-
PDF
- Split View
-
Views
-
Cite
Cite
Anna L. Meyer, Doris Malehsa, Christoph Bara, Axel Haverich, Martin Strueber, Implantation of rotary blood pumps into 115 patients: a single-centre experience, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 6, June 2013, Pages 1233–1236, https://doi.org/10.1093/ejcts/ezs568
Close - Share Icon Share
Abstract
From 2004 to 2009, rotary blood pumps were implanted for heart failure as a bridge to transplant or destination therapy in 101 male and 14 female patients at our institution. We report on our experiences of these patients with a follow-up of 132 patient years.
Seventy-four HeartMate II axial flow pumps and 41 HeartWare centrifugal pumps were implanted in patients with non-ischaemic (n = 70) or ischaemic cardiomyopathy (n = 45). The mean age of the patients was 50 ± 13 years. All patients were on inotropic support prior to implantation. Extracorporeal membrane oxygenation was used as a bridging procedure in 21 cases.
The perioperative mortality was 14%. Hospital discharge occurred on average after 46 ± 33 days. Twenty-two patients of this cohort received a heart transplant 492 ± 342 days after implantation of the device. Two patients died after heart transplantation. A 1-year survival of 73% and a 2-year survival of 69% were recorded, despite a low incidence of transplant procedures. The longest support time was 1686 days.
Modern left ventricular assist device technology can be used successfully for heart failure. The scarcity of donor hearts leads to prolonged periods on the device, and chronic ventricular assist device therapy has become a reality, although bridge to transplant was intended; therefore, sufficient support services for outpatient care of these patients are required.
INTRODUCTION
As donor hearts for transplantation continue to be limited in number, the role of implantable left ventricular assist devices (LVADs) becomes increasingly more important. Despite technical problems and failure of pulsatile systems, the survival rate among patients with an LVAD was higher in comparison to patients receiving medical therapy [1]. However, new generation continuous axial flow pumps have remedied former problems and resulted in significantly better clinical outcomes in patients suffering from heart failure. Slaughter et al. described a survival rate after 2 years of 58% in patients with continuous-flow devices vs 24% in patients with pulsatile-flow LVADs. Furthermore, significant differences in the 2-year survival rate without stroke and without reoperation due to device repair and/or replacement were reported (continuous-flow LVAD, 46%; and pulsatile LVAD, 11%) [2].
Initially, the effectiveness of non-pulsatile pumps was equivocal. However, the incidence of right heart dysfunction was similar for pulsatile and non-pulsatile systems [3], and equivalent degrees of haemodynamic support, postoperative renal function and exercise capacity were described [4]. It was shown that a greater left ventricular volume unloading without influence of early exercise performance was associated with pulsatile pumps [5]. On the practical side, non-pulsatile pumps are smaller, only half as large as pulsatile pumps, which makes them suitable for smaller patients. In addition, the non-pulsatile pumps are quieter and more durable owing to the loss of biological valves.
The first continuous flow pumps, HeartMate II (Thoratec, Pleasonton, CA, USA), a second-generation axial flow pump, were implanted in Hannover as part of a clinical trial in 2004. Since January 2007, HVAD™ (HeartWare Inc., Miramar, FL, USA), a third-generation implantable LVAD with a small centrifugal pump, has become available [6, 7]. The HeartMate II became commercially available in 2006, the HVAD in 2009. Thus, in this retrospective study, we analysed the outcome of patients implanted with continuous LVADs at the Hannover Medical School and report our experience.
PATIENTS AND METHODS
From 2004 to 2009, 115 rotary blood pumps (HeartMate II, n = 74; HeartWare, n = 41) were implanted for heart failure at Hanover Medical School. The first HeartMate II LVAD implantation was in February 2004. Since 2007, the HVAD from HeartWare has been used in patients with heart failure enrolled in the HeartWare study.
