-
PDF
- Split View
-
Views
-
Cite
Cite
Marco Di Eusanio, Paolo Berretta, Mariano Cefarelli, Roberto Di Bartolomeo, Root graft substitution after aortic valve replacement: sparing the valve prosthesis is a valid option, European Journal of Cardio-Thoracic Surgery, Volume 44, Issue 3, September 2013, Pages 427–430, https://doi.org/10.1093/ejcts/ezt034
Close - Share Icon Share
Abstract
Few case studies have shown the feasibility of the prosthesis-sparing operation in patients requiring aortic root replacement after aortic valve replacement. Such technique allows the sparing of a well-functioning aortic valve prosthesis and facilitates the root substitution with only a vascular graft. The aim of the present study was to assess short- and mid-term outcomes of the patients who underwent such procedures at our institution.
Between 2004 and 2012, 26 patients (mean age: 59 ± 13.6 years; male: 21, 80.8%) underwent the prosthesis-sparing operation in our institution. The mean time from previous aortic intervention was 20.1 ± 6.9 years; two patients were operated for a Type A acute aortic dissection.
Overall, two patients (7.7%) died during hospitalization: both were operated for a complicated Type A acute aortic dissection. None of the electively operated patients died or presented serious complications after surgery, except for one patient (3.8%) who required chest re-exploration for excessive bleeding due to coagulopathy. At follow-up (100% completed at 30 ± 24 months) two late deaths occurred: one due to lung cancer and one due to infective endocarditis. Kaplan-Meier estimates of 1- and 3-year survival were 92 and 85.4%, respectively. No late cardiac/aortic re-interventions were performed during follow-up, with a 5-year freedom from re-operation of 100%.
Our favourable short- and mid-term results indicate that the prosthesis-sparing operation is a valid treatment option in selected re-operative aortic root procedures.
INTRODUCTION
Following aortic valve replacement, aneurysmal growth and/or acute dissection of the aortic root may require secondary interventions and root substitution [1]. In this setting, the Bentall procedure—involving the removal of the aortic valve prosthesis and the replacement of the aortic root with a new composite graft—represents the most common surgical option for treatment [2]. Few case studies have shown the feasibility (or indicated the potential advantages) of the prosthesis-sparing operation that, in patients with well-functioning aortic valve prostheses, allows the graft replacement of the aortic root without removing the valve prosthesis [3–5].
The aim of the present study was to surpass feasibility how to do it study by assessing short- and mid-term outcomes in a larger group of patients who were operated in our institution.
MATERIAL AND METHODS
Patient characteristics
Between July 2004 and April 2012, 26 patients underwent re-operative aortic root procedures at our institution using the prosthesis-sparing operation. This technique was employed—according to the surgeon's preference—if the aortic valve prosthesis implanted at prior surgery was functioning well, without pannus or thrombus, as documented by both preoperative echocardiography and direct inspection.
Preoperative patient characteristics are set out in Table 1. Indications for root replacement included degenerative aneurysm (n = 22), chronic post-dissection aneurysm (n = 1), acute Type A aortic dissection (n = 2) and false aneurysm (n = 1). All patients had received a mechanical aortic valve prosthesis during prior surgery. The mean time from previous aortic intervention was 20.1 ± 6.9 years; four patients (15.4%) had already undergone more than two aortic/cardiac procedures via a mid-sternotomy.
| . | Frequency . | % . |
|---|---|---|
| Male | 21 | 80.8 |
| Age | 59 ± 13.6 | |
| NYHA class III-IV | 2 | 7.7 |
| Hypertension | 17 | 65.4 |
| Diabetes | 2 | 7.7 |
| Obesity | – | – |
| Smoking | 8 | 30.8 |
| COPD | 1 | 3.8 |
| Renal insufficiency | 1 | 3.8 |
| Peripheral vasculopathy | – | – |
| Cerebral vasculopathy | – | – |
| Coronary artery disease | 4 | 15.4 |
| Marfan | 1 | 3.8 |
| Urgent/emergent status | 2 | 7.7 |
| Redo >2 | 4 | 15.4 |
| Years from previous operation | 20.1 ± 6.9 |
| . | Frequency . | % . |
|---|---|---|
| Male | 21 | 80.8 |
| Age | 59 ± 13.6 | |
| NYHA class III-IV | 2 | 7.7 |
| Hypertension | 17 | 65.4 |
| Diabetes | 2 | 7.7 |
| Obesity | – | – |
| Smoking | 8 | 30.8 |
| COPD | 1 | 3.8 |
| Renal insufficiency | 1 | 3.8 |
| Peripheral vasculopathy | – | – |
| Cerebral vasculopathy | – | – |
| Coronary artery disease | 4 | 15.4 |
| Marfan | 1 | 3.8 |
| Urgent/emergent status | 2 | 7.7 |
| Redo >2 | 4 | 15.4 |
| Years from previous operation | 20.1 ± 6.9 |
COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association.
