-
PDF
- Split View
-
Views
-
Cite
Cite
Waël C. Hanna, Moira de Valence, Eshetu G. Atenafu, Marcelo Cypel, Thomas K. Waddell, Kazuhiro Yasufuku, Andrew Pierre, Marc De Perrot, Shaf Keshavjee, Gail E. Darling, Is video-assisted lobectomy for non-small-cell lung cancer oncologically equivalent to open lobectomy?, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 6, June 2013, Pages 1121–1125, https://doi.org/10.1093/ejcts/ezs623
Close - Share Icon Share
Abstract
The purpose of this study was to compare overall and disease-free survival after VATS and open lobectomy for clinical Stage I and II non-small-cell lung cancer (NSCLC).
A retrospective review of a prospective database of all patients undergoing VATS or open lobectomy for clinical Stage I or II NSCLC between 2002 and 2010 was performed. Postoperative outcomes, disease-free survival and overall survival were compared between the two groups after optimum 1:1 propensity matching for age, gender, tumour histology and pathological stage.
Over an 8-year period, 608 patients underwent lobectomy for NCSLC by VATS (n = 196, 32%) or open technique (n = 412, 68%). After matching, there were 190 patients in each group. Adenocarcinoma was found in 80% (open: 149, VATS: 152) and 55% of tumours were T1 (open: 108, VATS: 105). Pathological N1 disease was found in 21 and 19 patients in the open and VATS group, respectively. Disease-free 5-year survival was 69.1% for the open group vs 69.7% for VATS (P = 0.94). Cancer-specific 5-year survival was 82.9% for the open group vs 76.7% for VATS (P = 0.170). Five-year overall survival was 73% in the open group vs 64% in the VATS group (P = 0.17). Operative mortality and postoperative complications were not significantly different between groups.
Overall survival and disease-free survival are not significantly different when compared between VATS lobectomy and open lobectomy. VATS resection appears to provide an adequate oncological operation for patients with operable clinical Stage I and II NSCLC.
INTRODUCTION
Pulmonary resection by video-assisted thoracoscopic surgery (VATS) is becoming increasingly common in the treatment of non-small-cell lung cancer (NSCLC). While the efficacy and safety of VATS lobectomy have been established [1], the long-term outcomes and survival benefits are still uncertain [2]. The purpose of this study was to compare overall and disease-free survival (DFS) after VATS and open lobectomy for NSCLC.
METHODS
After ethics board approval, a prospective database of patients who underwent lobectomy for NSCLC at our institution between 2002 and 2010 was queried. The decision to perform lobectomy via VATS or the open approach was at the discretion of the operating surgeon. Patients who underwent complex reconstruction such as sleeve arterioplasty or bronchoplasty were excluded from the study, as well as patients with Stage III or higher NSCLC, those with multiple primaries, and those with tumours other than NSCLC. Patients with clinical Stage I or II disease, who were found to have N2 disease on final postoperative pathology, were still included in the analysis. Data were collected on patient demographics, comorbidities, smoking history, clinical and pathological stage of disease, operative details and postoperative complications (Fig. 1). Descriptive statistics were used to assess all variables of interest. Categorical variables were expressed as count and proportions and continuous variables as mean ± standard deviation. Propensity score matching was done by open vs VATS Lobectomy based on five variables: age, gender, tumour histology, pathological T-stage and pathological N-stage. The 6th edition of the UICC TNM Staging System was used to determine clinical and pathological stages [3]. Comorbidities were quantified and compared between cohorts using the Charlson comorbidity index CCI. Overall survival was calculated in months from the date of surgery to the date of death or the last follow-up. Disease-free survival (DFS) and cancer-specific survival (CSS) were calculated in months from the date of surgery to the date of recurrence or cancer-related death, respectively, or till the last follow-up if no recurrence or death occurred. Cancer-related death was defined as death from any reason in a patient with documented recurrence of lung cancer. The Kaplan–Meier product-limit method was used to calculate survival. All P-values were two-sided and results were considered significant if P < 0.05 (SAS v9.2, SAS Institute, Inc., Cary, NC, USA).

