-
PDF
- Split View
-
Views
-
Cite
Cite
Tomohiro Murakawa, Chihiro Konoeda, Takuya Ito, Yuta Inoue, Atsushi Sano, Kazuhiro Nagayama, Jun Nakajima, The ground glass opacity component can be eliminated from the T-factor assessment of lung adenocarcinoma, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 5, May 2013, Pages 925–932, https://doi.org/10.1093/ejcts/ezs467
Close - Share Icon Share
Abstract
The radiological ground glass opacity (GGO) component of an adenocarcinoma pathologically reflects a non-invasive adenocarcinoma in situ (AIS). Measuring the tumour diameter to include the GGO component may overestimate the T factor. In this retrospective study, we evaluated the effect of the GGO component on the recurrence of an adenocarcinoma.
We reviewed patients who underwent a surgical resection of a lung adenocarcinoma and were pathologically proven to be T1-2N0M0, from 1999 to 2009. We conducted four different types of analyses (multivariate analysis, receiver operating characteristic [ROC] analysis, survival analysis according to subcategories and survival analysis of propensity score-matched pairs) to evaluate the impacts of GGO and the solid component on recurrence.
The study included 241 patients, and there were 34 recurrences. Sixty-eight cases with AIS and minimally invasive adenocarcinoma exhibited 100% recurrence-free survival. A univariate and a multivariate analysis revealed that the maximum tumour diameter measured in the mediastinal window was a better prognostic factor than the maximum tumour diameter in the lung window. This finding was supported by an ROC curve analysis, a subgroup analysis and a propensity score-matched analysis. An ROC curve analysis revealed that GGO component exclusion resulted in improved prognostic performance for recurrence and pathological vessel invasion. A subgroup analysis and a propensity score-matched analysis demonstrated that tumours with similar solid component sizes and different GGO sizes exhibited equivalent recurrence-free survival.
The GGO component showed little influence on recurrence. Recurrence-free survival was solely dependent on the solid component size. A T factor measured by the solid component may be a more accurate prognostic parameter.
INTRODUCTION
A localized bronchioloalveolar carcinoma with or without foci of structural alveolar collapse is considered to be biologically in situ, and the formation of foci of active fibroblastic proliferation in the tumour parallels the acquisition of malignant potential [1, 2]. The radiological ground glass opacity (GGO) component of an adenocarcinoma pathologically reflects a non-invasive adenocarcinoma in situ (AIS) [3], and the percent of localized AIS vs invasive components in lung adenocarcinomas has been repeatedly reported to be prognostically important [4, 5]. Thus, measuring the tumour diameter to include the GGO, which is the current T-assessment method, may overestimate the T factor. In this study, we evaluated the impact of the GGO component on tumour recurrence.
MATERIALS AND METHODS
Patients
We reviewed patients who underwent a surgical resection of a lung adenocarcinoma and were pathologically proven to be T1-2N0M0, from 1999 to 2009. All resections were complete, and the completeness of resection was defined as a tumour-free surgical margin on pathological evaluation. The data set was limited to those patients for whom preoperative high-resolution computed tomography (HRCT) was available. Clinical and pathological staging was based on the 7th edition of the TNM classification of the Union for International Cancer Control (UICC) [6]. Resected specimens were examined by staining with haematoxylin and eosin and the Elastica van Gieson stain. Stained tissue sections were evaluated by pathologists for the histological type, the extent of lymph node involvement, and the existence of vessel invasion and pleural invasion. The histological classification was based on the IASLC/ATS/ERS international multidisciplinary classification of lung adenocarcinoma [7]. Pleural lavage cytology was performed at the time of exploration of the thoracic cavity. Interval between follow-ups was every 3 months for the first 2 years, then every 4 months for up to 5 years. Full examination and chest X-ray were carried out on each visit, and CT was checked annually. Other investigations were determined by clinical need. We reviewed the clinical records of all of these patients. Before the study, the Research Review Board at our institution examined and approved our research protocol in accordance with the Declaration of Helsinki. All patients provided written informed consent for review of their medical charts before the surgery.
Radiological assessment
For radiological analyses, we used thin-slice HRCT images taken just before surgery. The HRCT images taken in our hospital were all preserved in Digital Imaging and Communications in Medicine (DICOM) format and reviewed on computing systems. The protocol for HRCT image analysis was as follows: (i) a slice that maximizes tumour diameter in the lung window (window level: −600 Hounsfield units (HUs); window width, 1500 HUs) was selected, and the maximum and minimum diameters were measured (maxDinLW and minDinLW); and (ii) a slice that maximizes tumour diameter in the mediastinal window (window level: 40 HUs, window width: 250 HUs) was selected, and the maximum and minimum diameters were measured (maxDinMW and minDinMW). The area sizes were calculated by estimating the tumours as ellipses (areainMW and areainLW). The tumour shadow disappearance rate (TDR) was also calculated, as reported previously [8].
