| Elephant trunk | 3 (2) |
| Aortic valve surgery | 114 (87) |
| Repair (valve and/or root) | 70 (53) |
| Replacement | 44 (34) |
| Mitral valve surgery | 7 (5) |
| Repair | 6 (5) |
| Replacement | 1 (1) |
| CABG due to coronary heart disease | 33 (25) |
| Others | 6 (5) |
| CPB duration (min) | 177 ± 48 |
| Aortic cross-clamp time (min)a | 111 ± 34 |
| Circulatory arrest time (min) | 31 ± 11 |
| UCP time (min) | 36 ± 14 |
| Lowest rectal temperature (°C) | 30 ± 1.6 |
| Variables . | n (%) or mean ± SD (range) . |
|---|---|
| Extension of arch repair | |
| Repair 1 arch artery | 10 (8) |
| Repair 2 arch arteries | 58 (44) |
| Repair 3 arch arteries | 63 (48) |
| Ascending aorta replacement | 129 (98) |
| Supracoronary | 39 (29) |
| Root repair | 60 (46) |
| Valve conduit | 30 (23) |
| Descending aorta repair | 16 (12) |
| Replacement | 13 (10) |
| Elephant trunk | 3 (2) |
| Aortic valve surgery | 114 (87) |
| Repair (valve and/or root) | 70 (53) |
| Replacement | 44 (34) |
| Mitral valve surgery | 7 (5) |
| Repair | 6 (5) |
| Replacement | 1 (1) |
| CABG due to coronary heart disease | 33 (25) |
| Others | 6 (5) |
| CPB duration (min) | 177 ± 48 |
| Aortic cross-clamp time (min)a | 111 ± 34 |
| Circulatory arrest time (min) | 31 ± 11 |
| UCP time (min) | 36 ± 14 |
| Lowest rectal temperature (°C) | 30 ± 1.6 |
aIncluding circulatory arrest.
CABG: coronary artery bypass.
![(left) Preoperative sagittal reconstruction of contrast-enhanced computed tomography scan (angio-CT) showing aneurysm of the thoracic aorta in 62-year-old female. (right) Postoperative angio-CT of the same patient after complete thoracic aorta replacement via clamshell thoracotomy. Thoracic aorta was replaced with three vascular grafts to enable optimal surgical management and shortening of CA and UCP times by gradual re-perfusion technique. For technical details, see text and references [1, 19].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/44/3/10.1093_ejcts_ezt030/1/m_ezt03001.gif?Expires=1749133570&Signature=jkXb1MOuZe35FioMZTLKm~ORiJVwJL5E~wVaQ3RPhuX~eAPMIsvRgq~PcGN8d~U3QfN~eaAOtaT0Dp6oz4F8K3BfA7q9e~EbufR~vWrYFY3FLjn~0CgBIDl71y6L9Rkcp-nRy1BwHNRVPu2Ec9jWTJC6CUjNjJOKenO3wI4XZnsJiZPsAm1i-kRJ8OmHnJIUKqVgjYYbTKcUh6cZ8Wwmqy61Ft2wANYwvRAKDdi3T46D8zaw5J4ug0HYoD7WL5ayFRQMzIdYwTcpoHIQdl2xGVauRadndjbFt0kcQcbu07XOVm2y9vnxZpNQ38rOyUvtr0wk93r2s4dAf6MAxMSRFw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
(left) Preoperative sagittal reconstruction of contrast-enhanced computed tomography scan (angio-CT) showing aneurysm of the thoracic aorta in 62-year-old female. (right) Postoperative angio-CT of the same patient after complete thoracic aorta replacement via clamshell thoracotomy. Thoracic aorta was replaced with three vascular grafts to enable optimal surgical management and shortening of CA and UCP times by gradual re-perfusion technique. For technical details, see text and references [1, 19].
All operative data were prospectively collected, and an intention-to-treat analysis was performed. Adverse neurological outcome was defined as a permanent focal neurological deficit confirmed by a neurologist as well as computed tomography or magnetic resonance imaging or temporary neurological dysfunction (confusion, delirium, agitation or temporary focal deficits without evidence in computed tomography or magnetic resonance imaging). The follow-up duration of at least 90 days was the goal for survivors to allow a data analysis according to the reporting guidelines [6]. The patients were followed up by echocardiography and, if necessary, by CT angiography performed in our outpatient clinic, or by their cardiologist, from whom the written documents and images were requested and reviewed.
