-
PDF
- Split View
-
Views
-
Cite
Cite
Rajamiyer V. Venkateswaran, Vamsidhar Dronavalli, Val Patchell, Ian Wilson, Jorge Mascaro, Richard Thompson, John Coote, Robert S. Bonser, Measurement of extravascular lung water following human brain death: implications for lung donor assessment and transplantation, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 6, June 2013, Pages 1227–1232, https://doi.org/10.1093/ejcts/ezs657
Close - Share Icon Share
Abstract
The measurement of extravascular lung water could aid the assessment and guide the management of potential lung donors following brain death. We therefore sought to validate a single indicator thermodilution extravascular lung water index (EVLWI-T) measurement using gravimetry and to assess the impact and clinical correlates of elevated EVLWI-T in potential lung donors and transplant recipients.
In a prospective study, we measured serial EVLWI-T and haemodynamic and oxygenation data in 60 potential lung donors. To validate the EVLWI-T measurement, we measured in vivo thermodilution EVLWI (EVLWI-T) and gravimetric ex vivo EVLWI (EVLWI-G) in donor lungs rejected for transplant using the Holcroft and Trunkey modification of Pearce's method. We assessed the clinical correlates of elevated lung water and measured interleukin-8 and hepatocyte growth factor in bronchoalveolar lavage (BAL) fluid.
The mean EVLWI-T (n = 60) was 9.7 (4.5) ml kg−1, being >7–10 ml kg−1 in 23/60 and >10 ml kg−1 in 16/60 potential donors. Donor lungs with EVLWI >10 ml kg−1 were more likely to be receiving norepinephrine (P = 0.04), have higher pulmonary capillary wedge pressures (P = 0.008), be unsuitable for transplantation (P = 0.007) and, if transplanted, have worse survival (P = 0.04). Lungs submitted to gravimetric analysis [n = 20 in 11 donors (9 double and 2 single)] had EVWLI-T of 10.8 (2.7) and EVLWI-G was 10.1 (2.5). There was a strong correlation between EVLW-T and EVLW-G (r = 0.7; P = 0.014), but EVLWI-T over-predicted the EVLWI-G by ∼1 ml kg−1 (EVLW-T = 1.05 × EVLW-G). Cytokine levels in BAL fluid were elevated.
Elevated lung water is found in >50% of potential lung donors, predicts lung suitability for transplant and may adversely affect recipient outcome. Although EVLWI-T intrinsically overestimates gravimetric lung water, its measurement may aid the assessment of organ suitability. Lung water accumulation and the proinflammatory response may both be targets for modifying therapy.
INTRODUCTION
In the circumstances of brain death, extravascular lung water (EVLW) could increase due to direct lung injury associated with the cardiovascular events of coning [1] and secondary effects related to proinflammatory response or cardiac dysfunction [2]. Each of these could be exacerbated by over-zealous fluid replacement. Current, in vivo assessment of donor lungs is inexact and reliant on donor history, chest-X-ray evaluation, arterial and pulmonary venous blood gas measurement, bronchoscopy and direct inspection [3, 4]. As in other clinical scenarios, significant lung water increases might precede clinical, radiological and oxygenation manifestations of lung oedema and could potentiate the very significant increases in lung water observed immediately after transplantation [5]. The measurement of EVLW could thus be an additional facile and real-time method of assessment of potential lung donors following brain death [6].
EVLW can be measured in different ways including a single indicator trans-pulmonary thermodilution technique based on the Stewart-Hamilton principle [6, 7]. Using this thermodilution technique, the EVLW, indexed to predicted body weight (PBW; extravascular lung water index (EVLWI-T)), has a normal range of 3–7 ml kg−1 [8], and values >10 ml kg−1 are believed to indicate significant lung water excess [8–10] and predict adverse outcome in critically ill patients.
Early EVLW studies, using a thermal dye double indicator dilution technique suggested potential clinical utility in brain dead human heart–lung donors [11], but the single indicator technique has not been investigated or validated in this setting.