Statistical methods
The prognostic cofactors for recurrence-free survival were examined by univariate and multivariate analyses using the Cox proportional hazards model and included age, sex, smoking history, preoperative serum carcinoembryonic antigen (CEA) level, maxDinLW, maxDinMW, areainMW, GGO area size (areainLW − areainMW) to isolate the GGO component, TDR, the types of surgical procedures performed, histological type, pathological vessel invasion, pathological pleural invasion and pleural lavage cytology. The measurement of the survival periods began on the date of surgery. Patients without recurrence were censored from analysis at the time of their last negative follow-up visit. Only variables that were significant in the univariate analyses were included in the multivariate model. Because one-dimension and two-dimension assessments of tumour size overlap with each other as confounding factors, these factors were included in the separate multivariate models. The recurrence-free survival curves were estimated according to the Kaplan–Meier method, and the differences between groups were compared using a log-rank test. The continuous variables were categorized by assessing their correlation with recurrence using a receiver operating characteristic (ROC) curve analysis, and the optimal thresholds were identified by a Youden index.
The predictive performances of the GGO component and the solid component for recurrence, vessel invasion and pleural invasion were examined by comparing the areas under the ROC curves (AUCs). A one-dimensional study that compared maxDinLW and maxDinMW and a two-dimensional study that compared the area sizes in the lung and mediastinal windows were performed. The area sizes were calculated by estimating the tumours as ellipses. The predictive performance of TDR for recurrence was also evaluated.
To compare the recurrence-free survival rates among the similar solid tumour sizes (i.e. 0 mm < maxDinMW ≤ 10 mm; 10 mm < maxDinMW ≤ 20 mm; 20 mm < maxDinMW), the tumours were subdivided into three groups according to the calculated M/L (i.e. tumour area size in the mediastinal window/tumour area size in the lung window) ratio (i.e. 0 < M/L ≤ 0.4, 0.4 < M/L ≤ 0.8, 0.8 < M/L), and the survival rates were examined.
To overcome any bias caused by the different distributions of covariates among the patients from the two groups (GGO-predominant group: GGO group, and solid component-predominant group: SLD group), a one-to-one match was created using propensity score matching, and the recurrence-free survival rates of propensity score-matched pairs with similar solid component sizes from the two groups were compared. In this analysis, the GGO group was defined as the tumours with maxDinMW/maxDinLW ≤ 0.85, and the SLD group as the tumours with maxDinMW/maxDinLW > 0.85. Because there were no patients with maxDinMW >30 mm in GGO group, these patients were excluded. Radiologically pure GGOs were also excluded. There were 130 patients in the GGO group and 34 patients in the SLD group. The variables entered into the propensity score-matching model were age, sex, smoking history, preoperative CEA and maxDinMW. The model was then used to obtain a one-to-one match using the nearest-neighbor-matching method.
The data were analysed using JMP 9.0.0 (SAS Institute, Cary, NC, USA) and R version 2.14.0 (2011 The R Foundation for Statistical Computing) [9]. The pROC package for R [10] was used for the ROC analyses, and the MatchIt package for R [11] was used for propensity score matching. A P-value of <0.05 was considered statistically significant.
RESULTS
Patient characteristics
The study included 241 patients, and there were 34 recurrences (brain metastasis in 4 patients, bone metastasis in 7, intrapulmonary metastasis in 9, lymph node metastasis in 6, pleural dissemination in 5, local recurrence at the surgical stump in 1 segmentectomy patient and multiple local and distant metastases in 2). Ninety-five patients with adenocarcinoma were excluded because their preoperative HRCT scans were performed in other hospitals, and there were no appropriate DICOM data that we could analyse in our computer system. The baseline characteristics of the 241 patients are summarized in Table 1. The mean recurrence-free survival was 49.3 months (95% confidence interval [CI] 45.6–53.1), and the median recurrence-free survival was 42.0 months (range 0.37–125.5). Sixty-eight AIS and minimally invasive adenocarcinoma (MIA) cases showed 100% recurrence-free survival (Fig. 1). Five-year recurrence-free survival rates for AIS/MIA and invasive adenocarcinoma were 1.0 (95% CI 1.0–1.0) and 0.775 (95% CI 0.694–0.837), respectively.