An LVAD was necessary in 70 patients with non-ischaemic and in 45 with ischaemic cardiomyopathy. The mean age of the 101 male and 14 female patients was 50 ± 13 years. In nine patients, the LVAD was implanted as the destination therapy and in 106 patients as a bridge to transplantation. Extracorporeal membrane oxygenation (ECMO) was used as a bridging procedure in 21 cases, and 35 patients were on mechanical ventilation. Flucloxacillin and meropenem were used for perioperative antibiotic prophylaxis. The duration of administration was extended to the seventh postoperative day. Old intravenous lines were replaced on the day of implantation.
All patients were anticoagulated with heparin 24 h after implantation if the bleeding from the chest tubes was less than 50 ml/h. Heparin was switched to warfarin, with a target international normalized ratio (INR) from 2.0 to 2.5, aspirin (100 mg/day) in combination with clopidogrel (75 mg/day). In patients with a HeartMate II, the anticoagulation protocol was ultimately changed to a single treatment with warfarin owing to a high rate of bleeding complications and the findings of an acquired von Willebrand syndrome. Patients with a HeartWare were treated with 100 mg clopidogrel three times a week and warfarin with an INR between 2.0 and 2.5.
Red blood cell units were given if the haemoglobin was less than 9 g/dl and platelets administered if the platelet count was less than 50 × 103/µl.
The follow-up was a minimum of 6 months until June 2010. Regular outpatient visits took place every 3 months or more often as medically indicated.
Patients were treated with an ECMO prior to LVAD implantation in the case of secondary multiorgan failure, cardiogenic shock with right heart failure or an unknown cerebral status or for transportation from another centre after cardiotomy failure.
Statistical analysis
Data are reported as means ± SD. All statistical analyses were carried out using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Analysis of variance (ANOVA) was performed using the non-parametric Mann–Whitney U-test to detect significant statistical differences between parameters before ECMO and LVAD implantation. Statistical significance was accepted at P < 0.05.
RESULTS
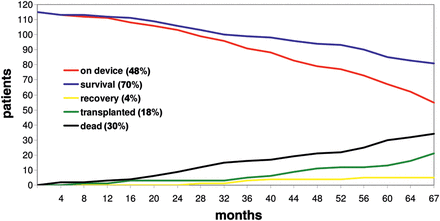
The 30-day and 2-year survivals of the patients with a rotary blood pump were 86 and 69%, respectively (Fig. 1). The median length of hospital stay after implantation was 33 days. Following LVAD implantation, 19% of patients (n = 22) were transplanted and 4% (n = 5) recovered, with subsequent LVAD explantation. The remaining 48% (n = 55) continued with the pump. The most common causes of death were multiorgan failure (n = 10) and sepsis (n = 6; Table 1). In four patients, the cause of death was unknown; however, patient application errors related to the LVAD were suspected.
| Cause of death . | Number of patients . |
|---|---|
| Multiorgan failure | 10 |
| Sepsis | 6 |
| Cerebral ischaemia | 5 |
| Cerebral bleeding | 5 |
| Right heart failure | 4 |
| Unknown | 4 |
| Others | 3 |
| Cause of death . | Number of patients . |
|---|---|
| Multiorgan failure | 10 |
| Sepsis | 6 |
| Cerebral ischaemia | 5 |
| Cerebral bleeding | 5 |
| Right heart failure | 4 |
| Unknown | 4 |
| Others | 3 |
| Cause of death . | Number of patients . |
|---|---|
| Multiorgan failure | 10 |
| Sepsis | 6 |
| Cerebral ischaemia | 5 |
| Cerebral bleeding | 5 |
| Right heart failure | 4 |
| Unknown | 4 |
| Others | 3 |
| Cause of death . | Number of patients . |
|---|---|
| Multiorgan failure | 10 |
| Sepsis | 6 |
| Cerebral ischaemia | 5 |
| Cerebral bleeding | 5 |
| Right heart failure | 4 |
| Unknown | 4 |
| Others | 3 |
The mean (±SD) preoperative cardiac index was 1.9 ± 0.50 l/m2 of body surface area, and the mean (±SD) body surface area was 1.96 ± 0.29 m2. All patients were on inotropic support prior to implantation, and 28 patients were on mechanical ventilation. Secondary organ failure was detected through elevated blood urea nitrogen, serum aspartate aminotransferase, serum creatinine, serum alanine aminotransferase and bilirubin.