In a previous report, we found that potential donor lungs deemed unsuitable for transplantation had higher EVLWI-T. The aims of the current study were therefore to validate the single indicator thermodilution measurement against gravimetric methodology in patients following brain death, and to assess the clinical and haemodynamic correlates of EVLWI-T measurements >10 ml kg−1.
METHODS
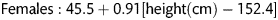
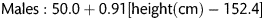
The underlying principles of this lung water assessment have been previously reported [6]. The pulmonary vascular permeability index (PVPI) is the EVLW to pulmonary blood volume ratio (normal range 1–3 kg−1). Elevated EVLW with a low PVPI implies a greater pulmonary blood volume and hydrostatic oedema, whereas a high EVLW with a high PVPI implies permeability oedema. A Swan-Ganz catheter was also inserted, allowing the measurement of pulmonary capillary wedge pressure (PCWP) and pulmonary vascular resistance.
A bronchoscopy was performed to assess anatomy, confirm endotracheal tube placement, aspirate secretions, and to obtain bronchoalveolar lavage (BAL) specimens. Blood and BAL specimens were kept on ice until return to the transplant centre where they were immediately processed. Blood samples were centrifuged and serum then frozen at −80°C for later analysis. One aliquot of BAL fluid was despatched for microbiological culture and the centrifuged supernatant of a second specimen frozen at −80°C for later cytokine analysis. ABG, EVLWI and PVPI measurements were obtained at baseline, repeated after bronchoscopy, 1 h thereafter and immediately preretrieval.
After initial measurements and BAL, donors received either T3, methylprednisolone, both T3 and MP or placebo (dextrose 5%) in a 1:1:1:1 randomization as previously described [12]. Details of blinding, randomization, donor management, a CONSORT flow diagram and hormonal group allocation outcomes for this trial have been previously reported [12]. Lung suitability for transplantation was predefined as an arterial PaO2/FiO2 ≥300 mmHg immediately prior to retrieval without lung trauma, aspiration, infection or non-recruitable atelectasis being identified during assessment at direct inspection. Interleukin-8 (IL-8) and hepatocyte growth factor (HGF) as possible markers of lung injury were measured in a single thaw cycle of the BAL supernatant sample using commercially available ELISA kits (Quantikine® by R&D Systems, Inc., MN, USA). As the kits were not previously validated for use on BAL samples, for each of the markers, spike/ recovery and linearity experiments were performed based on the manufacturer's protocol (R&D Systems, Inc.), ensuring measurement accuracy up to maximal allowed dilution.
Donor lungs for transplantation were flush perfused with a single pulmonary artery flush of blood-based pneumoplegia (Ringer's lactate 700 ml, human albumin solution (20%) 200 ml, mannitol solution (20%) 100 ml, sodium heparin 10 000 units, venesected donor blood 300 ml, citrate/phosphate/dextrose buffer 56 ml and a single dose of epoprostenol infusion (0.5 mg in 20 ml (Flolan; GlaxoSmithKline UK, Ltd)) and retrieved as a whole block after explanting the donor heart. The trachea was stapled after expansion of both lungs, and the block or individual lung transported in 4°C Ringer's lactate solution to the transplant centre.
Gravimetric measurement of lung water
Specific, additional next-of-kin consent was requested to retain lungs not accepted for transplantation for gravimetric lung water assessment. Gravimetric measurement of EVLW was determined using the Holcroft and Trunkey modification of the Pearce method [13]. Peripheral venous (5 ml) and pulmonary venous effluent blood (10 ml) were collected prior to retrieval of lungs for haemoglobin estimation. Lungs were then retrieved in a standard manner, but without pneumoplegia or epoprostenol infusion and transported, inflated at 4°C in Ringer's lactate solution. Within 8 h, lung blocks were separated, and non-parenchymal tissue excised and weighed. Large representative samples from upper and lower lobes were weighed and then homogenized with a known amount of distilled water and centrifuged at 12 000 rpm for 30 min at 4°C. The wet weights of supernatant, lung sediments and the pulmonary venous blood were obtained. Haemoglobin levels from the pulmonary venous effluent blood and supernatant samples were measured. Supernatant, blood and the lung sediments were then freeze dried for 72 h and weighed again to obtain their dry weight. EVLW was then calculated, linearly extrapolated to the total lung weight and indexed to predicted donor body weight.