Statistical analysis
The statistical analysis was performed with the SPSS software (SPSS, Inc., Chicago, IL, USA). Values in the tables and text are expressed as mean ± standard deviation unless otherwise indicated. Actuarial survival was estimated by the Kaplan–Meier method.
RESULTS
Operative data and early mortality and morbidity
Unilateral (130) or bilateral (1) cerebral perfusion at a blood temperature of 28°C (mean duration 36 ± 14, range: 16–80 min) was performed for brain protection during CA under mild-to-moderate hypothermia (mean rectal temperature 30.0 ± 1.6°C). Concomitant cardiac procedures, mostly on the aortic valve, were necessary in 121 (92%) patients. Among 114 patients necessitating aortic valve/root surgery, there were 70 aortic valve-preserving procedures (61%). The neurological condition could be assessed after surgery in all but 1 patient (female, 76 years), who died before regaining consciousness. She suffered from thoracic mega-aortic aneurysm combined with coronary heart disease and died after coronary artery bypass grafting and complete replacement of the entire thoracic aorta through clamshell thoracotomy. The cause of death was postoperative myocardial infarction due to a heparin-induced thrombocytopenia and coronary graft occlusion. Two further patients died during the postoperative 30-day period, resulting in a 2.3% early mortality. The second patient (female, 71 years) suffering from severe aortic valve stenosis combined with porcelain aorta died due to bowel ischaemia after complete ascending aorta and aortic arch replacement. At autopsy, a severe stenosis of the celiac and upper mesenteric artery was revealed. The third patient (male, 68 years) with a chronic aneurysm and an atherosclerotic ulcer penetrating the left lung and concomitant severe chronic obstructive pulmonary disease died because of pneumonia and subsequent sepsis.
Permanent deficit or temporary neurological dysfunctions occurred in 1 (0.8%) and 6 patients (4.6%), respectively. The neurological complications were not related to the duration of cerebral perfusion during CA, which lasted 29 min on average (range 20–66 min) and was shorter in these patients than in the entire study cohort.
Temporary dialysis was necessary primarily after surgery in 1 patient who had an increased creatinine level before surgery. Two further patients suffered cardiac tamponade and needed temporary dialysis subsequently to this event. Re-sternotomy was necessary in a total of 5 (3.8%) patients.
The most frequent postoperative complication, which occurred in 10 (7.6%) patients, was respiratory insufficiency necessitating prolonged ventilation or reintubation. Six of these patients required tracheotomy.
Survival
Clinical follow-up data were available for all patients. The mean follow-up was 37 ± 27 (range 0–97 months) totalling 404 patient-years. In addition to the early mortality mentioned above, there were 14 late deaths including 1 death that occurred during the first 3 months after surgery resulting in a 90-day mortality of 3.0%. This patient developed purulent mediastinitis after surgery and died during the hospital stay despite multiple surgical revisions, including omental plasty. Causes of all late deaths are listed in Table 3.
| Late death . | n (%) . |
|---|---|
| All | 14 (11) |
| Aortic rupturea | 3 (2.3) |
| Senile decay | 2 (1.5) |
| Cancer | 2 (1.5) |
| Mediastinitis | 1 (0.8) |
| Pneumonia | 1 (0.8) |
| Myocardial infarction | 1 (0.8) |
| Bronchial asthma | 1 (0.8) |
| Renal insufficiency | 1 (0.8) |
| Haemorrhageb | 1 (0.8) |
| Clostridium difficile-associated disease | 1 (0.8) |
| Late death . | n (%) . |
|---|---|
| All | 14 (11) |
| Aortic rupturea | 3 (2.3) |
| Senile decay | 2 (1.5) |
| Cancer | 2 (1.5) |
| Mediastinitis | 1 (0.8) |
| Pneumonia | 1 (0.8) |
| Myocardial infarction | 1 (0.8) |
| Bronchial asthma | 1 (0.8) |
| Renal insufficiency | 1 (0.8) |
| Haemorrhageb | 1 (0.8) |
| Clostridium difficile-associated disease | 1 (0.8) |
aOccurred distally to repaired aortic segment in all cases, for details see text.
