-
PDF
- Split View
-
Views
-
Cite
Cite
Shin-ichi Kosugi, Tatsuo Kanda, Kazuhito Yajima, Takashi Ishikawa, Kaoru Sakamoto, Risk factors influencing the pleural drainage volume after transthoracic oesophagectomy, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 6, June 2013, Pages 1116–1120, https://doi.org/10.1093/ejcts/ezs556
Close - Share Icon Share
Abstract
The objective of this study was to clarify the factors influencing pleural drainage volume after transthoracic oesophagectomy and to determine criteria for the selection of patients who would benefit from the early removal of chest drains.
Clinicopathological characteristics of 155 patients who underwent transthoracic oesophagectomy were prospectively collected, and the daily drainage volume of each patient was retrospectively reviewed. Potential risk factors were compared between the high-output group (n = 39) and low-output group (n = 116), which were dichotomized using the 75th percentile of total pleural drainage volume of the total study population. Multivariate logistic regression analyses were used to identify independent risk factors.
The median duration of drainage was 10 days, with a median total drainage volume of 2258 ml. Of 27 potential risk factors influencing the drainage volume, creatinine clearance (P = 0.04), operative approach (P = 0.03) and thoracic duct removal (P = 0.01) were significantly associated with the total pleural drainage volume. The removal of the thoracic duct (P = 0.02; odds ratio, 4.02; 95% confidence interval 1.20–13.41) and lower creatinine clearance (P = 0.04; odds ratio, 1.02; 95% confidence interval 1.00–1.04) was independent risk factors for increased pleural drainage volume after transthoracic oesophagectomy.
The early removal of chest drains may be possible in patients without these risk factors.
INTRODUCTION
Chest tube drainage after thoracic surgery is usually necessary to evacuate excessive air and fluid from the operated chest and to allow for full lung re-expansion [1]. However, these chest tubes are uncomfortable, reduce respiratory effort by causing significant pain and eventually hamper coughing and ambulation, which are important in preventing pulmonary complications and enhancing recovery after surgery [1, 2]. Therefore, the chest tubes must be removed as soon as it is safe and feasible [2, 3]. It is useful to know the risk factors influencing the pleural drainage volume for managing patients who have undergone thoracic surgery.
Lagarde et al. [2] reported that a transthoracic approach was the only independent predictive factor associated with prolonged chest drainage production after oesophagectomy. In their study, patients who underwent transhiatal oesophagectomy lost significantly less pleural fluid, both in terms of daily and total drainage volumes. Consistent with their results, we have omitted the use of a conventional chest tube after transhiatal oesophagectomy in daily practice. To our knowledge; however, no studies analysing the predictive factors associated with pleural drainage volume after transthoracic oesophagectomy have been performed.
The timing for tube removal is based on the surgeon's limited experience, with wide variation among thoracic surgeons [4]. As early removal of the chest tube after pulmonary resection has evolved, the threshold of the daily drainage volume prompting removal has increased from previously used volumes of 100 ml and 150–200 ml [4], 300 ml [5], 400 ml [3] and 450 ml [6], irrespective of the operative approach. After transthoracic oesophagectomy, it has widely been accepted that the chest tubes should be left in place until the drainage is <200 ml/day [7]. However, no clear evidence concerning the proper drainage volume with regard to the optimal timing for removal of the chest tube has been reported in the literature to date.
The objective of this study was to clarify potential risk factors influencing the pleural drainage volume after transthoracic oesophagectomy and to determine criteria for the selection of patients who would benefit from early removal of the chest drain.
PATIENTS AND METHODS
Study design and patients
We conducted a single centre case–control study comparing two groups of patients with high-output or low-output pleural drainage after transthoracic oesophagectomy. Two hundred and ninety-three consecutive patients underwent oesophagectomy for carcinoma of the oesophagus at Niigata University Medical and Dental Hospital between January 1999 and December 2011. Of these, oesophagectomy with extended mediastinal lymph node (LN) dissection via the transthoracic approach was performed in 185 (63%) patients. Thirty (16%) patients were excluded from this series because of specific complications that could delay removal of the chest tube, as follows: chyle leakage in 11, pyothorax in 5, pneumothorax in 5, postoperative intrathoracic haemorrhage in 2 and other causes in 1. In addition, 6 patients were excluded whose data concerning postoperative drainage volume were lost. Therefore, 155 patients were eventually included in this study.
Preoperative therapy and surgery
Fifty-six patients (36%) received preoperative therapy. Chemotherapy alone was administered in 45 patients; chemoradiotherapy was given in 11 patients, with a median radiation dose of 66 Gy (range 40–70 Gy). The chemotherapy regimen consisted mainly of 5-fluorouracil plus a platinum compound in each treatment.