Statistical analysis
Data were analyzed using SPSS v15.0 (Chicago, IL, USA) and STATA (Release 10; Stata Corp., College Station, TX). Continuous data were assessed for normality and are presented as mean (standard deviation) or median (25th, 75th quartiles). Normally distributed variables were compared using the paired or independent t-test as appropriate. Skewed data were tested using non-parametric tests (Mann–Whitney and Kruskal–Wallis test). Categorical data were analyzed using Chi-square and Fisher's exact tests.
Comparison between gravimetry and thermodilution was performed using Spearman's correlation and Bland–Altman analysis. We analysed donor EVLWI-T and corresponding lung EVLWI-G (averaged if both lungs were available for gravimetric analysis). The receiver operating curve characteristic for the ability of EVLWI-T to predict lung suitability for transplantation at retrieval assessment is reported as area under the curve (AUC; standard error). Statistical significance was assigned when P < 0.05, and all tests were two-sided.
RESULTS
Sixty donors, median age 47 (36, 56) years, underwent the in vivo studies (Table 1). The mean initial PaO2/FiO2 ratio was 396 (79) mmHg. Donors commenced investigations 2 (0.5, 3.5) h after consent, within 12.5 (8.1) h of coning. During a management period of 6.9 (1.2) h, donors received limited amounts of fluid [376 (353) ml of colloid and 27 (94) ml of crystalloid].
Characteristics of 60 potential lung donors subjected to EVLWI-T estimation
| Characteristics . | Result . |
|---|---|
| Age (years) | 47 (36, 56) |
| Sex (female:male) | 31:29 |
| Baseline PaO2/FiO2 ratio | 396 (79) (range 377–418) |
| Donor cause of death | |
| Vascular and tumour | 40/60 (67%) |
| Traumatic | 14/60 (23%) |
| Hypoxic | 3/60 (5%) |
| CNS infection | 3/60 (5%) |
| Duration of ventilation (hours) | 24 (24, 48) |
| Hospital stay (days) | 2 (1, 2) |
| Characteristics . | Result . |
|---|---|
| Age (years) | 47 (36, 56) |
| Sex (female:male) | 31:29 |
| Baseline PaO2/FiO2 ratio | 396 (79) (range 377–418) |
| Donor cause of death | |
| Vascular and tumour | 40/60 (67%) |
| Traumatic | 14/60 (23%) |
| Hypoxic | 3/60 (5%) |
| CNS infection | 3/60 (5%) |
| Duration of ventilation (hours) | 24 (24, 48) |
| Hospital stay (days) | 2 (1, 2) |
Data are presented in mean (standard deviation), median (25th, 75th percentiles) or number/denominator (%). CNS: central nervous system.
Characteristics of 60 potential lung donors subjected to EVLWI-T estimation
| Characteristics . | Result . |
|---|---|
| Age (years) | 47 (36, 56) |
| Sex (female:male) | 31:29 |
| Baseline PaO2/FiO2 ratio | 396 (79) (range 377–418) |
| Donor cause of death | |
| Vascular and tumour | 40/60 (67%) |
| Traumatic | 14/60 (23%) |
| Hypoxic | 3/60 (5%) |
| CNS infection | 3/60 (5%) |
| Duration of ventilation (hours) | 24 (24, 48) |
| Hospital stay (days) | 2 (1, 2) |
| Characteristics . | Result . |
|---|---|
| Age (years) | 47 (36, 56) |
| Sex (female:male) | 31:29 |
| Baseline PaO2/FiO2 ratio | 396 (79) (range 377–418) |
| Donor cause of death | |
| Vascular and tumour | 40/60 (67%) |
| Traumatic | 14/60 (23%) |
| Hypoxic | 3/60 (5%) |
| CNS infection | 3/60 (5%) |
| Duration of ventilation (hours) | 24 (24, 48) |
| Hospital stay (days) | 2 (1, 2) |
Data are presented in mean (standard deviation), median (25th, 75th percentiles) or number/denominator (%). CNS: central nervous system.