bUnder warfarin therapy due to atrial fibrillation.
| Late death . | n (%) . |
|---|---|
| All | 14 (11) |
| Aortic rupturea | 3 (2.3) |
| Senile decay | 2 (1.5) |
| Cancer | 2 (1.5) |
| Mediastinitis | 1 (0.8) |
| Pneumonia | 1 (0.8) |
| Myocardial infarction | 1 (0.8) |
| Bronchial asthma | 1 (0.8) |
| Renal insufficiency | 1 (0.8) |
| Haemorrhageb | 1 (0.8) |
| Clostridium difficile-associated disease | 1 (0.8) |
| Late death . | n (%) . |
|---|---|
| All | 14 (11) |
| Aortic rupturea | 3 (2.3) |
| Senile decay | 2 (1.5) |
| Cancer | 2 (1.5) |
| Mediastinitis | 1 (0.8) |
| Pneumonia | 1 (0.8) |
| Myocardial infarction | 1 (0.8) |
| Bronchial asthma | 1 (0.8) |
| Renal insufficiency | 1 (0.8) |
| Haemorrhageb | 1 (0.8) |
| Clostridium difficile-associated disease | 1 (0.8) |
aOccurred distally to repaired aortic segment in all cases, for details see text.
bUnder warfarin therapy due to atrial fibrillation.
The linearized death rate was 4.2%/year, and the actuarial survival, including 30-day mortality, was 81.9 ± 4.3% at 5 years (Fig. 2).
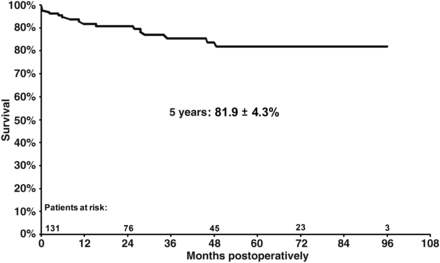
Actuarial survival without cardiac and/or aortic re-intervention.
Aortic events
No patient needed any reoperation or new intervention on the repaired segments of aorta; however, there were a total of three fatal aortic events. A 15-year-old Marfan patient underwent a root replacement elsewhere because of an acute type A dissection and a repair of the abdominal aorta due to an imminent rupture of a progressive abdominal aneurysm 8 weeks later. She was referred to our centre with a chronic dissected mega-aneurysm beginning at the ascending aorta and extending throughout the entire thoracoabdominal aorta. For this reason, a two-stage procedure was chosen. First, an ascending aorta and a complete aortic arch replacement using the elephant trunk technique were successfully performed. For unknown reasons, the patient living abroad chose not to take the opportunity for the planned repair of the thoracoabdominal aneurysm and died due to an aortic rupture a few months later. Two further patients, who underwent a successful valve-preserving root repair combined with complete arch replacement, suffered from concomitant abdominal aneurysms localized in the infrarenal aorta. The first of these patients also did not take the opportunity for the planned surgery of the abdominal aorta and died 1 year later because the aneurysm ruptured. The second patient suffered a rupture during stent-grafting of the abdominal aorta 36 months after primary surgery.
DISCUSSION
Although the results of open aortic arch surgery have improved dramatically in the last decade [1–3], this procedure is still considered high risk [7]. Unfortunately, the results from the 1990s or even the 1980s are frequently used to support the argumentation that the use of extracorporeal circulation and deep hypothermic CA, which are needed for open arch surgery, leads to increased mortality and morbidity [7]. Hence, thoracic endovascular aortic repair (TEVAR) of aortic arch pathologies, which is combined as a hybrid procedure with bypassing or re-routing (also called debranching) of supra-aortic arteries, has been recently proposed [7–9].
The pathology of the descending aorta frequently extends to the distal arch and demands, in such cases, the exclusion of the left subclavian artery during an endograft implantation, even if the descending aorta is the primary target of repair. In this case, a left carotid-subclavian bypass or transposition of the left subclavian artery to the left common carotid artery is recommended [10], which is the mildest form of an aortic arch hybrid procedure. The revascularization of the left subclavian artery on the neck is a simple and safe procedure and therefore justifies such proximal extension of the endograft.