An ECMO support was initiated in 18% of patients (n = 21) for stabilization and to screen out patients with cerebral contraindications, sepsis or irreversible multiorgan failure. The support time was 7 ± 5 days, and a maximal flow was aspired. Heparin was administered during ECMO support, with a target activated clotting time of 160–180 s. Comparison of parameters pre-ECMO and on the day of switching from ECMO to LVAD (Table 2) showed a significant normalization of creatinine (P = 0.009) and white blood cell count (P = 0.000), a reduction in alanine aminotransferase (P = 0.003), aspartate aminotransferase (P = 0.003) and platelets (P = 0.004) and an increased sodium (P = 0.017) and bilirubin value (P = 0.001). In contrast, blood urea nitrogen and haematocrit were not significantly changed. After implantation of the LVAD, the ECMO support was continued in six patients instead of a temporary right heart bypass. The ECMO flow was reduced to 2–3 l/min to guarantee a minimal LVAD flow of 3 l/min. The ECMO was implanted percutaneously in the femoral vessels, with a sheath for distal leg perfusion, so that a re-sternotomy could be avoided. The 30-day mortality in this patient group, who were supported pre-LVAD implantation with an ECMO, was 24%. Three patients died due to multiorgan failure, one due to sepsis and one due to right heart failure after ECMO explantation.
Parameters before ECMO implantation and after a mean support time of 7 ± 5 days
| Parameter . | Pre-ECMO implantation . | Pre-LVAD implantation . | P-value . |
|---|---|---|---|
| Serum sodium (mmol/l) | 139 ± 6 | 143 ± 7 | 0.017 |
| Serum creatinine (μmol/l) | 161 ± 83 | 106 ± 73 | 0.009 |
| Blood urea nitrogen (mmol/l) | 15 ± 10 | 11 ± 5 | 0.193 |
| Serum aspartate aminotransferase (U/l) | 1803 ± 2618 | 157 ± 156 | 0.003 |
| Serum alanine aminotransferase (U/l) | 1094 ± 1654 | 203 ± 170 | 0.003 |
| Serum total bilirubin (μmol/l) | 32 ± 21 | 63 ± 39 | 0.001 |
| Haematocrit (%) | 33 ± 4 | 30 ± 4 | 0.816 |
| White blood cell count (×103/μl) | 17 ± 7 | 11 ± 4 | 0.000 |
| Platelets (×103/μl) | 208 ± 119 | 84 ± 47 | 0.004 |
| Parameter . | Pre-ECMO implantation . | Pre-LVAD implantation . | P-value . |
|---|---|---|---|
| Serum sodium (mmol/l) | 139 ± 6 | 143 ± 7 | 0.017 |
| Serum creatinine (μmol/l) | 161 ± 83 | 106 ± 73 | 0.009 |
| Blood urea nitrogen (mmol/l) | 15 ± 10 | 11 ± 5 | 0.193 |
| Serum aspartate aminotransferase (U/l) | 1803 ± 2618 | 157 ± 156 | 0.003 |
| Serum alanine aminotransferase (U/l) | 1094 ± 1654 | 203 ± 170 | 0.003 |
| Serum total bilirubin (μmol/l) | 32 ± 21 | 63 ± 39 | 0.001 |
| Haematocrit (%) | 33 ± 4 | 30 ± 4 | 0.816 |
| White blood cell count (×103/μl) | 17 ± 7 | 11 ± 4 | 0.000 |
| Platelets (×103/μl) | 208 ± 119 | 84 ± 47 | 0.004 |
ECMO: extracorporeal membrane oxygenation; LVAD: left ventricular assist device.