Tumour stages and residual tumour status were grouped according to the International Union against Cancer tumour-node-metastasis classification [8]. The transthoracic approach was defined as mobilization of the thoracic oesophagus under a right fourth intercostal thoracotomy. Starting in January 2007, thoracoscopic mobilization was performed in 20 patients with clinical T1 tumours, all of whom are included in this study. The dissected nodes were defined using the nomenclature of the Japanese Classification of Esophageal Cancer [9]. The following LN stations were removed en bloc during thoracic oesophageal mobilization: upper, middle and lower thoracic paraoesophageal LNs; bilateral thoracic paratracheal LNs including recurrent nerve LNs and tracheobronchial LNs; subcarinal LNs; bilateral main bronchus LNs; supradiaphragmatic LNs and posterior mediastinal LNs. In addition to the mediastinal LN dissection, 75 patients underwent systematic bilateral cervical and abdominal LN dissection (three-field LN dissection) and 80 underwent abdominal LN dissection (two-field LN dissection). Removal or preservation of the thoracic duct mainly depended on the depth of tumour invasion and intraoperative findings; the most common pattern was resection from the thoracic inlet to just above the diaphragm together with a mobilized thoracic oesophagus and dissected mediastinal LNs. The gastric tube (n = 144), colon (n = 10) or jejunum (n = 1) was used as an oesophageal substitute via the posterior mediastinal (n = 81), retrosternal (n = 72) or subcutaneous (n = 2) route with cervical (n = 129) or intrathoracic (n = 26) anastomosis.
Chest tube management
A 28-Fr chest tube (Sumitomo Bakelite, Japan) was routinely placed apically and posteriorly in the right thoracic cavity before chest closure throughout this study period. It was connected to a Chest tube Vac™ (Sumitomo Bakelite), with negative 12-cm water suction applied. In addition, two 10-mm flat Blake™ silicone drains (Ethicon, Japan) were inserted via the supradiaphragmatic route into the right and left thoracic cavities separately after the abdominal procedure and connected to one J-VAC™ suction reservoir (Ethicon). The placement of a third drain or thoracentesis was performed if the chest X-ray showed pleural effusion despite drainage. The total drainage volume from all drains was calculated in this study, based on records taken at least every 8 h by the nursing staff. The drainage volume from all drains was measured exactly using a graduated cylinder. Although we had no clinical criteria regarding the chest drain removal during this study period, the drain was generally removed at the operating surgeon's discretion when the daily drainage volume dropped to <100 ml.
Data analysis and statistics
Clinicopathological characteristics of the study patients were prospectively collected, and the daily drainage volume of each patient was retrospectively reviewed from the medical records. Potential risk factors that would influence the drainage volume were selected as follows: age, gender, body mass index, co-morbidities, tumour location, operative approach, extent of LN dissection, number of dissected mediastinal LNs, residual tumour status, tumour stage, removal or preservation of the thoracic duct, reconstruction route, operative time, blood loss and the presence or absence of preoperative therapy. Co-morbidities included cardiovascular disease requiring antihypertensive drugs, antiplatelet agents and anticoagulants; chronic obstructive pulmonary disease with forced expiratory volume at 1 s <70% of predicted normal; chronic liver disease and established diagnosis of diabetes mellitus requiring medical intervention. Preoperative serum total protein, albumin and creatinine levels were regarded as risk factors and were included in the analysis. Preoperative creatinine clearance was calculated according to the Cockcroft–Gault formula. Patient characteristics are shown in Table 1.
| Age (years, range) | 63 (43–82) |
| Male | 139 (90%) |
| Body mass index (kg/m2)a | 21.4 ± 3.0 |
| Tumour location | |
| Upper thoracic | 25 (16%) |
| Mid-thoracic | 110 (71%) |
| Lower thoracic | 20 (13%) |
| Operative approach | |
| Transthoracic | 135 (87%) |
| Thoracoscopic | 20 (13%) |
| Lymph node dissection | |
| Three-field | 80 (52%) |
| Two-field | 75 (48%) |
| Number of dissected mediastinal LNs (range) | 17 (0–61) |
| Residual tumour status (UICC) | |
| R0 | 143 (92%) |
| R1 | 7 (5%) |
| R2 | 5 (3%) |
| Pathological tumour stage (UICC) | |
| 0–I | 47 (30%) |
| II | 35 (23%) |
| III | 63 (41%) |
| IV | 9 (6%) |
| Thoracic duct | |
| Removed | 115 (74%) |
| Preserved | 40 (26%) |
| Reconstruction route | |
| Subcutaneous | 2 (1%) |
| Retrosternal | 71 (46%) |
| Posterior mediastinal | 82 (53%) |
| Operative time (min)a | 461 ± 110 |
| Blood loss (ml)a | 696 ± 480 |
| Preoperative therapy | |
| None | 99 (64%) |
| Chemotherapy | 45 (29%) |
| Chemoradiotherapy | 11 (7%) |
| Duration of drainage (days, range) | 10 (4–26) |
| Total drainage volume (ml, IQR) | 2258 (1643) |
| Age (years, range) | 63 (43–82) |
| Male | 139 (90%) |
| Body mass index (kg/m2)a | 21.4 ± 3.0 |
| Tumour location | |
| Upper thoracic | 25 (16%) |
| Mid-thoracic | 110 (71%) |
| Lower thoracic | 20 (13%) |
| Operative approach | |
| Transthoracic | 135 (87%) |
| Thoracoscopic | 20 (13%) |
| Lymph node dissection | |
| Three-field | 80 (52%) |
| Two-field | 75 (48%) |
| Number of dissected mediastinal LNs (range) | 17 (0–61) |
| Residual tumour status (UICC) | |
| R0 | 143 (92%) |
| R1 | 7 (5%) |
| R2 | 5 (3%) |
| Pathological tumour stage (UICC) | |
| 0–I | 47 (30%) |
| II | 35 (23%) |
| III | 63 (41%) |
| IV | 9 (6%) |
| Thoracic duct | |
| Removed | 115 (74%) |
| Preserved | 40 (26%) |
| Reconstruction route | |
| Subcutaneous | 2 (1%) |
| Retrosternal | 71 (46%) |
| Posterior mediastinal | 82 (53%) |
| Operative time (min)a | 461 ± 110 |
| Blood loss (ml)a | 696 ± 480 |
| Preoperative therapy | |
| None | 99 (64%) |
| Chemotherapy | 45 (29%) |
| Chemoradiotherapy | 11 (7%) |
| Duration of drainage (days, range) | 10 (4–26) |
| Total drainage volume (ml, IQR) | 2258 (1643) |
aValues depicted are mean ± standard deviations.