| Total patients | 241 |
| Observation period (day) | |
| Median | 1261 |
| Range | 11–3765 |
| Age (years) | |
| Median | 67 |
| Range | 17–85 |
| Sex | |
| Male | 133 (55.2%) |
| Female | 108 (44.8%) |
| Smoking history | |
| No | 103 (42.7%) |
| Yes | 138 (57.3%) |
| CEA (ng/ml) | |
| ≤5 | 172 (71.4%) |
| >5 | 69 (28.6%) |
| Maximum tumour diameter measured in the lung window (mm) | |
| Median | 18.3 |
| Range | 5.0–53.0 |
| ≤22.87 | 161 (66.8%) |
| >22.87 | 80 (33.2%) |
| Maximum tumour diameter measured in the mediastinal window (mm) | |
| Median | 9.05 |
| Range | 0–51.0 |
| ≤12.52 | 149 (61.8%) |
| >12.52 | 92 (38.2%) |
| Area size measured in the lung window (areainLW) (mm2) | |
| Median | 194.2 |
| Range | 14.1–1193 |
| ≤122.8 | 73 (30.3%) |
| >122.8 | 168 (69.7%) |
| Area size measured in the mediastinal window (areainMW) (mm2) | |
| Median | 47.1 |
| Range | 0.0–1193 |
| ≤90.3 | 153 (63.5%) |
| >90.3 | 88 (36.5%) |
| GGO area size (areainLW − areainMW) (mm2) | |
| Median | 103.6 |
| Range | 0.0–883.2 |
| ≤167.0 | 168 (69.7%) |
| >167.0 | 73 (30.3%) |
| TDR | |
| Median | 0.36 |
| Range | 0.0–1.0 |
| ≤0.778 | 124 (51.5%) |
| >0.778 | 117 (48.5%) |
| Surgical procedure | |
| Wedge resection | 62 (25.7%) |
| Segmentectomy | 12 (5.0%) |
| Lobectomy | 167 (69.3%) |
| Histology | |
| AIS and MIA | 68 (28.2%) |
| Wedge resection | 34 |
| Segmentectomy | 7 |
| Lobectomy | 27 |
| Invasive adenocarcinoma | 173 (71.8%) |
| Wedge resection | 28 |
| Segmentectomy | 5 |
| Lobectomy | 140 |
| Vessel invasion | |
| No | 190 (78.8%) |
| Yes | 51 (21.2%) |
| Pleural invasion | |
| No | 184 (76.3%) |
| Yes | 57 (23.7%) |
| Pleural lavage cytology | |
| Negative | 236 (97.9%) |
| Positive | 5 (2.1%) |
| Recurrence | |
| No | 207 (85.9%) |
| Wedge resection | 53 |
| Segmentectomy | 10 |
| Lobectomy | 144 |
| Yes | 34 (14.1%) |
| Wedge resection | 9 |
| Segmentectomy | 2 |
| Lobectomy | 23 |
| Total patients | 241 |
| Observation period (day) | |
| Median | 1261 |
| Range | 11–3765 |
| Age (years) | |
| Median | 67 |
| Range | 17–85 |
| Sex | |
| Male | 133 (55.2%) |
| Female | 108 (44.8%) |
| Smoking history | |
| No | 103 (42.7%) |
| Yes | 138 (57.3%) |
| CEA (ng/ml) | |
| ≤5 | 172 (71.4%) |
| >5 | 69 (28.6%) |
| Maximum tumour diameter measured in the lung window (mm) | |
| Median | 18.3 |
| Range | 5.0–53.0 |
| ≤22.87 | 161 (66.8%) |
| >22.87 | 80 (33.2%) |
| Maximum tumour diameter measured in the mediastinal window (mm) | |
| Median | 9.05 |
| Range | 0–51.0 |
| ≤12.52 | 149 (61.8%) |
| >12.52 | 92 (38.2%) |
| Area size measured in the lung window (areainLW) (mm2) | |
| Median | 194.2 |
| Range | 14.1–1193 |
| ≤122.8 | 73 (30.3%) |
| >122.8 | 168 (69.7%) |
| Area size measured in the mediastinal window (areainMW) (mm2) | |
| Median | 47.1 |
| Range | 0.0–1193 |
| ≤90.3 | 153 (63.5%) |
| >90.3 | 88 (36.5%) |
| GGO area size (areainLW − areainMW) (mm2) | |
| Median | 103.6 |
| Range | 0.0–883.2 |
| ≤167.0 | 168 (69.7%) |
| >167.0 | 73 (30.3%) |
| TDR | |
| Median | 0.36 |
| Range | 0.0–1.0 |
| ≤0.778 | 124 (51.5%) |
| >0.778 | 117 (48.5%) |
| Surgical procedure | |
| Wedge resection | 62 (25.7%) |
| Segmentectomy | 12 (5.0%) |
| Lobectomy | 167 (69.3%) |
| Histology | |
| AIS and MIA | 68 (28.2%) |
| Wedge resection | 34 |
| Segmentectomy | 7 |
| Lobectomy | 27 |
| Invasive adenocarcinoma | 173 (71.8%) |
| Wedge resection | 28 |
| Segmentectomy | 5 |
| Lobectomy | 140 |
| Vessel invasion | |
| No | 190 (78.8%) |
| Yes | 51 (21.2%) |
| Pleural invasion | |
| No | 184 (76.3%) |
| Yes | 57 (23.7%) |
| Pleural lavage cytology | |
| Negative | 236 (97.9%) |
| Positive | 5 (2.1%) |
| Recurrence | |
| No | 207 (85.9%) |
| Wedge resection | 53 |
| Segmentectomy | 10 |
| Lobectomy | 144 |
| Yes | 34 (14.1%) |
| Wedge resection | 9 |
| Segmentectomy | 2 |
| Lobectomy | 23 |
CEA: carcinoembryonic antigen; AIS: adenocarcinoma in situ; MIA: minimally invasive adenocarcinoma; GGO: ground glass opacity; TDR: tumor shadow disappearance rate.