On the other hand, there are very complex pathologies of the thoracic aorta in which the open ascending aorta replacement is combined with a re-routing of the arch arteries and antegrade or retrograde stent-grafting of the arch and descending aorta [7–9, 11]. This procedure is, per definition, an aortic arch hybrid procedure. But it is also an open one and is actually a hybrid procedure of the descending aorta rather than the aortic arch. The extent of surgery is absolutely identical with conventional arch replacement combined with repair of the descending aorta using the elephant trunk technique. The difference between both procedures is that, in the first, cerebral perfusion is generally not used for the re-routing of the arch arteries. Furthermore, re-routing enables a proximalward moving of the distal aortic anastomosis, which, from a surgical point of view, may be convenient in some cases. For example, the distal anastomosis is not located deeply in the chest, and a simultaneous or staged stent-grafting of the descending aorta, if necessary, can be performed throughout the aortic arch, regardless of whether an antegrade or retrograde approach is used [8, 11]. The endografting of the descending aorta in this way is, of course, also possible after conventional complete arch replacement, because the distance between the distal anastomosis and the origin of the last arch branch enables safe anchoring of the stent graft in the arch prosthesis (Fig. 3).
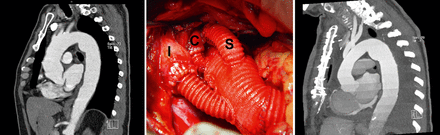
(left) Preoperative sagittal reconstruction of contrast-enhanced computed tomography scan (angio-CT) showing aneurysm of the ascending aorta and dilatation of the atherosclerotic aortic arch in 73-year-old male. Even if pathology of the descending aorta (atherosclerosis and mild-to-moderate dilatation) did not demand any procedure at time of surgery, necessity of such procedure at later date cannot be ruled out. (middle) Intraoperative photograph after aortic root repair and proximal aortic replacement depicting the moving of supra-aortic arteries away from distal anastomosis between arch graft and descending aorta. (right) Postoperative angio-CT of the same patient. Proximalward moving of supra-aortic arteries enables safe anchoring of the stent graft in arch prosthesis should endografting of the descending aorta become necessary in future. I: innominate artery; C: left common carotid artery; S: left subclavian artery. For technical details see text.
Nevertheless, the aortic arch surgeries mentioned above require the use of extracorporeal circulation and CA, and it is therefore not surprising that, in the last report from the Transcontinental Registry about total arch re-routing, almost 60% of patients required the use of CPB, and this rate was even higher in the recent meta-analysis [7, 9]. Avoiding open surgery in patients with aortic arch pathology is seldom possible, because it is mostly combined with pathology of the ascending aorta. Even an aortic arch aneurysm that seems to be isolated is frequently combined with atherosclerosis and calcifications that are spread out in the entire proximal aorta (Fig. 4). Given that the proximal aorta is a main source of cerebrovascular embolism, not only should a tangential clamping of the ascending aorta be avoided in such cases, but its complete replacement is even indicated [12–14]. Aortic arch pathology is very frequently associated with an aortic valve defect, and because it is mostly pure insufficiency, a valve-sparing surgery can be performed. In our series, 87% of the patients needed aortic valve and/or root surgery concomitantly to arch repair, and the valve could be preserved in more than half of them.
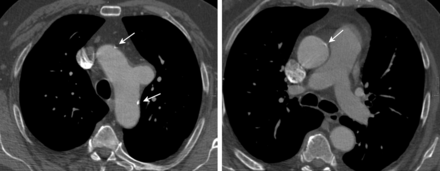
Preoperative axial images of contrast-enhanced computed tomography scan (angio-CT) showing saccular aneurysm of the aortic arch (left) and mild dilatation of the atherosclerotic ascending aorta (right) in 72-year-old male. Arrows indicate calcifications in both aortic arch and ascending aorta.