EVLWI-T and gravimetric lung water validation
Consent was obtained in 11 donors (20 lungs; 9 lung pairs and 2 single lungs) for gravimetric analysis. When comparing techniques, the EVLWI-T and PaO2/FiO2 ratio measured immediately before lung explantation were considered. The gravimetric lung study donors had a similar EVLWI-T [10.8 (2.7)] when compared with others [10.6 (5.4) ml kg−1], but as expected, a lower PaO2/FiO2 ratio (265 (135) vs 373 (111) mmHg) than non-gravimetrically studied lungs. There was strong, direct correlation between EVLWI-T and EVLWI-G (Fig. 1; r = 0.7; Spearman's correlation P = 0.014; regression analysis EVLW-T = 1.05 × EVLW-G) with modest over-prediction [Bland–Altmann analysis of 0.5 (2.1) ml kg−1] and increased positive bias with higher EVLWI measurements (Fig. 2). The upper and lower limits of agreement (±2 standard deviations) were 4.6 and −3.7 ml kg−1, respectively.
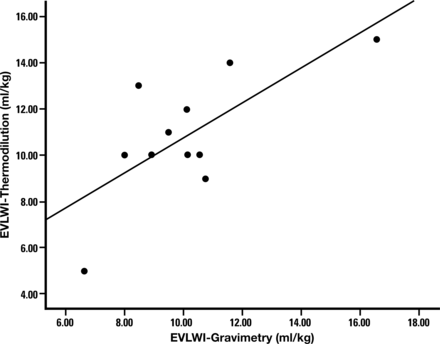
Correlation between EVLWI measurement by thermodilution and gravimetry (r = 0.7; Spearman's correlation P = 0.002).
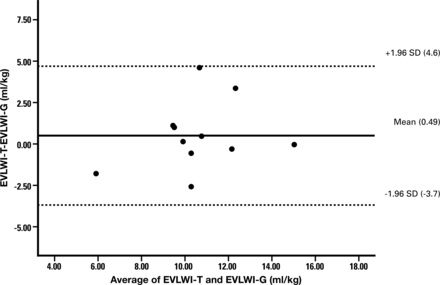
Bland–Altman plot demonstrating limits of agreement between EVLWI measurement by thermodilution and gravimetry. EVLWI-T over-predicted the EVLWI-G and there was a positive bias which increased with higher EVLWI-T. However the limits of agreement values lie between the two standard deviation.
Single indicator thermodilution EVLWI-T measurement
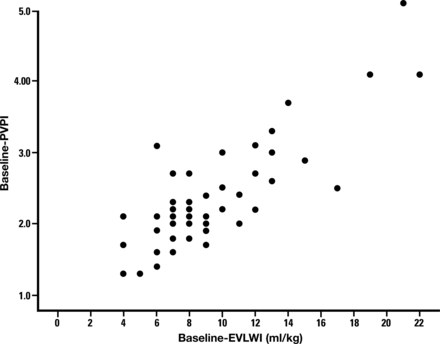
The initial EVLWI-T was 9.7 (4.5) ml kg−1; 85% (51/60) donors had EVLWI-T >7 ml kg−1, with 27% (16/60) having values >10 ml kg−1. PVPI values rose in parallel with increasing EVLWI-T (Fig. 3). Clinical and laboratory data according to EVLWI-T dichotomized as ≤ or >10 ml kg−1 are shown in Table 2. High EVLW-T donors had higher PVPI, higher PCWP and were more likely to be receiving a higher dose of norepinephrine and less likely to be suitable for transplantation at end inspection. Positive BAL culture rates were not different between lung water groups. Additionally, BAL levels of IL-8 [1472 (223, >2000) vs 2000 (663, >2000) pg ml−1] and HGF [325 (125, 1160) vs 402 (222–1707) pg ml−1; P = 0.47] were observed for the ≤10 and >10 ml kg−1 groups, respectively. IL-8 levels were highly elevated and were beyond the measurable range despite maximal allowed dilution precluding statistical comparison.