UICC: International Union against Cancer; IQR: interquartile range.
| Age (years, range) | 63 (43–82) |
| Male | 139 (90%) |
| Body mass index (kg/m2)a | 21.4 ± 3.0 |
| Tumour location | |
| Upper thoracic | 25 (16%) |
| Mid-thoracic | 110 (71%) |
| Lower thoracic | 20 (13%) |
| Operative approach | |
| Transthoracic | 135 (87%) |
| Thoracoscopic | 20 (13%) |
| Lymph node dissection | |
| Three-field | 80 (52%) |
| Two-field | 75 (48%) |
| Number of dissected mediastinal LNs (range) | 17 (0–61) |
| Residual tumour status (UICC) | |
| R0 | 143 (92%) |
| R1 | 7 (5%) |
| R2 | 5 (3%) |
| Pathological tumour stage (UICC) | |
| 0–I | 47 (30%) |
| II | 35 (23%) |
| III | 63 (41%) |
| IV | 9 (6%) |
| Thoracic duct | |
| Removed | 115 (74%) |
| Preserved | 40 (26%) |
| Reconstruction route | |
| Subcutaneous | 2 (1%) |
| Retrosternal | 71 (46%) |
| Posterior mediastinal | 82 (53%) |
| Operative time (min)a | 461 ± 110 |
| Blood loss (ml)a | 696 ± 480 |
| Preoperative therapy | |
| None | 99 (64%) |
| Chemotherapy | 45 (29%) |
| Chemoradiotherapy | 11 (7%) |
| Duration of drainage (days, range) | 10 (4–26) |
| Total drainage volume (ml, IQR) | 2258 (1643) |
| Age (years, range) | 63 (43–82) |
| Male | 139 (90%) |
| Body mass index (kg/m2)a | 21.4 ± 3.0 |
| Tumour location | |
| Upper thoracic | 25 (16%) |
| Mid-thoracic | 110 (71%) |
| Lower thoracic | 20 (13%) |
| Operative approach | |
| Transthoracic | 135 (87%) |
| Thoracoscopic | 20 (13%) |
| Lymph node dissection | |
| Three-field | 80 (52%) |
| Two-field | 75 (48%) |
| Number of dissected mediastinal LNs (range) | 17 (0–61) |
| Residual tumour status (UICC) | |
| R0 | 143 (92%) |
| R1 | 7 (5%) |
| R2 | 5 (3%) |
| Pathological tumour stage (UICC) | |
| 0–I | 47 (30%) |
| II | 35 (23%) |
| III | 63 (41%) |
| IV | 9 (6%) |
| Thoracic duct | |
| Removed | 115 (74%) |
| Preserved | 40 (26%) |
| Reconstruction route | |
| Subcutaneous | 2 (1%) |
| Retrosternal | 71 (46%) |
| Posterior mediastinal | 82 (53%) |
| Operative time (min)a | 461 ± 110 |
| Blood loss (ml)a | 696 ± 480 |
| Preoperative therapy | |
| None | 99 (64%) |
| Chemotherapy | 45 (29%) |
| Chemoradiotherapy | 11 (7%) |
| Duration of drainage (days, range) | 10 (4–26) |
| Total drainage volume (ml, IQR) | 2258 (1643) |
aValues depicted are mean ± standard deviations.
UICC: International Union against Cancer; IQR: interquartile range.