All this suggests that indications for a classic aortic arch hybrid procedure consisting of re-routing the supra-aortic arteries and subsequent arch endografting are rare, and the comparability of such procedures with conventional arch surgery is very limited. The authors of the Transcontinental Registry claimed that only 12% of their patients were suitable for open repair because of ‘multisegmental aortic disease or comorbidities’ [9]. This assessment seems to be very subjective, though open surgery with CPB was used in 60% of the patients. Trying to designate inoperable patients in our cohort according to such a definition of inoperability would have only been a polemic. Nevertheless, 5 patients were referred to our centre because the surgery was refused elsewhere or the operative risk, as assessed elsewhere, had not been accepted by the patients. All 5 patients survived surgery and are still alive.
Even the classic aortic arch hybrid procedure (without the use of CPB) is burdened with specific procedural problems, which are clearly mentioned by Czerny et al. [9] in their paper. The history of acute dissection caused by a stent graft is almost as old as the procedure itself, and this aspect has not lost relevance despite the technical progress [9, 15, 16]. The tangential clamping of the ascending aorta has also been considered as a cause of dissection [9], although an increased neurological morbidity as a consequence of cerebral embolism is much more probable during this manoeuvre [12, 13]. Lastly, the stent malpositions or migrations and early and late endoleaks may demand re-interventions or even challenging open surgeries [17, 18].
In contrast, the refined technique of conventional aortic arch repair, although more invasive in some cases, provides definitive repair with excellent clinical results and offers the possibility of repairing concomitant cardiac pathologies simultaneously. Even if there is no doubt that CA, CPB, and surgery times are the clear predictors of an increased risk in cardiovascular surgery, the considerable shortening of all these aspects could be achieved by the recent improvements in surgical and perfusion strategies.
Nevertheless, it seems that conventional arch repair (which can be and is combined with TEVAR, if appropriate) as well as most TEVAR techniques combined with so-called hybrid aortic arch procedures are open techniques and differ from each other just because of their different approach to the pathology of the adjacent segments of the aorta [1, 8, 11]. We are convinced that endovascular techniques may be a practical enrichment of the surgical armamentarium, but we consider the use of CPB and cerebral perfusion as a decisive tool to ensure the full control in each phase of surgery, especially during anastomosing the vascular grafts with supra-aortic arteries, rather than a general risk factor. Fortunately, surgical progress and evolving technologies have led to the development of different surgical approaches, which can be applied depending on the clinical situation, aortic and cardiac pathology and surgeon's experience. The variety of cardiac and aortic pathologies as well as the clinical parameters demand very individual therapy, which is essential for optimal outcome; and therefore, a randomization would not have been justified in most of these patients. Nevertheless, the lack of randomization and patient selection, which may have introduced a selection bias, can be considered a limitation of the study; however, all patients referred for surgery were enrolled for observational assessment in which all relevant perioperative parameters were collected prospectively. Moreover, no patient was refused surgery because of the severity of cardiac disease.
In conclusion, conventional arch surgery offers definitive repair and can be safely performed using current perfusion and operative techniques. Open surgery ensures simultaneous aortic valve repair, which is frequently necessary, and can be performed by reconstruction in more than half of the cases. The use of refined surgical techniques with cerebral perfusion allows the avoidance of deep hypothermia with all its negative side effects and leads to excellent outcomes against which the results of alternative approaches should be compared.
ACKNOWLEDGEMENTS
The authors thank Melissa Lindner, Alexandra Metz and Bianca Müller for the assistance in preparing this article.
Conflict of interest: Paul P. Urbanski is a consultant for MAQUET Cardiovascular, Inc.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr H. Jakob(Essen, Germany): Earlier this year you published a series of 347 patients who had undergone arch surgery with a 30-day mortality of 0.9%. Out of these, 77 patients (22%) had a subtotal or total arch replacement. So the vast majority had what we call open arch anastomosis or hemiarch.
The more complex subgroup has now been extended over a two and a half year period to 131 patients. Out of these, 63 patients, or 48%, had total arch replacement and eight patients underwent additional descending aortic treatment, three classic elephant trunks and five descending replacements via clamshell thoracotomy. The reported 30-day mortality was 2.3% (three patients). And since these casualties have already been described in the recent paper, you obviously must have operated upon these additional 54 patients in the presented series with zero mortality. Actuarial 5-year survival was 82%.