Characteristics of donors and transplant outcome according to initially measured lung water content
| Parameters . | EVLWI ≤10 ml kg−1 (n = 16) . | EVLWI > 10 ml kg−1 (n = 44) . | P-value . |
|---|---|---|---|
| Age (years) | 45.3 (29) | 41.2 (19) | 0.29 |
| Gender (female) | 21 | 10 | 0.39 |
| Positive smoking history | 14 (32%) | 6 (38%) | 0.04 |
| Cause of death | |||
| Head injury | 10 | 4 | 0.41 |
| Central nervous system infection | 1 | 2 | |
| Hypoxic brain injury | 2 | 1 | |
| Vascular/tumour | 31 | 9 | |
| Time from coning | 12.7 (8.2) | 13.1 (6.9) | 0.87 |
| Initial EVLWI-T | 7.6 (1.6) | 15.6 (4.7) | <0.001 |
| Δ-EVLWI-T | −1.3 (1.7) | −0.25 (5.1) | 0.22 |
| PaO2:FiO2 ratio | 387 (75) | 428 (83) | 0.08 |
| Δ-PaO2:FiO2 ratio | 4.3 (15.9) | 7.2 (20.8) | 0.58 |
| PVPI | 2.1 (0.4) | 3.4 (1.3) | <0.001 |
| Δ-PVPI | −0.19 (0.5) | −0.48 (1.5) | 0.28 |
| Initial pulmonary vascular resistance | 111 (66) | 161 (67) | 0.11 |
| Δ-PVR | −20.3 (100) | 21.2 (66) | 0.16 |
| PCWP | 7.4 (3.6) | 11.3 (6.9) | 0.008 |
| Δ-PCWP | −2.3 (5.2) | −3.9 (3.9) | 0.31 |
| Norepinephrine dose (µg kg−1 min−2) | 0.13 (0.03) | 0.25 (0.06) | <0.001 |
| Norepinephrine > 0.06 µg kg−1 min−2 | 14/44 | 6/16 | 0.04 |
| Positive bronchoalveolar lavage culture | 27/39 | 11/13 | 0.47 |
| Time from initial EVLWI to retrieval assessment (h) | 6.5 (1.2) | 7.1 (1.2) | 0.09 |
| Lungs matching suitability | 27/44 | 3/16 | 0.007 |
| Parameters . | EVLWI ≤10 ml kg−1 (n = 16) . | EVLWI > 10 ml kg−1 (n = 44) . | P-value . |
|---|---|---|---|
| Age (years) | 45.3 (29) | 41.2 (19) | 0.29 |
| Gender (female) | 21 | 10 | 0.39 |
| Positive smoking history | 14 (32%) | 6 (38%) | 0.04 |
| Cause of death | |||
| Head injury | 10 | 4 | 0.41 |
| Central nervous system infection | 1 | 2 | |
| Hypoxic brain injury | 2 | 1 | |
| Vascular/tumour | 31 | 9 | |
| Time from coning | 12.7 (8.2) | 13.1 (6.9) | 0.87 |
| Initial EVLWI-T | 7.6 (1.6) | 15.6 (4.7) | <0.001 |
| Δ-EVLWI-T | −1.3 (1.7) | −0.25 (5.1) | 0.22 |
| PaO2:FiO2 ratio | 387 (75) | 428 (83) | 0.08 |
| Δ-PaO2:FiO2 ratio | 4.3 (15.9) | 7.2 (20.8) | 0.58 |
| PVPI | 2.1 (0.4) | 3.4 (1.3) | <0.001 |
| Δ-PVPI | −0.19 (0.5) | −0.48 (1.5) | 0.28 |
| Initial pulmonary vascular resistance | 111 (66) | 161 (67) | 0.11 |
| Δ-PVR | −20.3 (100) | 21.2 (66) | 0.16 |
| PCWP | 7.4 (3.6) | 11.3 (6.9) | 0.008 |
| Δ-PCWP | −2.3 (5.2) | −3.9 (3.9) | 0.31 |
| Norepinephrine dose (µg kg−1 min−2) | 0.13 (0.03) | 0.25 (0.06) | <0.001 |
| Norepinephrine > 0.06 µg kg−1 min−2 | 14/44 | 6/16 | 0.04 |
| Positive bronchoalveolar lavage culture | 27/39 | 11/13 | 0.47 |
| Time from initial EVLWI to retrieval assessment (h) | 6.5 (1.2) | 7.1 (1.2) | 0.09 |
| Lungs matching suitability | 27/44 | 3/16 | 0.007 |
The assessment time represents the period between initial and final measurements. The prefix Δ indicates the change in measurement between final and initial sampling. Lungs matching suitability indicates those attaining the predefined transplant suitability criteria (see text).