Continuous variables were usually summarized as median (range) for the non-normally distributed data and mean ± standard deviation for normally distributed data. The pleural drainage volume was summarized as median (interquartile range [IQR]) instead because the data included extreme values and the IQR was considered less likely to be distorted by such outliers. IQR was calculated as the distance between the first quartile (25th percentile) and third quartile (75th percentile). We defined two groups of patients with high-output or low-output pleural drainage. The cut-off for the dichotomized variable was the 75th percentile of total pleural drainage volume of the total study population, which reflected the upper quartile of the study population. Potential risk factors were compared between the two groups, the differences of which were assessed using Student's t-test or Mann–Whitney U-test for continuous variables and the χ2 test or Fisher's exact test for categorical variables as univariate analyses. The Spearman rank correlation coefficient was used to assess the correlation between the radiation dose given and pleural drainage volume in 11 patients who received preoperative chemoradiotherapy. Two-tailed P-values <0.05 were considered statistically significant. Multivariate logistic regression analyses were used to identify independent risk factors influencing the pleural drainage volume after transthoracic oesophagectomy. Factors with a P-value <0.1 in univariate analyses were entered into the multivariate model. The Hosmer–Lemeshow test was used to examine the model's goodness of fit. All but one analysis was performed with StatView for Windows version 5.0 (SAS Institute, Inc., Cary, NC, USA); the Hosmer–Lemeshow test was performed with Statistical Package for Social Sciences (SPSS) version 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Drainage duration and drainage volume
The median duration of pleural drainage was 10 (range 4–26) days; those of the chest tube and vacuum system were 8 (range 1–21) and 9 (range 3–26) days, respectively. There was a significant difference in drainage duration between the chest tube and the vacuum system (P < 0.01). The median total drainage volume was 2258 (IQR 1643) ml; those of the chest tube and vacuum system were 945 (IQR 916) and 1313 (IQR 1121) ml, respectively. There was a significant difference in drainage volume between the chest tube and vacuum system (P < 0.01). The daily and cumulative drainage volumes up to postoperative day (POD) 7 are shown in Fig. 1.
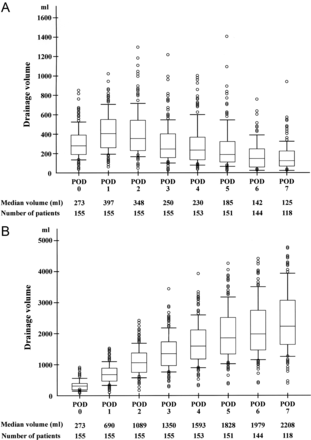
Daily drainage volume (A) and cumulative drainage volume (B) up to POD 7 are shown in graph form. The box plot shows IQRs. The upper and lower bars indicate 90th percentile and 10th percentile, respectively; the circles are outliers. Median drainage volume and numbers of patients for each POD are also appended.
Using the 75th percentile of the total drainage volume as a cut-off value, we dichotomized the study population: 39 patients with a total drainage volume of 3324 ml or more were classified into the high-output group and the remaining 116 patients were classified into the low-output group. The median durations of pleural drainage in the high-output group and low-output group were 13 (range 7–26) and 9 (range 4–16) days, respectively. There was a significant difference in drainage duration between the two groups (P < 0.01).
Risk factors influencing the drainage volume
Of 27 potential risk factors influencing the drainage volume, the creatinine clearance (P = 0.04), operative approach (P = 0.03) and thoracic duct removal (P = 0.01) were significantly associated with the total pleural drainage volume (Tables 2 and 3). In addition, chronic obstructive pulmonary disease and the reconstruction route tended to be associated with the total pleural drainage volume (P = 0.07 and 0.10, respectively). The median total drainage volume in 11 patients who received preoperative chemoradiotherapy was 2462 (IQR 1716) ml, and there was no significant correlation between the radiation dose given and the pleural drainage volume (P = 0.80). Because