In the study design, propensity score matching was done by open vs VATS lobectomy to create comparable patient cohorts based on age, gender, tumour histology, pathological T-stage and pathological N-stage.
RESULTS
Demographic criteria
Between 2002 and 2012, 608 patients who met the study inclusion criteria were identified in the thoracic surgery database at our institution. After propensity matching, two cohorts of 190 patients were selected for statistical comparison. The VATS and open lobectomy cohorts were statistically similar in gender, age, tumour histology, pathological T-stage and pathological N-stage (Fig. 2). The majority of patients had pathological Stage I disease. Adjuvant chemotherapy was administered to 42/190 patients in the VATS cohort and 34/190 patients in the open cohort. Measurement of the CCI revealed that the life expectancy of patients in the VATS cohort (mean CCI = 10, IQR 6–14) was not statistically different from that of patients in the open cohort (mean CCI = 9, IQR 6–13, P = 0.461). In the VATS cohort, 71% of patients were smokers, compared with 73% in the open group (P = 0.322).
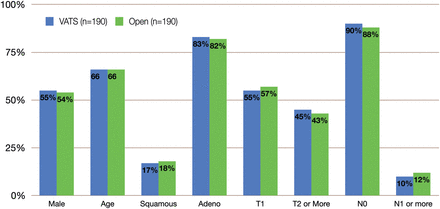
Cohort characteristics after matching. Blue columns indicate VATS lobectomy and green columns indicate open lobectomy.
Preoperative staging and lymph node sampling
The preoperative staging regimen for both groups comprised computed tomography of the chest and abdomen, whole body positron emission tomography and magnetic resonance imaging of the brain. In the VATS group, 4.2% of patients (8/190) were suspected to have clinical N1 disease vs 5.3% (10/190) in the open group (P = 0.236). Preoperative lymph node sampling was done by mediastinoscopy, endobronchial ultrasound-guided fine needle aspiration or both. Patients also underwent intraoperative systematic lymph node sampling at the time of lobectomy. Rates of lymph node sampling were compared (combined preoperative and intraoperative) between the two cohorts to ensure proper pathological staging. Table 1 shows the percentage of patients who had documented lymph node sampling at each of the relevant stations, according to the location of the primary tumour and type of resection (open vs VATS). There were no statistical differences in the rates of lymph nodes sampled between both cohorts at any of the lymph node stations. After lymph node sampling, 5.8% (11/190) of patients from the VATS cohort were upstaged from clinical stage N0 to pathological stage N1, and 1 patient was upstaged from clinical stage N1 to pathological stage N2. In the open cohort, 7.4% (14/190) of patients were upstaged from clinical stage N0 to pathological stage N1, and 1 patient was upstaged from clinical stage N1 to pathological stage N2. After the final pathological staging, 10% of VATS patients and 12% of open patients had lymph node positive disease (P = 0.412). There were no complications that were attributed to the lymph node sampling.