PVPI: pulmonary vascular permeability index: PCWP: initial pulmonary capillary wedge pressure.
Characteristics of donors and transplant outcome according to initially measured lung water content
| Parameters . | EVLWI ≤10 ml kg−1 (n = 16) . | EVLWI > 10 ml kg−1 (n = 44) . | P-value . |
|---|---|---|---|
| Age (years) | 45.3 (29) | 41.2 (19) | 0.29 |
| Gender (female) | 21 | 10 | 0.39 |
| Positive smoking history | 14 (32%) | 6 (38%) | 0.04 |
| Cause of death | |||
| Head injury | 10 | 4 | 0.41 |
| Central nervous system infection | 1 | 2 | |
| Hypoxic brain injury | 2 | 1 | |
| Vascular/tumour | 31 | 9 | |
| Time from coning | 12.7 (8.2) | 13.1 (6.9) | 0.87 |
| Initial EVLWI-T | 7.6 (1.6) | 15.6 (4.7) | <0.001 |
| Δ-EVLWI-T | −1.3 (1.7) | −0.25 (5.1) | 0.22 |
| PaO2:FiO2 ratio | 387 (75) | 428 (83) | 0.08 |
| Δ-PaO2:FiO2 ratio | 4.3 (15.9) | 7.2 (20.8) | 0.58 |
| PVPI | 2.1 (0.4) | 3.4 (1.3) | <0.001 |
| Δ-PVPI | −0.19 (0.5) | −0.48 (1.5) | 0.28 |
| Initial pulmonary vascular resistance | 111 (66) | 161 (67) | 0.11 |
| Δ-PVR | −20.3 (100) | 21.2 (66) | 0.16 |
| PCWP | 7.4 (3.6) | 11.3 (6.9) | 0.008 |
| Δ-PCWP | −2.3 (5.2) | −3.9 (3.9) | 0.31 |
| Norepinephrine dose (µg kg−1 min−2) | 0.13 (0.03) | 0.25 (0.06) | <0.001 |
| Norepinephrine > 0.06 µg kg−1 min−2 | 14/44 | 6/16 | 0.04 |
| Positive bronchoalveolar lavage culture | 27/39 | 11/13 | 0.47 |
| Time from initial EVLWI to retrieval assessment (h) | 6.5 (1.2) | 7.1 (1.2) | 0.09 |
| Lungs matching suitability | 27/44 | 3/16 | 0.007 |
| Parameters . | EVLWI ≤10 ml kg−1 (n = 16) . | EVLWI > 10 ml kg−1 (n = 44) . | P-value . |
|---|---|---|---|
| Age (years) | 45.3 (29) | 41.2 (19) | 0.29 |
| Gender (female) | 21 | 10 | 0.39 |
| Positive smoking history | 14 (32%) | 6 (38%) | 0.04 |
| Cause of death | |||
| Head injury | 10 | 4 | 0.41 |
| Central nervous system infection | 1 | 2 | |
| Hypoxic brain injury | 2 | 1 | |
| Vascular/tumour | 31 | 9 | |
| Time from coning | 12.7 (8.2) | 13.1 (6.9) | 0.87 |
| Initial EVLWI-T | 7.6 (1.6) | 15.6 (4.7) | <0.001 |
| Δ-EVLWI-T | −1.3 (1.7) | −0.25 (5.1) | 0.22 |
| PaO2:FiO2 ratio | 387 (75) | 428 (83) | 0.08 |
| Δ-PaO2:FiO2 ratio | 4.3 (15.9) | 7.2 (20.8) | 0.58 |
| PVPI | 2.1 (0.4) | 3.4 (1.3) | <0.001 |
| Δ-PVPI | −0.19 (0.5) | −0.48 (1.5) | 0.28 |
| Initial pulmonary vascular resistance | 111 (66) | 161 (67) | 0.11 |
| Δ-PVR | −20.3 (100) | 21.2 (66) | 0.16 |
| PCWP | 7.4 (3.6) | 11.3 (6.9) | 0.008 |
| Δ-PCWP | −2.3 (5.2) | −3.9 (3.9) | 0.31 |
| Norepinephrine dose (µg kg−1 min−2) | 0.13 (0.03) | 0.25 (0.06) | <0.001 |
| Norepinephrine > 0.06 µg kg−1 min−2 | 14/44 | 6/16 | 0.04 |
| Positive bronchoalveolar lavage culture | 27/39 | 11/13 | 0.47 |
| Time from initial EVLWI to retrieval assessment (h) | 6.5 (1.2) | 7.1 (1.2) | 0.09 |