the thoracoscopic approach is closely associated with preservation of the thoracic duct and reconstruction via the posterior mediastinal route, an operative approach was excluded from the multivariate model. Of the remaining four variables, removal of the thoracic duct (P = 0.02; odds ratio 4.02; 95% confidence interval 1.20–13.41) and lower creatinine clearance (P = 0.04; odds ratio, 1.02; 95% confidence interval 1.00–1.04) were independent risk factors for increased pleural drainage volume after transthoracic oesophagectomy (Table 4). The Hosmer–Lemeshow χ2 was 9.36 with a P-value of 0.313, indicating reasonable model fit.
| Factors . | Low-output group (n = 116) . | High-output group (n = 39) . | P-value . |
|---|---|---|---|
| Agea | 63.7 ± 8.3 | 65.3 ± 7.1 | 0.28 |
| Male | 103 (89%) | 36 (92%) | 0.76 |
| Body mass index (kg/m2)a | 21.6 ± 3.1 | 20.9 ± 2.5 | 0.26 |
| Co-morbidities | |||
| Hypertension | 34 (29%) | 16 (41%) | 0.55 |
| Ischaemic heart disease | 6 (5%) | 2 (5%) | 1.00 |
| Atrial fibrillation | 3 (3%) | 3 (8%) | 0.17 |
| Anticoagulants | 5 (4%) | 1 (3%) | 1.00 |
| Antiplatelet agent | 5 (4%) | 2 (5%) | 1.00 |
| Peripheral vascular disease | 5 (4%) | 1 (3%) | 1.00 |
| COPD | 29 (25%) | 16 (41%) | 0.07 |
| Chronic liver disease | 7 (6%) | 1 (3%) | 0.68 |
| Diabetes mellitus | 9 (8%) | 4 (10%) | 0.74 |
| Total protein (g/dl)a | 6.9 ± 0.5 | 6.8 ± 0.5 | 0.21 |
| Albumin (g/dl)a | 4.0 ± 0.4 | 4.0 ± 0.4 | 0.73 |
| Creatinine (mg/dl)a | 0.7 ± 0.2 | 0.8 ± 0.1 | 0.22 |
| Creatinine clearance (ml/min)a | 84.0 ± 20.9 | 76.4 ± 17.1 | 0.04 |
| Tumour location | |||
| Upper thoracic | 16 (14%) | 4 (10%) | 0.82 |
| Mid-thoracic | 82 (71%) | 28 (72%) | |
| Lower thoracic | 18 (15%) | 7 (18%) | |
| Pathological tumour stage (UICC) | |||
| 0–I | 37 (32%) | 10 (26%) | 0.65 |
| II | 25 (21%) | 10 (26%) | |
| III | 46 (40%) | 17 (45%) | |
| IV | 8 (7%) | 1 (3%) | |
| Preoperative therapy | |||
| None | 73 (63%) | 26 (67%) | 0.49 |
| Chemotherapy | 36 (31%) | 9 (23%) | |
| Chemoradiotherapy | 7 (6%) | 4 (10%) | |
| Factors . | Low-output group (n = 116) . | High-output group (n = 39) . | P-value . |
|---|---|---|---|
| Agea | 63.7 ± 8.3 | 65.3 ± 7.1 | 0.28 |
| Male | 103 (89%) | 36 (92%) | 0.76 |
| Body mass index (kg/m2)a | 21.6 ± 3.1 | 20.9 ± 2.5 | 0.26 |
| Co-morbidities | |||
| Hypertension | 34 (29%) | 16 (41%) | 0.55 |
| Ischaemic heart disease | 6 (5%) | 2 (5%) | 1.00 |
| Atrial fibrillation | 3 (3%) | 3 (8%) | 0.17 |
| Anticoagulants | 5 (4%) | 1 (3%) | 1.00 |
| Antiplatelet agent | 5 (4%) | 2 (5%) | 1.00 |
| Peripheral vascular disease | 5 (4%) | 1 (3%) | 1.00 |
| COPD | 29 (25%) | 16 (41%) | 0.07 |
| Chronic liver disease | 7 (6%) | 1 (3%) | 0.68 |
| Diabetes mellitus | 9 (8%) | 4 (10%) | 0.74 |
| Total protein (g/dl)a | 6.9 ± 0.5 | 6.8 ± 0.5 | 0.21 |
| Albumin (g/dl)a | 4.0 ± 0.4 | 4.0 ± 0.4 | 0.73 |
| Creatinine (mg/dl)a | 0.7 ± 0.2 | 0.8 ± 0.1 | 0.22 |
| Creatinine clearance (ml/min)a | 84.0 ± 20.9 | 76.4 ± 17.1 | 0.04 |
| Tumour location | |||
| Upper thoracic | 16 (14%) | 4 (10%) | 0.82 |
| Mid-thoracic | 82 (71%) | 28 (72%) | |
| Lower thoracic | 18 (15%) | 7 (18%) | |
| Pathological tumour stage (UICC) | |||
| 0–I | 37 (32%) | 10 (26%) | 0.65 |
| II | 25 (21%) | 10 (26%) | |
| III | 46 (40%) | 17 (45%) | |
| IV | 8 (7%) | 1 (3%) | |
| Preoperative therapy | |||
| None | 73 (63%) | 26 (67%) | 0.49 |
| Chemotherapy | 36 (31%) | 9 (23%) | |
| Chemoradiotherapy | 7 (6%) | 4 (10%) | |
aValues depicted are mean ± standard deviations.
COPD: chronic obstructive pulmonary disease; UICC: International Union against Cancer.
| Factors . | Low-output group (n = 116) . | High-output group (n = 39) . | P-value . |
|---|---|---|---|
| Agea | 63.7 ± 8.3 | 65.3 ± 7.1 | 0.28 |
| Male | 103 (89%) | 36 (92%) | 0.76 |
| Body mass index (kg/m2)a | 21.6 ± 3.1 | 20.9 ± 2.5 | 0.26 |
| Co-morbidities | |||
| Hypertension | 34 (29%) | 16 (41%) | 0.55 |
| Ischaemic heart disease | 6 (5%) | 2 (5%) | 1.00 |
| Atrial fibrillation | 3 (3%) | 3 (8%) | 0.17 |
| Anticoagulants | 5 (4%) | 1 (3%) | 1.00 |
| Antiplatelet agent | 5 (4%) | 2 (5%) | 1.00 |
| Peripheral vascular disease | 5 (4%) | 1 (3%) | 1.00 |