| . | Frequency . | % . |
|---|---|---|
| Male | 21 | 80.8 |
| Age | 59 ± 13.6 | |
| NYHA class III-IV | 2 | 7.7 |
| Hypertension | 17 | 65.4 |
| Diabetes | 2 | 7.7 |
| Obesity | – | – |
| Smoking | 8 | 30.8 |
| COPD | 1 | 3.8 |
| Renal insufficiency | 1 | 3.8 |
| Peripheral vasculopathy | – | – |
| Cerebral vasculopathy | – | – |
| Coronary artery disease | 4 | 15.4 |
| Marfan | 1 | 3.8 |
| Urgent/emergent status | 2 | 7.7 |
| Redo >2 | 4 | 15.4 |
| Years from previous operation | 20.1 ± 6.9 |
| . | Frequency . | % . |
|---|---|---|
| Male | 21 | 80.8 |
| Age | 59 ± 13.6 | |
| NYHA class III-IV | 2 | 7.7 |
| Hypertension | 17 | 65.4 |
| Diabetes | 2 | 7.7 |
| Obesity | – | – |
| Smoking | 8 | 30.8 |
| COPD | 1 | 3.8 |
| Renal insufficiency | 1 | 3.8 |
| Peripheral vasculopathy | – | – |
| Cerebral vasculopathy | – | – |
| Coronary artery disease | 4 | 15.4 |
| Marfan | 1 | 3.8 |
| Urgent/emergent status | 2 | 7.7 |
| Redo >2 | 4 | 15.4 |
| Years from previous operation | 20.1 ± 6.9 |
COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association.
Surgical technique
Our surgical technique for the prosthesis-sparing operation has previously been described [3]. Put briefly, after cardiopulmonary bypass was instituted and cardioplegic arrest achieved, the aneurysm was resected and the coronary ostia fully detached. The base of the aorta was trimmed to leave a 4 mm remnant and the Dacron graft size (Valsalva graft; Vascutek, Inchinnan, Scotland) was selected, adding 5 mm to the size of the aortic valve prosthesis. Horizontal Teflon felt pledgeted 2-0 mattress sutures were placed from outside the aortic annulus to the sewing ring of the previously implanted aortic prosthesis and then to the Dacron conduit. The graft was parachuted and sutures tied. The 4 mm remnant of the aortic wall was sutured to the proximal end of the Dacron graft using a 4/0 polypropylene running suture to improve haemostasis [6]. The left and right coronary ostia were re-implanted using a 5/0 polypropylene running suture. The coronary anastomoses and the graft/aortic coupling were tested by infusion of cardioplegic solution into the graft. The distal anastomosis between the graft and the distal aortic stump (ascending aorta or arch) was performed using a 4/0 polypropylene running suture and reinforced using double Teflon felt. The surgical technique is shown in Figure 1 and Supplementary Video 1.
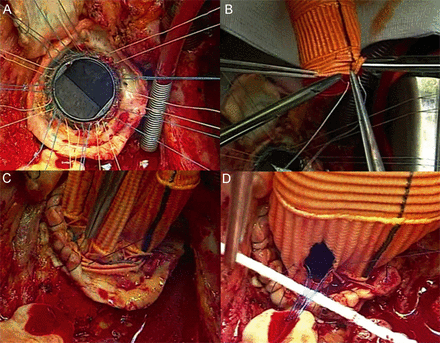
(A) Horizontal Teflon felt pledgeted 2-0 mattress sutures were placed from outside the aortic annulus to the sewing ring of the previously implanted aortic prosthesis and then (B) to the Dacron conduit. The graft was parachuted and sutures tied. (C) The 4-mm remnant of the aortic wall was sutured to the proximal end of the Dacron graft using a 4/0 polypropylene running suture to improve haemostasis. (D) The left and right coronary ostia were re-implanted using a 5/0 polypropylene running suture.