The rates of lymph node sampling at each nodal station relative to the site of resection in the VATS and open group
| Nodal station . | Left upper . | Left lower . | Right upper and middle . | Right lower . | ||||
|---|---|---|---|---|---|---|---|---|
| VATS . | Open . | VATS . | Open . | VATS . | Open . | VATS . | Open . | |
| 2R | 79% | 77% | 55% | 52% | ||||
| 2L | ||||||||
| 4R | 95% | 91% | 73% | 79% | ||||
| 4L | 52% | 64% | 73% | 74% | ||||
| 5 | 64% | 53% | 50% | 30% | ||||
| 6 | 17% | 6% | 8% | 11% | ||||
| 7 | 73% | 70% | 97% | 93% | 94% | 99% | 90% | 90% |
| 8 | 17% | 0% | 7% | 7% | ||||
| 9 | 79% | 48% | 21% | 38% | ||||
| 10R | 62% | 46% | 31% | 38% | ||||
| 10L | 47% | 58% | 54% | 56% | ||||
| 11R | 72% | 54% | 69% | 76% | ||||
| 11L | 64% | 70% | 92% | 81% | ||||
| 12R | 93% | 79% | 83% | 93% | ||||
| 12L | 87% | 83% | 54% | 89% | ||||
| Nodal station . | Left upper . | Left lower . | Right upper and middle . | Right lower . | ||||
|---|---|---|---|---|---|---|---|---|
| VATS . | Open . | VATS . | Open . | VATS . | Open . | VATS . | Open . | |
| 2R | 79% | 77% | 55% | 52% | ||||
| 2L | ||||||||
| 4R | 95% | 91% | 73% | 79% | ||||
| 4L | 52% | 64% | 73% | 74% | ||||
| 5 | 64% | 53% | 50% | 30% | ||||
| 6 | 17% | 6% | 8% | 11% | ||||
| 7 | 73% | 70% | 97% | 93% | 94% | 99% | 90% | 90% |
| 8 | 17% | 0% | 7% | 7% | ||||
| 9 | 79% | 48% | 21% | 38% | ||||
| 10R | 62% | 46% | 31% | 38% | ||||
| 10L | 47% | 58% | 54% | 56% | ||||
| 11R | 72% | 54% | 69% | 76% | ||||
| 11L | 64% | 70% | 92% | 81% | ||||
| 12R | 93% | 79% | 83% | 93% | ||||
| 12L | 87% | 83% | 54% | 89% | ||||
The rates of lymph node sampling at each nodal station relative to the site of resection in the VATS and open group
| Nodal station . | Left upper . | Left lower . | Right upper and middle . | Right lower . | ||||
|---|---|---|---|---|---|---|---|---|
| VATS . | Open . | VATS . | Open . | VATS . | Open . | VATS . | Open . | |
| 2R | 79% | 77% | 55% | 52% | ||||
| 2L | ||||||||
| 4R | 95% | 91% | 73% | 79% | ||||
| 4L | 52% | 64% | 73% | 74% | ||||
| 5 | 64% | 53% | 50% | 30% | ||||
| 6 | 17% | 6% | 8% | 11% | ||||
| 7 | 73% | 70% | 97% | 93% | 94% | 99% | 90% | 90% |
| 8 | 17% | 0% | 7% | 7% | ||||
| 9 | 79% | 48% | 21% | 38% | ||||
| 10R | 62% | 46% | 31% | 38% | ||||
| 10L | 47% | 58% | 54% | 56% | ||||
| 11R | 72% | 54% | 69% | 76% | ||||
| 11L | 64% | 70% | 92% | 81% | ||||
| 12R | 93% | 79% | 83% | 93% | ||||
| 12L | 87% | 83% | 54% | 89% | ||||
| Nodal station . | Left upper . | Left lower . | Right upper and middle . | Right lower . | ||||
|---|---|---|---|---|---|---|---|---|
| VATS . | Open . | VATS . | Open . | VATS . | Open . | VATS . | Open . | |
| 2R | 79% | 77% | 55% | 52% | ||||
| 2L | ||||||||
| 4R | 95% | 91% | 73% | 79% | ||||
| 4L | 52% | 64% | 73% | 74% | ||||
| 5 | 64% | 53% | 50% | 30% | ||||
| 6 | 17% | 6% | 8% | 11% | ||||
| 7 | 73% | 70% | 97% | 93% | 94% | 99% | 90% | 90% |
| 8 | 17% | 0% | 7% | 7% | ||||
| 9 | 79% | 48% | 21% | 38% | ||||
| 10R | 62% | 46% | 31% | 38% | ||||
| 10L | 47% | 58% | 54% | 56% | ||||
| 11R | 72% | 54% | 69% | 76% | ||||
| 11L | 64% | 70% | 92% | 81% | ||||
| 12R | 93% | 79% | 83% | 93% | ||||
| 12L | 87% | 83% | 54% | 89% | ||||
Postoperative outcomes
The overall complication rate, including surgical site infection, pulmonary infection, arrhythmias, cardiovascular events, neurological events, bleeding, reoperation and prolonged air leakage, was similar in both cohorts (VATS = 26% vs open = 28%, P = 0.068). There were no 30-day mortalities in either group. The median length of stay in-hospital was 4 (2–14) days for the VATS group and 6 (3–20) days for the open group (P = 0.067). Although statistical significance was not reached (P = 0.067), these results suggest a slightly lower complication rate and a shorter postoperative course for VATS lobectomy. The median follow-up was 24.6 months for the VATS group and 40.6 months for the open group.