| COPD | 29 (25%) | 16 (41%) | 0.07 |
| Chronic liver disease | 7 (6%) | 1 (3%) | 0.68 |
| Diabetes mellitus | 9 (8%) | 4 (10%) | 0.74 |
| Total protein (g/dl)a | 6.9 ± 0.5 | 6.8 ± 0.5 | 0.21 |
| Albumin (g/dl)a | 4.0 ± 0.4 | 4.0 ± 0.4 | 0.73 |
| Creatinine (mg/dl)a | 0.7 ± 0.2 | 0.8 ± 0.1 | 0.22 |
| Creatinine clearance (ml/min)a | 84.0 ± 20.9 | 76.4 ± 17.1 | 0.04 |
| Tumour location | |||
| Upper thoracic | 16 (14%) | 4 (10%) | 0.82 |
| Mid-thoracic | 82 (71%) | 28 (72%) | |
| Lower thoracic | 18 (15%) | 7 (18%) | |
| Pathological tumour stage (UICC) | |||
| 0–I | 37 (32%) | 10 (26%) | 0.65 |
| II | 25 (21%) | 10 (26%) | |
| III | 46 (40%) | 17 (45%) | |
| IV | 8 (7%) | 1 (3%) | |
| Preoperative therapy | |||
| None | 73 (63%) | 26 (67%) | 0.49 |
| Chemotherapy | 36 (31%) | 9 (23%) | |
| Chemoradiotherapy | 7 (6%) | 4 (10%) | |
| Factors . | Low-output group (n = 116) . | High-output group (n = 39) . | P-value . |
|---|---|---|---|
| Agea | 63.7 ± 8.3 | 65.3 ± 7.1 | 0.28 |
| Male | 103 (89%) | 36 (92%) | 0.76 |
| Body mass index (kg/m2)a | 21.6 ± 3.1 | 20.9 ± 2.5 | 0.26 |
| Co-morbidities | |||
| Hypertension | 34 (29%) | 16 (41%) | 0.55 |
| Ischaemic heart disease | 6 (5%) | 2 (5%) | 1.00 |
| Atrial fibrillation | 3 (3%) | 3 (8%) | 0.17 |
| Anticoagulants | 5 (4%) | 1 (3%) | 1.00 |
| Antiplatelet agent | 5 (4%) | 2 (5%) | 1.00 |
| Peripheral vascular disease | 5 (4%) | 1 (3%) | 1.00 |
| COPD | 29 (25%) | 16 (41%) | 0.07 |
| Chronic liver disease | 7 (6%) | 1 (3%) | 0.68 |
| Diabetes mellitus | 9 (8%) | 4 (10%) | 0.74 |
| Total protein (g/dl)a | 6.9 ± 0.5 | 6.8 ± 0.5 | 0.21 |
| Albumin (g/dl)a | 4.0 ± 0.4 | 4.0 ± 0.4 | 0.73 |
| Creatinine (mg/dl)a | 0.7 ± 0.2 | 0.8 ± 0.1 | 0.22 |
| Creatinine clearance (ml/min)a | 84.0 ± 20.9 | 76.4 ± 17.1 | 0.04 |
| Tumour location | |||
| Upper thoracic | 16 (14%) | 4 (10%) | 0.82 |
| Mid-thoracic | 82 (71%) | 28 (72%) | |
| Lower thoracic | 18 (15%) | 7 (18%) | |
| Pathological tumour stage (UICC) | |||
| 0–I | 37 (32%) | 10 (26%) | 0.65 |
| II | 25 (21%) | 10 (26%) | |
| III | 46 (40%) | 17 (45%) | |
| IV | 8 (7%) | 1 (3%) | |
| Preoperative therapy | |||
| None | 73 (63%) | 26 (67%) | 0.49 |
| Chemotherapy | 36 (31%) | 9 (23%) | |
| Chemoradiotherapy | 7 (6%) | 4 (10%) | |
aValues depicted are mean ± standard deviations.
COPD: chronic obstructive pulmonary disease; UICC: International Union against Cancer.
| Factors . | Low-output group (n = 116) . | High-output group (n = 39) . | P-value . |
|---|---|---|---|
| Approach | |||
| Right thoracotomy | 97 (84%) | 38 (97%) | 0.03 |
| Thoracoscopy | 19 (16%) | 1 (3%) | |
| Lymph node dissection | |||
| Three-field | 54 (47%) | 21 (54%) | 0.46 |
| Two-field | 62 (53%) | 18 (46%) | |
| Dissected mediastinal LNsa | 19 ± 10 | 20 ± 11 | 0.39 |
| Residual tumour status (UICC) | |||
| R0 | 107 (92%) | 36 (92%) | 1.00 |
| R1/R2 | 9 (8%) | 3 (8%) | |
| Thoracic duct preservation | |||
| Removed | 36 (31%) | 4 (10%) | 0.01 |
| Preserved | 80 (69%) | 35 (90%) | |
| Reconstruction route | |||
| Subcutaneous/retrosternal | 50 (43%) | 23 (59%) | 0.10 |
| Posterior mediastinal | 66 (57%) | 16 (41%) | |
| Operative time (min)a | 460 ± 116 | 465 ± 93 | 0.84 |
| Blood loss (ml)a | 684 ± 491 | 734 ± 451 | 0.57 |
| Factors . | Low-output group (n = 116) . | High-output group (n = 39) . | P-value . |
|---|---|---|---|
| Approach | |||
| Right thoracotomy | 97 (84%) | 38 (97%) | 0.03 |
| Thoracoscopy | 19 (16%) | 1 (3%) | |
| Lymph node dissection | |||
| Three-field | 54 (47%) | 21 (54%) | 0.46 |
| Two-field | 62 (53%) | 18 (46%) | |
| Dissected mediastinal LNsa | 19 ± 10 | 20 ± 11 | 0.39 |
| Residual tumour status (UICC) | |||
| R0 | 107 (92%) | 36 (92%) | 1.00 |
| R1/R2 | 9 (8%) | 3 (8%) | |
| Thoracic duct preservation | |||
| Removed | 36 (31%) | 4 (10%) | 0.01 |
| Preserved | 80 (69%) | 35 (90%) | |
| Reconstruction route | |||
| Subcutaneous/retrosternal | 50 (43%) | 23 (59%) | 0.10 |
| Posterior mediastinal | 66 (57%) | 16 (41%) | |
| Operative time (min)a | 460 ± 116 | 465 ± 93 | 0.84 |
| Blood loss (ml)a | 684 ± 491 | 734 ± 451 | 0.57 |
aValues depicted are mean ± standard deviations.