Though still representing a patient group with a somewhat milder arch pathology (all acute and chronic dissection cases were excluded and more complex arch pathologies were rare), the presented results are outstanding and you and your team are to be congratulated for your elaborated approach using classic but optimized perfusion techniques with short ischaemic times at only mildly lowered temperatures.
I now have two questions. If you are confronted with severe atherosclerotic or chronically dissected ascending aorta, arch and descending aorta, how would you choose your operative strategy? Can your technique be extended to all pathologies?
Dr Urbanski: I think that we had eight clamshells, if I remember correctly. We excluded the patients with acute aortic dissection but not with chronic. Acute dissection is a very different group because there are a lot of patients with malperfusion and some symptoms which influences the results. For this reason we took into the evaluation only chronic aortic diseases, but, of course, chronic dissections are also present. Regarding the surgical strategy, we would consider the pathology and the age of the patients. When a patient is younger, I would prefer conventional surgery but with the clamshell approach, for example, to replace the entire thoracic aorta; we have included such patients in this evaluation.
In patients with advanced age or with a lot of comorbidities, a combination of two surgical approaches can be considered. For example, conventional arch combined with an elephant trunk, conventional or even stented trunk. In chronic dissection, it's seldom possible to place the endograft and to close the false lumen because it can cause a malperfusion. In such situations I would prefer conventional surgery with resection of the membrane between both lumens.
Dr Jakob: Second question, what is your follow-up strategy? Are you tracking all your patients with repeat imaging to detect downstream problems at an early stage? What is your therapeutic concept in the event of progressing descending or thoracoabdominal aortic disease? Are you going for stent-grafting or for a second operation, thoracoabdominal replacement?
Dr Urbanski: We see it as a problem in Germany and we should try to be very aggressive with the follow-up, especially in patients after surgery for acute dissection. Unfortunately, we see that a very small number of patients receive postoperative examinations after surgery, even after explicit recommendation. For this reason we strive to contact the patients at least once a year and to persuade the patients and the family doctors to perform control examinations, because we know that the disease can progress. Unfortunately, we don't have the possibility to follow up all patients from our centre, but we do it for special groups, for example, for acute dissection or for patients with extensive aortic surgery, such as complete aortic arch repair, or for study purposes. But it is a very important aspect and I would like to emphasize that it is really necessary to undertake a very close follow-up of this patient group.
Dr Jakob: We also ask all patients to come back to our centre and we have a 100% follow-up with imaging on all our patients, which I think is the way we have to deal with this kind of disease. Nevertheless, your results are outstanding and this is the benchmark for any upcoming endovascular therapies.
Dr Y. Okita(Kobe, Japan): I have to raise a question about the temperature. You perfuse the patients at 28 degrees. I noted that one patient had massive gastrointestinal tract necrosis. We had a similar patient, a 75-year-old woman with a massive gastrointestinal tract necrosis. Her rectal temperature was 31, but the brain temperature was, if I remember, 27 or 26. So, especially in patients with a very, very atherosclerotic gastrointestinal tract artery, very calcified, I think moderate hypothermia is very dangerous for protecting the gastrointestinal tract.
Dr Urbanski: Yes, it can be dangerous if the diagnostics are not properly done. In the case you referred to, I showed the slide with the CT angiography of the case as a note of caution. We did not find the problem with the upper mesenteric artery until autopsy, but we should take these changes into consideration before surgery. Currently, if we were to see atherosclerotic calcifications at abdominal level on CT angiography, we would perform abdominal angiography to look at the visceral vessels. And in such patients we would now consider revascularization of the mesenteric artery prior to elective surgery of the thoracic aorta.
Dr Okita: Would you go to deeper hypothermia?
Dr Urbanski: I'm not sure if the deep hypothermia would be the only solution to resolve this problem. I think the problem should be resolved by proper diagnostics and proper therapy.
Author notes
Presented at the 26th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 27–31 October 2012.
- aorta
- aortic arch
- aortic valve
- cardiac surgery procedures
- hypothermia, natural
- repair of aortic valve
- follow-up
- perfusion
- reconstructive surgical procedures
- repeat surgery
- surgical procedures, operative
- brain
- heart
- mortality
- pathology
- temperature
- neuroprotection
- avoidance behavior
- cerebral perfusion
- circulatory arrest