Parameters before ECMO implantation and after a mean support time of 7 ± 5 days
| Parameter . | Pre-ECMO implantation . | Pre-LVAD implantation . | P-value . |
|---|---|---|---|
| Serum sodium (mmol/l) | 139 ± 6 | 143 ± 7 | 0.017 |
| Serum creatinine (μmol/l) | 161 ± 83 | 106 ± 73 | 0.009 |
| Blood urea nitrogen (mmol/l) | 15 ± 10 | 11 ± 5 | 0.193 |
| Serum aspartate aminotransferase (U/l) | 1803 ± 2618 | 157 ± 156 | 0.003 |
| Serum alanine aminotransferase (U/l) | 1094 ± 1654 | 203 ± 170 | 0.003 |
| Serum total bilirubin (μmol/l) | 32 ± 21 | 63 ± 39 | 0.001 |
| Haematocrit (%) | 33 ± 4 | 30 ± 4 | 0.816 |
| White blood cell count (×103/μl) | 17 ± 7 | 11 ± 4 | 0.000 |
| Platelets (×103/μl) | 208 ± 119 | 84 ± 47 | 0.004 |
| Parameter . | Pre-ECMO implantation . | Pre-LVAD implantation . | P-value . |
|---|---|---|---|
| Serum sodium (mmol/l) | 139 ± 6 | 143 ± 7 | 0.017 |
| Serum creatinine (μmol/l) | 161 ± 83 | 106 ± 73 | 0.009 |
| Blood urea nitrogen (mmol/l) | 15 ± 10 | 11 ± 5 | 0.193 |
| Serum aspartate aminotransferase (U/l) | 1803 ± 2618 | 157 ± 156 | 0.003 |
| Serum alanine aminotransferase (U/l) | 1094 ± 1654 | 203 ± 170 | 0.003 |
| Serum total bilirubin (μmol/l) | 32 ± 21 | 63 ± 39 | 0.001 |
| Haematocrit (%) | 33 ± 4 | 30 ± 4 | 0.816 |
| White blood cell count (×103/μl) | 17 ± 7 | 11 ± 4 | 0.000 |
| Platelets (×103/μl) | 208 ± 119 | 84 ± 47 | 0.004 |
ECMO: extracorporeal membrane oxygenation; LVAD: left ventricular assist device.
Levosimendan (Orion Pharma, Espoo, Finland), a calcium sensitizer, was administered for preconditioning in 54% (n = 62) of patients before implantation. The 30-day mortality in patients treated with levosimendan was 5%, in contrast to 33% in patients without levosimendan.
The overall running time for all LVADs implanted was 419 ± 383 days, or 132 patient years, with 117 patient years after discharge home. The adverse events documented during this period are presented in Table 3. The most serious and prevalent adverse event was bleeding that required operative correction (45%, n = 52). Driveline infections were documented in 32 patients, sternal wound in infection in three, and ventricular assist device pocket infection in one patient. Cardiac arrhythmias were reported in 37 patients. Over 50% of the patients with respiratory dysfunction were on mechanical ventilation before LVAD implantation.
| Adverse events . | Number of patients . | Per patient year . |
|---|---|---|
| Bleeding requiring operation | 52 | 0.394 |
| Driveline infections | 32 | 0.242 |
| Cardiac arrhythmias | 37 | 0.280 |
| Renal failure | 10 | 0.076 |
| Respiratory dysfunction | 7 | 0.053 |
| VAD thrombus | 5 | 0.038 |
| Adverse events . | Number of patients . | Per patient year . |
|---|---|---|
| Bleeding requiring operation | 52 | 0.394 |
| Driveline infections | 32 | 0.242 |
| Cardiac arrhythmias | 37 | 0.280 |
| Renal failure | 10 | 0.076 |
| Respiratory dysfunction | 7 | 0.053 |
| VAD thrombus | 5 | 0.038 |
VAD: ventricular assist device.
| Adverse events . | Number of patients . | Per patient year . |
|---|---|---|
| Bleeding requiring operation | 52 | 0.394 |
| Driveline infections | 32 | 0.242 |
| Cardiac arrhythmias | 37 | 0.280 |
| Renal failure | 10 | 0.076 |
| Respiratory dysfunction | 7 | 0.053 |
| VAD thrombus | 5 | 0.038 |
| Adverse events . | Number of patients . | Per patient year . |
|---|---|---|
| Bleeding requiring operation | 52 | 0.394 |
| Driveline infections | 32 | 0.242 |
| Cardiac arrhythmias | 37 | 0.280 |
| Renal failure | 10 | 0.076 |
| Respiratory dysfunction | 7 | 0.053 |
| VAD thrombus | 5 | 0.038 |
VAD: ventricular assist device.