UICC: International Union against Cancer.
| Factors . | Low-output group (n = 116) . | High-output group (n = 39) . | P-value . |
|---|---|---|---|
| Approach | |||
| Right thoracotomy | 97 (84%) | 38 (97%) | 0.03 |
| Thoracoscopy | 19 (16%) | 1 (3%) | |
| Lymph node dissection | |||
| Three-field | 54 (47%) | 21 (54%) | 0.46 |
| Two-field | 62 (53%) | 18 (46%) | |
| Dissected mediastinal LNsa | 19 ± 10 | 20 ± 11 | 0.39 |
| Residual tumour status (UICC) | |||
| R0 | 107 (92%) | 36 (92%) | 1.00 |
| R1/R2 | 9 (8%) | 3 (8%) | |
| Thoracic duct preservation | |||
| Removed | 36 (31%) | 4 (10%) | 0.01 |
| Preserved | 80 (69%) | 35 (90%) | |
| Reconstruction route | |||
| Subcutaneous/retrosternal | 50 (43%) | 23 (59%) | 0.10 |
| Posterior mediastinal | 66 (57%) | 16 (41%) | |
| Operative time (min)a | 460 ± 116 | 465 ± 93 | 0.84 |
| Blood loss (ml)a | 684 ± 491 | 734 ± 451 | 0.57 |
| Factors . | Low-output group (n = 116) . | High-output group (n = 39) . | P-value . |
|---|---|---|---|
| Approach | |||
| Right thoracotomy | 97 (84%) | 38 (97%) | 0.03 |
| Thoracoscopy | 19 (16%) | 1 (3%) | |
| Lymph node dissection | |||
| Three-field | 54 (47%) | 21 (54%) | 0.46 |
| Two-field | 62 (53%) | 18 (46%) | |
| Dissected mediastinal LNsa | 19 ± 10 | 20 ± 11 | 0.39 |
| Residual tumour status (UICC) | |||
| R0 | 107 (92%) | 36 (92%) | 1.00 |
| R1/R2 | 9 (8%) | 3 (8%) | |
| Thoracic duct preservation | |||
| Removed | 36 (31%) | 4 (10%) | 0.01 |
| Preserved | 80 (69%) | 35 (90%) | |
| Reconstruction route | |||
| Subcutaneous/retrosternal | 50 (43%) | 23 (59%) | 0.10 |
| Posterior mediastinal | 66 (57%) | 16 (41%) | |
| Operative time (min)a | 460 ± 116 | 465 ± 93 | 0.84 |
| Blood loss (ml)a | 684 ± 491 | 734 ± 451 | 0.57 |
aValues depicted are mean ± standard deviations.
UICC: International Union against Cancer.
| Factors . | Odds ratio . | 95% Confidence interval . | P-value . |
|---|---|---|---|
| COPD | 2.19 | 0.95–4.77 | 0.07 |
| Creatinine clearance | 1.02 | 1.00–1.04 | 0.04 |
| Posterior mediastinal route | 0.73 | 0.32–1.66 | 0.45 |
| Thoracic duct removed | 4.02 | 1.20–13.41 | 0.02 |
| Factors . | Odds ratio . | 95% Confidence interval . | P-value . |
|---|---|---|---|
| COPD | 2.19 | 0.95–4.77 | 0.07 |
| Creatinine clearance | 1.02 | 1.00–1.04 | 0.04 |
| Posterior mediastinal route | 0.73 | 0.32–1.66 | 0.45 |
| Thoracic duct removed | 4.02 | 1.20–13.41 | 0.02 |
COPD: chronic obstructive pulmonary disease.
| Factors . | Odds ratio . | 95% Confidence interval . | P-value . |
|---|---|---|---|
| COPD | 2.19 | 0.95–4.77 | 0.07 |
| Creatinine clearance | 1.02 | 1.00–1.04 | 0.04 |
| Posterior mediastinal route | 0.73 | 0.32–1.66 | 0.45 |
| Thoracic duct removed | 4.02 | 1.20–13.41 | 0.02 |
| Factors . | Odds ratio . | 95% Confidence interval . | P-value . |
|---|---|---|---|
| COPD | 2.19 | 0.95–4.77 | 0.07 |
| Creatinine clearance | 1.02 | 1.00–1.04 | 0.04 |
| Posterior mediastinal route | 0.73 | 0.32–1.66 | 0.45 |
| Thoracic duct removed | 4.02 | 1.20–13.41 | 0.02 |
COPD: chronic obstructive pulmonary disease.
Additional drainage
Additional drainage was performed in 19 (12%) patients. A chest tube was inserted in 6 patients, with left-sided placement in 5 patients and right-sided in 1, at a median timing of POD 5 (range POD 1–9). Fifteen patients underwent thoracentesis. The total number of centeses was 25, with 13 centeses performed on the right side and 12 on the left side, and the median timing being POD 14 (range POD 2–48). The median total drainage volume of the thoracentesis was 500 (IQR 600) ml. The median duration of pleural drainage in patients who underwent additional drainage and patients who did not was 11 (range 7–21) and 10 (range 4–26) days, respectively, showing no significant difference (P = 0.52). The median total drainage volumes in patients who underwent additional drainage and patients who did not were significantly different (P = 0.04) at 2882 (IQR 1638) and 2204 (IQR 1634) ml, respectively. Regarding the 27 potential risk factors, there was no significant difference between patients who underwent additional drainage and patients who did not (data not shown).