| Total patients | 241 |
| Observation period (day) | |
| Median | 1261 |
| Range | 11–3765 |
| Age (years) | |
| Median | 67 |
| Range | 17–85 |
| Sex | |
| Male | 133 (55.2%) |
| Female | 108 (44.8%) |
| Smoking history | |
| No | 103 (42.7%) |
| Yes | 138 (57.3%) |
| CEA (ng/ml) | |
| ≤5 | 172 (71.4%) |
| >5 | 69 (28.6%) |
| Maximum tumour diameter measured in the lung window (mm) | |
| Median | 18.3 |
| Range | 5.0–53.0 |
| ≤22.87 | 161 (66.8%) |
| >22.87 | 80 (33.2%) |
| Maximum tumour diameter measured in the mediastinal window (mm) | |
| Median | 9.05 |
| Range | 0–51.0 |
| ≤12.52 | 149 (61.8%) |
| >12.52 | 92 (38.2%) |
| Area size measured in the lung window (areainLW) (mm2) | |
| Median | 194.2 |
| Range | 14.1–1193 |
| ≤122.8 | 73 (30.3%) |
| >122.8 | 168 (69.7%) |
| Area size measured in the mediastinal window (areainMW) (mm2) | |
| Median | 47.1 |
| Range | 0.0–1193 |
| ≤90.3 | 153 (63.5%) |
| >90.3 | 88 (36.5%) |
| GGO area size (areainLW − areainMW) (mm2) | |
| Median | 103.6 |
| Range | 0.0–883.2 |
| ≤167.0 | 168 (69.7%) |
| >167.0 | 73 (30.3%) |
| TDR | |
| Median | 0.36 |
| Range | 0.0–1.0 |
| ≤0.778 | 124 (51.5%) |
| >0.778 | 117 (48.5%) |
| Surgical procedure | |
| Wedge resection | 62 (25.7%) |
| Segmentectomy | 12 (5.0%) |
| Lobectomy | 167 (69.3%) |
| Histology | |
| AIS and MIA | 68 (28.2%) |
| Wedge resection | 34 |
| Segmentectomy | 7 |
| Lobectomy | 27 |
| Invasive adenocarcinoma | 173 (71.8%) |
| Wedge resection | 28 |
| Segmentectomy | 5 |
| Lobectomy | 140 |
| Vessel invasion | |
| No | 190 (78.8%) |
| Yes | 51 (21.2%) |
| Pleural invasion | |
| No | 184 (76.3%) |
| Yes | 57 (23.7%) |
| Pleural lavage cytology | |
| Negative | 236 (97.9%) |
| Positive | 5 (2.1%) |
| Recurrence | |
| No | 207 (85.9%) |
| Wedge resection | 53 |
| Segmentectomy | 10 |
| Lobectomy | 144 |
| Yes | 34 (14.1%) |
| Wedge resection | 9 |
| Segmentectomy | 2 |
| Lobectomy | 23 |
| Total patients | 241 |
| Observation period (day) | |
| Median | 1261 |
| Range | 11–3765 |
| Age (years) | |
| Median | 67 |
| Range | 17–85 |
| Sex | |
| Male | 133 (55.2%) |
| Female | 108 (44.8%) |
| Smoking history | |
| No | 103 (42.7%) |
| Yes | 138 (57.3%) |
| CEA (ng/ml) | |
| ≤5 | 172 (71.4%) |
| >5 | 69 (28.6%) |
| Maximum tumour diameter measured in the lung window (mm) | |
| Median | 18.3 |
| Range | 5.0–53.0 |
| ≤22.87 | 161 (66.8%) |
| >22.87 | 80 (33.2%) |
| Maximum tumour diameter measured in the mediastinal window (mm) | |
| Median | 9.05 |
| Range | 0–51.0 |
| ≤12.52 | 149 (61.8%) |
| >12.52 | 92 (38.2%) |
| Area size measured in the lung window (areainLW) (mm2) | |
| Median | 194.2 |
| Range | 14.1–1193 |
| ≤122.8 | 73 (30.3%) |
| >122.8 | 168 (69.7%) |
| Area size measured in the mediastinal window (areainMW) (mm2) | |
| Median | 47.1 |
| Range | 0.0–1193 |
| ≤90.3 | 153 (63.5%) |
| >90.3 | 88 (36.5%) |
| GGO area size (areainLW − areainMW) (mm2) | |
| Median | 103.6 |
| Range | 0.0–883.2 |
| ≤167.0 | 168 (69.7%) |
| >167.0 | 73 (30.3%) |
| TDR | |
| Median | 0.36 |
| Range | 0.0–1.0 |
| ≤0.778 | 124 (51.5%) |