Formation of a thrombus (n = 5) was seen mainly in HeartWare patients and required a pump exchange. The mortality after exchange was 40% (n = 2) due to either right heart failure or sepsis.
The 1-year survival of the 22 patients receiving heart transplantations was 91%. Two patients died as a result of cerebral ischaemia and sepsis.
DISCUSSION
These results reflect our institutional learning curve with rotary blood pump technology for long-term support. It started in 2004, with the pilot trial of the HeartMate II for CE certification. When the HeartMate II became commercially available, pulsatile devices were abandoned from our clinic. The HVAD added to this experience, first again as a pilot trial for CE certification and then after commercialization.
The role of pretreatment became obvious in order to reduce secondary organ failure after implantation of the ventricular assist device. Pretreatment with levosimendan or even ECMO became part of the programme, even though a protective effect of levosimendan has not been shown in a randomized study [8]. Patients who were treated with levosimendan in our study cohort had surgery in the last period, which means that the better outcome could be influenced by our greater experience.
However, ECMO support may significantly lower the risk of secondary organ failure at the cost of lowering platelets and increasing bilirubin, ultimately leading to an increase in the number of transfusions required after LVAD implantation. Furthermore, a platelet count lower than 148 × 10³/μl has been identified as a risk factor for increased mortality [9]. Despite this, identification of irreversible secondary organ failure and cerebral damage under ECMO support may prevent subsequent economic burdens associated with LVAD implantation.
Our patient cohort showed no mechanical pump failure for any device. This highlights the main advantage of the new technology. However, analysis of the patient population describes the adverse events of long-term ventricular assist device therapy; for example, stroke, renal and right heart failure can be detected during concurrent LVAD treatment.
Renal and right heart failure rates were the same compared with data from other studies [10]. Neurological dysfunction was reported only if it caused death. The same rates were reported by INTERMACS for continuous flow pumps [11]. Furthermore, four patients died as a result of battery disconnection, which emphasizes the need for safer and more ‘user-friendly’ external equipment. The 30-day mortality in our centre is comparable to that of other centres. [12, 13].
Improvements in anticoagulation protocols may aid in decreasing the high rate of thrombus formation and higher bleeding event rate as have been shown for other centres [14]. For this patient cohort, we reported a 100% incidence of acquired von Willebrand syndrome, suggesting a disorder of the anticoagulation system in LVAD patients [15]. As a consequence, we stopped all antiplatelet therapy in the HeartMate II patients. In addition, Slaughter et al. described an anticoagulation approach without heparin, which resulted in a lower rate of thrombus formation and fewer bleeding events requiring transfusions [16]. The higher rate of thrombus formation in HVAD patients led to reintroduction of antiplatelet therapy for these patients, as well as to changes in the inflow cannula of the device.
Despite continued success with improved LVADs, patient quality of life after heart transplantation remains better. The low transplant rate in our cohort documents the trend that bridge to transplant and destination therapy indications can no longer be distinguished. Owing to the mechanical stability of the devices, the avoidance of long-term complications led to initiation of a long-term care facility for in- and outpatients.
In summary, we assess changes in the management of end-stage heart failure patients due to preoperative treatment with levosimendan and ECMO. A refinement of the anticoagulation protocol was necessary, and the new ventricular assist device technology changed our approach in patients with chronic heart failure [6, 7]. Limitations of this study include the single-centre retrospective setting, with the disadvantages of this type of research. So far, the treatment of LVAD patients has not been uniform. The International Society of Heart and Lung Transplantation is working on standardized guidelines.
Conflict of interest: Martin Strueber is expert consultant to Heartware Inc.
REFERENCES
- ischemia
- heart transplantation
- extracorporeal membrane oxygenation
- heart failure
- ischemic cardiomyopathy
- ventricular assist device
- ambulatory care services
- follow-up
- objective (goal)
- patient discharge
- heart
- transplantation
- medical devices
- axial flow heart assist system
- surgical mortality
- centrifugal pump
- inotropic support
- donors