| Lungs matching suitability | 27/44 | 3/16 | 0.007 |
The assessment time represents the period between initial and final measurements. The prefix Δ indicates the change in measurement between final and initial sampling. Lungs matching suitability indicates those attaining the predefined transplant suitability criteria (see text).
PVPI: pulmonary vascular permeability index: PCWP: initial pulmonary capillary wedge pressure.
Overall, both EVLWI-T and PVPI increased significantly between initial and preretrieval measurements from 9.7 (4.5) to 10.8 (5.2); P = 0.009 and 2.4 (0.9) to 2.7 (1.2); P = 0.025, respectively, while the PaO2/FiO2 ratio fell [397 (78) to 359 (126); P = 0.028] and PCWP increased [8.4 (4.9) to 11.1 (4.2); P < 0.001]. On receiver operating characteristic curve analysis, initial EVLWI [AUC = 0.67 (0.07); 95% CI 0.53, 0.81; P = 0.025] (Fig. 4) was predictive of suitability at final inspection with a positive and negative predictive value of 72 and 63%, respectively.
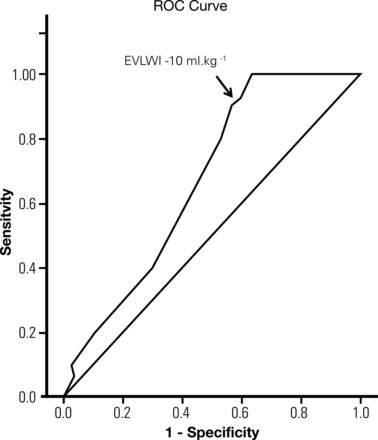
Receiver operating curve characteristic assessing lung suitability and extravascular lung water index. The area under the curve is 0.67 (standard error 0.07); P = 0.025.
A total of 27 lung transplants were performed from the study cohort; 30-day survival (60%) was lower in recipients of lungs with EVLWI-T >10 ml kg−1 (n = 5); P = 0.03 vs those with lower EVLWI-T values (100%).
DISCUSSION
Following transplantation, there is a disturbing incidence (15–35%) of post-transplantation lung injury with impaired gas exchange and pulmonary infiltrates and worse outcome [14, 15]. Increased lung water occurs uniformly [5]. Our study suggests that the process of oedema generation begins in the donor. We have demonstrated that EVLWI-T is elevated (>7 ml kg−1) in the majority of potential lung donors following brain death and is significantly elevated in ∼25%. EVLWI-T measurement, undertaken ∼6 h prior to final inspection, predicts the ultimate suitability of lungs for transplantation and recipient outcome.
Experimentally, EVLWI measured using thermodilution methods (EVLWI-T) have been shown to correlate well with lung water measured by gravimetry (EVLW-G) [6, 16, 17]. A number of experimental studies have allowed validation of the single indicator thermodilution method used in this study vs gravimetric measurement [13, 18, 19]. In each study, EVLWI-T has been shown to over-predict EVLWI-G by 2–5 ml kg−1. In our study, the first to attempt to validate the measurement in potential donor lungs, we noted a similar over-prediction of gravimetric values, a positive bias that increases with higher EVLWI values, but which may not limit the clinical value of this measurement.