Seven patients required a hemi-arch replacement using antegrade selective cerebral perfusion and moderate hypothermia as a method of brain protection and three patients underwent planned coronary artery bypass grafting [7]. Operative data are shown in Table 2.
| min | |
| Cardiopulmonary bypass time | 168.4 ± 48.3 |
| Myocardial ischaemic time | 132.4 ± 31.8 |
| Antegrade selective cerebral perfusion time | 25.6 ± 6.3 |
| min | |
| Cardiopulmonary bypass time | 168.4 ± 48.3 |
| Myocardial ischaemic time | 132.4 ± 31.8 |
| Antegrade selective cerebral perfusion time | 25.6 ± 6.3 |
| min | |
| Cardiopulmonary bypass time | 168.4 ± 48.3 |
| Myocardial ischaemic time | 132.4 ± 31.8 |
| Antegrade selective cerebral perfusion time | 25.6 ± 6.3 |
| min | |
| Cardiopulmonary bypass time | 168.4 ± 48.3 |
| Myocardial ischaemic time | 132.4 ± 31.8 |
| Antegrade selective cerebral perfusion time | 25.6 ± 6.3 |
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables were presented as percentages.
Estimates of 5-year survival and of 5-year freedom from re-operation (including hospital deaths) were obtained using Kaplan-Meier methods. Statistical analysis was performed using SPSS 20.0 software (IBM Corp.)
RESULTS
Hospital outcomes
Overall, two patients died during hospitalization: both were operated for Type A acute aortic dissection. The first was an 81-year-old man whose preoperative clinical status was burdened by severe left ventricular dysfunction (ejection fraction: 30%), severe chronic obstructive pulmonary disease and renal insufficiency (preoperative creatinine: 1.8 mg/dl). Postoperatively, he was never extubated and required continuous renal function replacement with dialysis. He eventually died on postoperative day (POD) 46 due to multi-organ failure.
The second patient presented with shock and was urgently intubated on admission in our hospital. He died on POD 1 due to mesenteric ischaemia.
Following elective surgery, no patients died or presented serious neurological, renal or respiratory complications. One patient underwent chest re-exploration for excessive bleeding due to coagulopathy. In elective patients, the mean intubation time was 11.0 ± 8.2 h (range: 2–40) and mean ICU stay was 1.8 ± 1.0 days (range: 1–4).
Follow-up
All patients underwent imaging assessment (CT or MRI) of the aorta prior to discharge. Clinical and CT or MRI imaging follow-up was performed at regular intervals (usually 3 months after surgery and then annually) in the outpatient clinic or by telephone interview of the patient or a family member.
Follow-up was 100% complete at a mean of 30 ± 24 months following the procedure (range: 0.07–89.7). Overall, two deaths occurred, one due to lung cancer and one due to late infective endocarditis, complicated by multiple myocardial and cerebral septic embolization, which eventually contraindicated surgical re-repair.
Kaplan-Meier estimates of 1- and 3-year survival were 92 ± 5.4% and 85.4 ± 8.1%, respectively (Fig. 2).
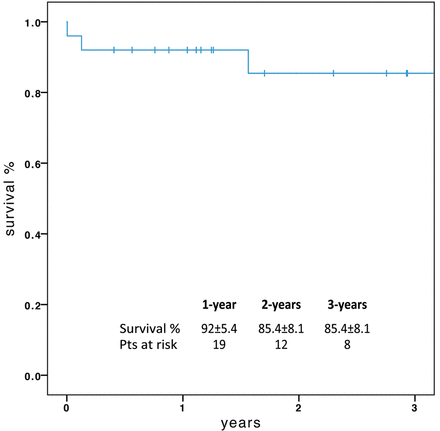
No root pseudo-aneurysms were detected by imaging exams and no cardiac/aortic re-interventions were performed during follow-up, with a 5-year freedom from re-operation of 100%.