| >0.778 | 117 (48.5%) |
| Surgical procedure | |
| Wedge resection | 62 (25.7%) |
| Segmentectomy | 12 (5.0%) |
| Lobectomy | 167 (69.3%) |
| Histology | |
| AIS and MIA | 68 (28.2%) |
| Wedge resection | 34 |
| Segmentectomy | 7 |
| Lobectomy | 27 |
| Invasive adenocarcinoma | 173 (71.8%) |
| Wedge resection | 28 |
| Segmentectomy | 5 |
| Lobectomy | 140 |
| Vessel invasion | |
| No | 190 (78.8%) |
| Yes | 51 (21.2%) |
| Pleural invasion | |
| No | 184 (76.3%) |
| Yes | 57 (23.7%) |
| Pleural lavage cytology | |
| Negative | 236 (97.9%) |
| Positive | 5 (2.1%) |
| Recurrence | |
| No | 207 (85.9%) |
| Wedge resection | 53 |
| Segmentectomy | 10 |
| Lobectomy | 144 |
| Yes | 34 (14.1%) |
| Wedge resection | 9 |
| Segmentectomy | 2 |
| Lobectomy | 23 |
CEA: carcinoembryonic antigen; AIS: adenocarcinoma in situ; MIA: minimally invasive adenocarcinoma; GGO: ground glass opacity; TDR: tumor shadow disappearance rate.
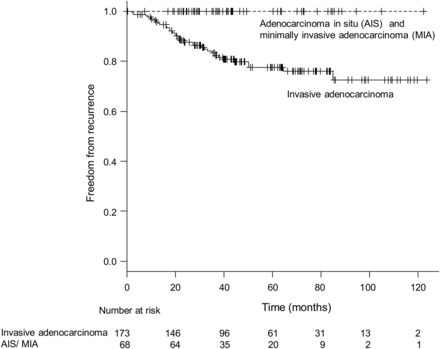
Recurrence-free survival curves according to histology. No recurrences were observed in cases of AIS and MIA.
Univariate and multivariate analyses
A univariate analysis revealed that high CEA (P = 0.0003), maxDinLW (P = 0.0036), maxDinMW (P < 0.0001), areainMW (P < 0.0001), GGO area size (P = 0.0027), TDR (P < 0.0001), positive vessel invasion (P < 0.0001), positive pleural invasion (P < 0.0001) and positive pleural lavage cytology (P = 0.0205) were predictive factors for recurrence (Table 2).
Univariate and multivariate analyses of recurrence-free survival (Cox proportional hazard model)
| . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Univariate analysis results | |||
| Age (>67) | 1.1127 | 0.7937–1.5669 | 0.5340 |
| Sex (female) | 0.7922 | 0.5484–1.1166 | 0.1861 |
| Smoking history (no) | 0.8813 | 0.6155–1.2381 | 0.4700 |
| CEA (>5) | 3.4969 | 1.7800–6.9952 | 0.0003 |
| Maximum tumour diameter measured in the lung window (>22.87) | 1.6550 | 1.1746–2.3545 | 0.0036 |
| Maximum tumour diameter measured in the mediastinal window (>12.52) | 2.6526 | 1.7992–4.1892 | <.0001 |
| Procedure | 0.9262 | ||
| Wedge resection | 1 | ||
| Lobectomy | 0.8889 | 0.5230–1.7466 | |
| Segmentectomy | 1.1827 | 0.3501–2.6496 | |
| Vessel invasion (yes) | 5.7248 | 2.9146–11.450 | <0.0001 |
| Pleural invasion (yes) | 4.6497 | 2.3666–9.3025 | <0.0001 |
| Lavage cytology (positive) | 5.8025 | 1.3858–16.411 | 0.0205 |
| 1. Multivariate analysis results: tumour size assessment by one-dimensional measurement | |||
| CEA (>5) | 2.5515 | 1.2737–5.1982 | 0.0084 |
| Maximum tumour diameter measured in the lung window (>22.87) | 0.8542 | 0.5684–1.3005 | 0.455 |
| Maximum tumour diameter measured in the mediastinal window (>12.52) | 2.0830 | 1.2547–3.5846 | 0.0042 |
| Vessel invasion (yes) | 1.9875 | 0.9105–4.5147 | 0.0850 |
| Pleural invasion (yes) | 3.1140 | 1.4907–6.5355 | 0.0028 |
| Lavage cytology (positive) | 1.3536 | 0.3057–4.3067 | 0.6520 |
| 2. Multivariate analysis results: tumour size assessment by two-dimensional measurement | |||
| CEA (>5) | 2.3043 | 1.1495–4.6962 | 0.0188 |