DISCUSSION
A pleural effusion will develop when the amount of pleural fluid that enters the pleural space from the capillaries in the parietal pleura exceeds the amount that can be removed via the lymphatics in the parietal pleura [10]. In a previous study that was part of a randomized clinical trial comparing limited transhiatal with extended transthoracic oesophagectomy, only the transthoracic approach was an independent determinant of thoracic drainage output [2]. This result was intuitively plausible because extended mediastinal lymphadenectomy was exclusively performed in patients who underwent the transthoracic approach. The difference in drainage output could most probably be explained by the larger surgical trauma to the lymphatic vessels in the mediastinal pleura, which could cause a combination of increased pleural fluid formation and decreased lymphatic clearance from the pleural space [2, 10]. However, pleural drainage volume varies among patients who undergo transthoracic oesophagectomy with extended lymphadenectomy; therefore, we conducted this retrospective cohort study to elucidate the factors influencing the pleural drainage volume after transthoracic oesophagectomy.
In this study, removal of the thoracic duct was an independent risk factor for increased pleural drainage volume after transthoracic oesophagectomy. The thoracic duct receives branches from the intercostal space of both sides via several connecting trunks and also branches from posterior mediastinal structures [11]; therefore, non-chylous lymph leakage may develop from these branches after removal of the thoracic duct. It remains an unresolved issue whether the thoracic duct should be removed or preserved during transthoracic oesophagectomy. En bloc resection seems oncologically sound though firm evidence is unavailable and the risk of chyle leakage becomes lower when the proximal stump is securely ligated. However, removal of the thoracic duct causes retroperitoneal lymphoedema and hypovolaemia. In addition, the increased pleural drainage volume makes it difficult to maintain an adequate body water balance. Although the risk of chyle leakage becomes higher, it is better to preserve the thoracic duct unless it shows primary tumour or metastatic node involvement.
On the other hand, it is uncertain whether impaired renal function really causes increased pleural drainage volume. Uraemia may be complicated by a fibrous pleuritis and pleural effusion [10]; however, renal dysfunction in the high-output group was relatively mild. One possible explanation is that postoperative fluid overload is apt to develop in patients with renal dysfunction, which causes increased hydrostatic pressure of systemic circulation and transudative pleural effusion. Further investigation is required to elucidate the correlation between renal function and pleural drainage volume after transthoracic oesophagectomy.
In this study, the operative approach was significantly associated with the total pleural drainage volume. In the field of lung surgery, Nakanishi et al. [12] reported in their prospective study that early removal of the chest tube on the day after thoracoscopic lobectomy was safe in well-selected patients, independent of the drainage volume. The use of the thoracoscopic approach may lower the surgical trauma due to smaller incisions without rib-spreading instruments, probably resulting in less fluid production and the retained capacity to absorb the fluid [3]. The benefits of thoracoscopic oesophagectomy in postoperative management should be examined in larger-scale studies in the future.
Because of the retrospective nature of this study, no definitive conclusion can be drawn regarding factors that influence the pleural drainage volume after transthoracic oesophagectomy. This study population was not homogeneous and could not control for many factors that could influence the postoperative drainage volume. We therefore performed multivariate analysis using a logistic regression model with goodness of fit to examine the influence of these factors on dichotomous drainage volume. In addition, the optimal timing to remove the chest drains remains unclear. The median duration of pleural drainage was 10 days in this study period, which seems very long from the viewpoint of recent daily practice. Because we had no clinical criteria regarding the chest drain removal and it depended on the operating surgeon's discretion, the chest drains tended to be left in place for a long time. Although the drain was generally removed when the daily drainage volume dropped to <100 ml, information bias regarding this point was not negligible. Nineteen (12%) patients required additional pleural drainage; however, the potential risk factors of pleural fluid reaccumulation were unavailable. To determine the optimal timing to remove the chest drains after transthoracic oesophagectomy, a prospective randomized study should be conducted that evaluates hospitalization time, overall costs and the rate of pleural fluid reaccumulation requiring additional drainage after removal of the chest drains using some possible thresholds [4]. This study may provide useful information to understand the natural course of pleural fluid production and to decide a possible threshold. As shown in Fig. 1, the median daily drainage volume reached a maximum of 397 ml on POD 1 and decreased gradually thereafter. For example, a daily drainage volume of 400 ml may be one of the possible thresholds. Cerfolio et al. [13] use a daily drainage volume of 450 ml on POD 3 as a threshold to remove the chest tube in their fast-tracking protocol after Ivor Lewis oesophagogastrectomy.
In conclusion, removal of the thoracic duct and lower creatinine clearance was independent risk factors for increased pleural drainage volume after transthoracic oesophagectomy. Early removal of the chest drains may be possible in patients without these risk factors.
Conflict of interest: none declared.