Disease-free survival
The 5-year DFS was 69.7% in the VATS group and 69.1% in the open group, with no significant differences found between the survival curves (P = 0.938). There were no notable differences in the pattern of recurrence (distant vs loco-regional) between the two groups. DFS rates remained similar between both cohorts throughout the period of follow-up (Fig. 3).
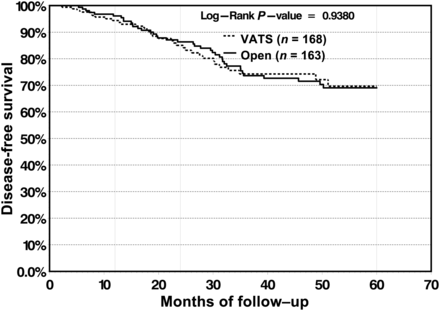
DFS is not significantly different between the VATS and open group.
Cancer-specific survival
The CSS was 98 and 91.3% at 1 and 2 years, respectively, for the VATS cohort and 99.4 and 94.8%, respectively, for the open cohort. At 5-years, there was slight divergence in survival rate in favour of open lobectomy (82.9%) vs VATS lobectomy (76.7%); however, the survival curves did not reach statistical significance (P = 0.139) (Fig. 4). For patients with N1 and N2 disease, 5-year survival was 48.9% for VATS lobectomy vs 48.5% for open lobectomy, and the differences in the survival curves were not statistically significant (P = 0.5148) (Fig. 5).
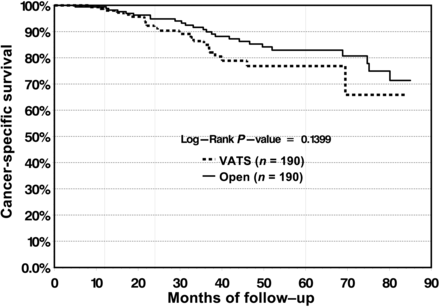
CSS is not significantly different between the VATS and open group.
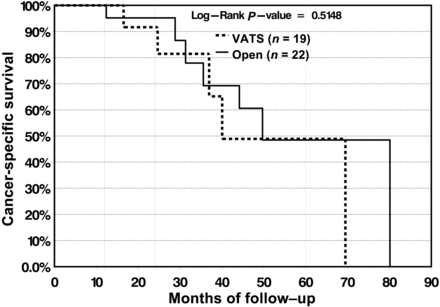
In patients with lymph node positive disease, CSS does not differ significantly between the VATS and open group.
Overall survival
In the VATS cohort, 5-year overall survival was measured at 66.4%, compared with 73.1% in the open cohort, with no significant difference found between the survival curves (P = 0.2099) (Fig. 6). At our institution, VATS lobectomy was first performed in 2002 and became well established in 2006. To test the effect of the learning curve of VATS lobectomy on survival, overall survival was measured for the subgroup of patients who were operated on between 2002 and 2006. In this subgroup, 5-year overall survival for the VATS group was 67.3 vs 69.3% for the open group, and no significant differences were found between the survival curves (Fig. 7).
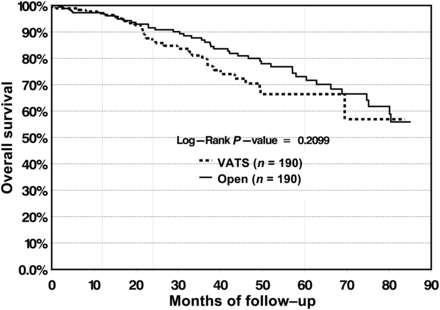
Overall survival does not differ significantly between the VATS and open group.