| Area size measured in the mediastinal window (>90.3) | 1.8700 | 1.1673–3.1138 | 0.0089 |
| GGO area size (>167.0) | 0.5507 | 0.2988–0.8834 | 0.0111 |
| Vessel invasion (yes) | 1.8771 | 0.8511–4.2975 | 0.1193 |
| Pleural invasion (yes) | 2.8057 | 1.3542–5.8451 | 0.0059 |
| Lavage cytology (positive) | 1.3990 | 0.3154–4.4551 | 0.6186 |
| 3. Multivariate analysis results: tumour size assessment by area size ratio (TDR) | |||
| CEA (>5) | 2.4791 | 1.2506–5.0016 | 0.0095 |
| TDR (>0.778) | 0.3247 | 0.1293–0.6056 | <0.0001 |
| Vessel invasion (yes) | 2.2617 | 1.0095–4.7257 | 0.0267 |
| Pleural invasion (yes) | 2.6987 | 1.3090–5.5957 | 0.0076 |
| Lavage cytology (positive) | 1.3301 | 0.3045–4.1136 | 0.6660 |
| . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Univariate analysis results | |||
| Age (>67) | 1.1127 | 0.7937–1.5669 | 0.5340 |
| Sex (female) | 0.7922 | 0.5484–1.1166 | 0.1861 |
| Smoking history (no) | 0.8813 | 0.6155–1.2381 | 0.4700 |
| CEA (>5) | 3.4969 | 1.7800–6.9952 | 0.0003 |
| Maximum tumour diameter measured in the lung window (>22.87) | 1.6550 | 1.1746–2.3545 | 0.0036 |
| Maximum tumour diameter measured in the mediastinal window (>12.52) | 2.6526 | 1.7992–4.1892 | <.0001 |
| Procedure | 0.9262 | ||
| Wedge resection | 1 | ||
| Lobectomy | 0.8889 | 0.5230–1.7466 | |
| Segmentectomy | 1.1827 | 0.3501–2.6496 | |
| Vessel invasion (yes) | 5.7248 | 2.9146–11.450 | <0.0001 |
| Pleural invasion (yes) | 4.6497 | 2.3666–9.3025 | <0.0001 |
| Lavage cytology (positive) | 5.8025 | 1.3858–16.411 | 0.0205 |
| 1. Multivariate analysis results: tumour size assessment by one-dimensional measurement | |||
| CEA (>5) | 2.5515 | 1.2737–5.1982 | 0.0084 |
| Maximum tumour diameter measured in the lung window (>22.87) | 0.8542 | 0.5684–1.3005 | 0.455 |
| Maximum tumour diameter measured in the mediastinal window (>12.52) | 2.0830 | 1.2547–3.5846 | 0.0042 |
| Vessel invasion (yes) | 1.9875 | 0.9105–4.5147 | 0.0850 |
| Pleural invasion (yes) | 3.1140 | 1.4907–6.5355 | 0.0028 |
| Lavage cytology (positive) | 1.3536 | 0.3057–4.3067 | 0.6520 |
| 2. Multivariate analysis results: tumour size assessment by two-dimensional measurement | |||
| CEA (>5) | 2.3043 | 1.1495–4.6962 | 0.0188 |
| Area size measured in the mediastinal window (>90.3) | 1.8700 | 1.1673–3.1138 | 0.0089 |
| GGO area size (>167.0) | 0.5507 | 0.2988–0.8834 | 0.0111 |
| Vessel invasion (yes) | 1.8771 | 0.8511–4.2975 | 0.1193 |
| Pleural invasion (yes) | 2.8057 | 1.3542–5.8451 | 0.0059 |
| Lavage cytology (positive) | 1.3990 | 0.3154–4.4551 | 0.6186 |
| 3. Multivariate analysis results: tumour size assessment by area size ratio (TDR) | |||
| CEA (>5) | 2.4791 | 1.2506–5.0016 | 0.0095 |
| TDR (>0.778) | 0.3247 | 0.1293–0.6056 | <0.0001 |
| Vessel invasion (yes) | 2.2617 | 1.0095–4.7257 | 0.0267 |
| Pleural invasion (yes) | 2.6987 | 1.3090–5.5957 | 0.0076 |
| Lavage cytology (positive) | 1.3301 | 0.3045–4.1136 | 0.6660 |
TDR: tumour shadow disappearance rate; GGO: ground glass opacity; CEA: carcinoembryonic antigen; AIS: adenocarcinoma in situ; MIA: minimally invasive adenocarcinoma.