Lung water may increase in donor lungs by various mechanisms of injury such as trauma, aspiration, infection, fluid overload and ventilatory barotrauma. These may occur before or after brain death, and donor smoking may be an additional risk factor. Also, during the circulatory changes associated with brain death, haemodynamic shear forces and pro-oedematous hydrostatic pressures may cause direct injury. Each of these modes of injury may be further exacerbated by a proinflammatory post-BSD environment, and our finding of elevated PVPI in higher lung water donors suggests that this may be occurring although the validity of PVPI as an index of permeability oedema has not been validated. All the mechanisms described could lead to pulmonary oedema and worsening lung function, and our data are consistent with findings in lungs rejected for transplantation [20]. The higher PCWP in the >10 ml kg−1 group is consistent with greater overall fluid overload or associated left ventricular dysfunction.
The huge circulating levels of catecholamines during the ‘storm’ of brainstem death may provoke pulmonary endothelial injury and combined hydrostatic and permeability oedema. Thereafter, vasoparetic hypotension is commonly treated by NE infusion, a potent but cardiotoxic α-adrenergic agonist. Donor pressor support with NE has been associated with a worse prognosis in heart and lung transplantation and inferior post-transplant gas exchange. In our study, more donors in the EVLWI-T >10 ml kg−1 group were receiving NE at higher dosage. Higher NE exposure could certainly influence lung water via hydrostatic mechanisms that increase fluid filtration, including post-capillary vasconstriction [21], and cardiac dysfunction with left atrial hypertension.
The epithelial Na+/K+ channels in alveolar epithelium have an important role in the clearance of excessive fluid from the alveolar space. Alveolar fluid clearance (AFC) mechanisms are known to be maintained following brainstem death and retrieval and may respond to pharmacological manipulation. Both thyroid hormone and steroids may up-regulate AFC and could be of benefit in this clinical scenario [22, 23]. However, we previously reported that in this cohort, T3 had no effect on EVLWI-T while MP [12] attenuated the increased Δ-EVLWI-T observed in non-treated donors. Early steroid administration is reported to improve oxygenation and yield and, in this regard, augmentation of AFC rather than an anti-inflammatory effect may be the dominant mechanism.
Importantly, our finding of elevated EVLWI-T in >50% donors provides an opportunity for modulation using diuretics to counteract fluid overload and nebulized β-adrenergic agonists to augment AFC. As post-transplantation increases in EVLWI occur uniformly following transplantation [5], commencement of AFC augmentation in the donor holds promise as a protective strategy [24]. Post-transplantation AFC manipulation has been shown to be associated with rapid resolution of hypoxia and improvement in radiological changes [14].
Our study suggests that the measurement of EVLWI-T may be a facile tool that may improve donor care. It may guide donor fluid management and prompt measures to improve AFC. The study was performed prior to the widespread introduction of ex vivo lung resuscitation [25], but we speculate that the identification of adverse lung water measurements, rather than being a prompt for lung rejection, may allow direct transfer to lung reconditioning.
Limitations
Our study has a number of limitations. Although our sample size is larger than many laboratory experimental studies, the heterogeneity of the population may have affected our findings. Gravimetric validation was performed on lungs rejected for transplantation that had worse gas exchange than those transplanted. Caution must therefore be advised when extrapolating these data clinically. As patients were transplanted in a number of centres, we used recipient survival as a surrogate measure of graft function and cannot report whether differences in outcome were solely graft-related.
In conclusion, we have validated the simple measurement of EVLWI by a single indicator thermodilution technique in post-brainstem death potential lung donors. Measurement many hours prior to retrieval may aid the prediction of organ suitability and recipient outcome. Elevated EVLWI-T represents a therapeutic target for modifying therapy during optimization.
Conflict of interest: none declared.
REFERENCES
Author notes
Prof. Robert Bonser unfortunately passed away after this paper was accepted for publication.