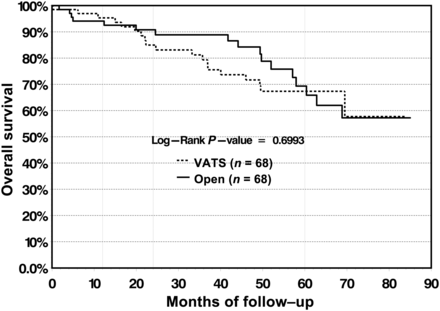
Overall survival for patients operated on in the early days of VATS (2002–06) does not differ significantly between the VATS and open group.
DISCUSSION
As more surgeons are choosing to perform VATS lobectomy for the treatment of early stage NSCLC, it is important to ensure that VATS lobectomy offers an oncologically equivalent operation to open lobectomy. While many authors have demonstrated the feasibility of an adequate lymph node dissection by VATS [4, 5], and operative morbidity and mortality are similar [6–8], long-term survival is the key outcome measure used to evaluate the effectiveness of any cancer therapy.
To date, only one prospective randomized trial has directly compared oncological outcomes between VATS and open lobectomy [9]. With 52 patients in the VATS cohort and 48 patients in the open cohort, Sugi et al. demonstrated that 3- and 5-year survival were equivalent in both groups. However, this study was restricted to Stage IA patients and has not been reproduced despite being published more than a decade ago. More recently, two large systematic reviews, with >40 retrospective studies combined [2, 10], have shown either similar or better survival with VATS lobectomy. Although encouraging, these results have been criticized for failing to address exaggeration of bias, as only six studies—all from Japan and all restricted to Stage IA patients—have undertaken a direct comparison of survival between VATS and open lobectomy [9, 11–15].
The most significant criticism of previous studies is selection bias, which has prevented any significant conclusion in favour of VATS lobectomy [8, 16]. Because of logistical issues and gradually waning equipoise, a prospective randomized controlled trial comparing survival of VATS and open lobectomy will likely never take place [10], and a well-designed retrospective study becomes the next best available means. In our study, selection bias is minimized by propensity matching based on oncological criteria. The cohorts that were compared were essentially identical in terms of age, cancer histology and pathological staging. To counter the effect of comorbidity on overall survival, we ensured that both cohorts have equivalent rates of preoperative probability of mortality as reflected by the absence of statistical difference between mean Charlson comorbidity scores. To minimize bias created by differences in preoperative staging, we demonstrate comparable preoperative and intraoperative lymph node sampling between both cohorts. Although we have chosen to match our patients according to pathological stage—which is a more accurate representation of the true stage of disease—we do not suspect that stage migration played any significant role in influencing our results. In essence, this study compares two cohorts of patients in whom the only measurable difference is whether they underwent VATS or open lobectomy, and demonstrates that survival is not different between both groups.
A few limitations to this study are worth noting. First, although selection bias has been minimized by propensity matching, lead time bias remains an issue in any study that measures survival. Secondly, the small number of patients included in the study may render it beyond the sensitivity of the survival analysis to detect significant changes in overall survival. Thirdly, the small proportion of patients with lymph node positive disease prevents any meaningful conclusion on the survival of patients with Stage II disease. Despite those limitations, this study is larger than any previously published work that directly compares survival between VATS and open lobectomy. Furthermore, it is the only study that includes Stage IB and Stage II patients.
Based on these results, VATS lobectomy has become the standard operation for Stage I and Stage II NSCLC at our institution. Rare exceptions would include patients with bulky lymphadenopathy or large (T2b) tumours, and patients requiring complex arterial or bronchial reconstruction.
CONCLUSIONS
VATS lobectomy appears to be an oncologically equivalent operation to open lobectomy. In patients with early stage NSCLC, overall survival and DFS are not significantly different when compared between VATS lobectomy and open lobectomy. VATS resection can be offered to patients with operable NSCLC without concerns about oncological validity.
Funding
Gail E. Darling receives support from the Kress Family Chair in Esophageal Cancer.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 20th European Conference on General Thoracic Surgery, Essen, Germany, 10–13 June 2012.