Univariate and multivariate analyses of recurrence-free survival (Cox proportional hazard model)
| . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Univariate analysis results | |||
| Age (>67) | 1.1127 | 0.7937–1.5669 | 0.5340 |
| Sex (female) | 0.7922 | 0.5484–1.1166 | 0.1861 |
| Smoking history (no) | 0.8813 | 0.6155–1.2381 | 0.4700 |
| CEA (>5) | 3.4969 | 1.7800–6.9952 | 0.0003 |
| Maximum tumour diameter measured in the lung window (>22.87) | 1.6550 | 1.1746–2.3545 | 0.0036 |
| Maximum tumour diameter measured in the mediastinal window (>12.52) | 2.6526 | 1.7992–4.1892 | <.0001 |
| Procedure | 0.9262 | ||
| Wedge resection | 1 | ||
| Lobectomy | 0.8889 | 0.5230–1.7466 | |
| Segmentectomy | 1.1827 | 0.3501–2.6496 | |
| Vessel invasion (yes) | 5.7248 | 2.9146–11.450 | <0.0001 |
| Pleural invasion (yes) | 4.6497 | 2.3666–9.3025 | <0.0001 |
| Lavage cytology (positive) | 5.8025 | 1.3858–16.411 | 0.0205 |
| 1. Multivariate analysis results: tumour size assessment by one-dimensional measurement | |||
| CEA (>5) | 2.5515 | 1.2737–5.1982 | 0.0084 |
| Maximum tumour diameter measured in the lung window (>22.87) | 0.8542 | 0.5684–1.3005 | 0.455 |
| Maximum tumour diameter measured in the mediastinal window (>12.52) | 2.0830 | 1.2547–3.5846 | 0.0042 |
| Vessel invasion (yes) | 1.9875 | 0.9105–4.5147 | 0.0850 |
| Pleural invasion (yes) | 3.1140 | 1.4907–6.5355 | 0.0028 |
| Lavage cytology (positive) | 1.3536 | 0.3057–4.3067 | 0.6520 |
| 2. Multivariate analysis results: tumour size assessment by two-dimensional measurement | |||
| CEA (>5) | 2.3043 | 1.1495–4.6962 | 0.0188 |
| Area size measured in the mediastinal window (>90.3) | 1.8700 | 1.1673–3.1138 | 0.0089 |
| GGO area size (>167.0) | 0.5507 | 0.2988–0.8834 | 0.0111 |
| Vessel invasion (yes) | 1.8771 | 0.8511–4.2975 | 0.1193 |
| Pleural invasion (yes) | 2.8057 | 1.3542–5.8451 | 0.0059 |
| Lavage cytology (positive) | 1.3990 | 0.3154–4.4551 | 0.6186 |
| 3. Multivariate analysis results: tumour size assessment by area size ratio (TDR) | |||
| CEA (>5) | 2.4791 | 1.2506–5.0016 | 0.0095 |
| TDR (>0.778) | 0.3247 | 0.1293–0.6056 | <0.0001 |
| Vessel invasion (yes) | 2.2617 | 1.0095–4.7257 | 0.0267 |
| Pleural invasion (yes) | 2.6987 | 1.3090–5.5957 | 0.0076 |
| Lavage cytology (positive) | 1.3301 | 0.3045–4.1136 | 0.6660 |
| . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Univariate analysis results | |||
| Age (>67) | 1.1127 | 0.7937–1.5669 | 0.5340 |
| Sex (female) | 0.7922 | 0.5484–1.1166 | 0.1861 |
| Smoking history (no) | 0.8813 | 0.6155–1.2381 | 0.4700 |
| CEA (>5) | 3.4969 | 1.7800–6.9952 | 0.0003 |
| Maximum tumour diameter measured in the lung window (>22.87) | 1.6550 | 1.1746–2.3545 | 0.0036 |
| Maximum tumour diameter measured in the mediastinal window (>12.52) | 2.6526 | 1.7992–4.1892 | <.0001 |
| Procedure | 0.9262 | ||
| Wedge resection | 1 | ||
| Lobectomy | 0.8889 | 0.5230–1.7466 | |
| Segmentectomy | 1.1827 | 0.3501–2.6496 | |
| Vessel invasion (yes) | 5.7248 | 2.9146–11.450 | <0.0001 |
| Pleural invasion (yes) | 4.6497 | 2.3666–9.3025 | <0.0001 |
| Lavage cytology (positive) | 5.8025 | 1.3858–16.411 | 0.0205 |
| 1. Multivariate analysis results: tumour size assessment by one-dimensional measurement | |||
| CEA (>5) | 2.5515 | 1.2737–5.1982 | 0.0084 |
| Maximum tumour diameter measured in the lung window (>22.87) | 0.8542 | 0.5684–1.3005 | 0.455 |
| Maximum tumour diameter measured in the mediastinal window (>12.52) | 2.0830 | 1.2547–3.5846 | 0.0042 |
| Vessel invasion (yes) | 1.9875 | 0.9105–4.5147 | 0.0850 |