DISCUSSION
Several disorders may dictate root replacement after aortic valve replacement. These most likely include root aneurysmal growth, acute dissection, infective endocarditis and pseudo-aneurysm at the aortic suture-lines.
In this setting—regardless of the proper functioning of the aortic valve prosthesis—the Bentall procedure, with removal of the old valve prosthesis and implantation of a new composite graft, represents the most common surgical solution [8, 9].
We recently described our technique for a procedure that spares the well-functioning aortic valve prosthesis, implanted during prior surgery, and allows the root substitution with only a vascular graft. To prevent the removal of the previously implanted aortic valve prosthesis, this technique avoids tractions, torsions and distortions of the aortic annulus and periannular structures, which may result in sub-optimal seating of the new composite graft (sometimes necessarily smaller in size), proximal leakages or pseudo-aneurysms and atrio-ventricular block. Furthermore, clamp and cardiopulmonary bypass durations can be somewhat reduced.
Obviously, the proper functioning of the valve prosthesis and the absence of infective endocarditis are necessary prerequisites for the implementation of this technique.
In the present series, all patients had received a mechanical valve during previous operations and, in our experience with re-operative root operations, all biological valves have been removed and not spared. However, in the presence of a favourable ratio (>1) between the expected freedom from valve degeneration and the patient's estimated life expectancy, we might consider sparing biological valve prostheses in future.
One might also argue that such conservative treatment could augment the risk of subsequent re-interventions in patients with long-standing mechanical valves, due to increased likelihood of prosthesis dysfunction. In the present series, half of the patients had had their artificial valves implanted 20 years earlier. In this regard, we recommend an accurate (echocardiographic and direct) assessment of the valve substitute; to avoid its manipulation; to always consider patient's expectancy of life and, eventually, to side-step when imperfect valves or sub-optimal conditions are encountered.
What may appear as the weak (and leaking) point of the procedure is probably the connection between the old valve prosthesis and the new root graft. Nevertheless, by suturing the invariably thick and robust remnant of the aortic wall to the proximal end of the Dacron graft (Fig. 1), proximal bleeding has never been an issue and no pseudo-aneurysm formation has been noted in follow-up imaging studies, as confirmed by the 100% freedom from aortic and cardiac re-operation of our patients at 5 years.
Very few clinical data are available in literature on the prosthetic sparing operation with—to the best of our knowledge—only seven cases reported to date [4, 5]. In our 26 patients, such conservative technique has produced favourable short- and mid-term outcomes. Apart from two deaths, which occurred in patients with Type A acute aortic dissection, presenting with severe comorbidities and complications, the postoperative course of elective patients was almost completely uneventful, with only one chest re-exploration performed for excessive bleeding (coagulopathy), leading to a total adverse event rate of only 3.7%.
At follow-up, the good early results have been confirmed by only one cardiac/aortic death, due to late infective endocarditis, and no late cardiac/aortic re-interventions: overall, Kaplan-Meier estimates of survival were 92 and 85.4% at 1 and 3 years, respectively.
The main limitations of the present study are its retrospective nature and the limited size of the study population. The latter also discouraged us from comparing patients' outcomes after prosthesis-sparing and Bentall operations. Nevertheless, to the best of our knowledge, this is the largest series of prosthesis-sparing operations reported so far and we believe that future, larger series with longer follow-up will confirm our initial favourable observations. Thus, although the described procedure remains challenging and requires advanced technical skills, we felt it was important to share our experience and hope this may contribute to expanding the surgical armamentarium available for those patients who may require root graft substitution after aortic valve replacement.
In conclusion, our encouraging short- and mid-term results indicate that the prosthesis-sparing operation is a valid treatment option in selected re-operative aortic root procedures.
SUPPLEMENTARY MATERIAL
Supplementary material (Video 1) is available at EJCTS online.
Video 1: Surgical technique.
Conflict of interest: none declared.
REFERENCES
- aorta
- dissection of aorta
- bacterial endocarditis
- hemorrhage
- aortic valve replacement
- blood coagulation disorders
- follow-up
- objective (goal)
- repeat surgery
- surgical procedures, operative
- tissue transplants
- heart
- surgery specialty
- chest
- lung cancer
- stent/grafts, vascular
- supraaortic valve area
- aortic root replacement
- prostheses
- aortic valve prosthesis