| Pleural invasion (yes) | 3.1140 | 1.4907–6.5355 | 0.0028 |
| Lavage cytology (positive) | 1.3536 | 0.3057–4.3067 | 0.6520 |
| 2. Multivariate analysis results: tumour size assessment by two-dimensional measurement | |||
| CEA (>5) | 2.3043 | 1.1495–4.6962 | 0.0188 |
| Area size measured in the mediastinal window (>90.3) | 1.8700 | 1.1673–3.1138 | 0.0089 |
| GGO area size (>167.0) | 0.5507 | 0.2988–0.8834 | 0.0111 |
| Vessel invasion (yes) | 1.8771 | 0.8511–4.2975 | 0.1193 |
| Pleural invasion (yes) | 2.8057 | 1.3542–5.8451 | 0.0059 |
| Lavage cytology (positive) | 1.3990 | 0.3154–4.4551 | 0.6186 |
| 3. Multivariate analysis results: tumour size assessment by area size ratio (TDR) | |||
| CEA (>5) | 2.4791 | 1.2506–5.0016 | 0.0095 |
| TDR (>0.778) | 0.3247 | 0.1293–0.6056 | <0.0001 |
| Vessel invasion (yes) | 2.2617 | 1.0095–4.7257 | 0.0267 |
| Pleural invasion (yes) | 2.6987 | 1.3090–5.5957 | 0.0076 |
| Lavage cytology (positive) | 1.3301 | 0.3045–4.1136 | 0.6660 |
TDR: tumour shadow disappearance rate; GGO: ground glass opacity; CEA: carcinoembryonic antigen; AIS: adenocarcinoma in situ; MIA: minimally invasive adenocarcinoma.
A multivariate analysis using one-dimensional measurement (maxDinLW and maxDinMW) as tumour size assessment identified high CEA (P = 0.0084), maxDinMW (P = 0.0042) and positive pleural invasion (P = 0.0028) as independent prognostic factors (Table 2). Positive vessel invasion was a marginal predictive factor for recurrence (P = 0.085), and maxDinLW was not identified as a predictive factor (P = 0.455).
A multivariate analysis using two-dimensional measurement (areainMW and GGO area size) as tumour size assessment identified high CEA (P = 0.0188), areainMW (P = 0.0089), larger GGO area size (P = 0.0111) and positive pleural invasion (P = 0.0059) as independent prognostic factors (Table 2).
A multivariate analysis using area size ratio (TDR) as tumour size assessment identified high CEA (P = 0.0095), larger TDR (P < 0.0001), positive vessel invasion (P = 0.0267) and positive pleural invasion (P = 0.0076) as independent prognostic factors (Table 2).
ROC Analysis
The area sizes measured for the ROC analysis were as follows: tumour area size in the lung window, median 194 mm2 (range 14.1–1193 mm2); tumour area size in the mediastinal window, median 47.1 mm2 (range 0–1193 mm2) and GGO area size [(tumour area size in the lung window) − (tumour area size in the mediastinal window)], median 103.6 mm2 (range 0–883.2 mm2; Table 1). The one-dimensional and two-dimensional analyses revealed that excluding the GGO component from the tumour size measurement could yield better prognostic performances for recurrence and pathological vessel invasion compared with including the GGO component (Fig. 2a and b). For pathological pleural invasion, the maxDinLW and maxDinMW were equivalent (Fig. 2c). The AUCs by GGO area size for recurrence, vessel invasion and pleural invasion were 0.612 (95% CI 0.713–0.511), 0.584 (95% CI 0.486–0.682) and 0.510 (95% CI 0.424–0.596), respectively. GGO area size alone did not show any predictive power. The AUC by TDR for recurrence was 0.808 (95% CI 0.742–0.875).
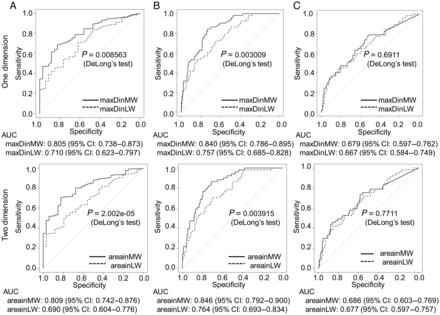
ROC curves and the area under the curves (AUCs) for predicting tumour recurrence (A), pathological vessel invasion (B) and pathological pleural invasion (C) according to one-dimensionally and two-dimensionally measured tumour sizes. Exclusion of the GGO component could yield better prognostic performance for recurrence and pathological vessel invasion. The size of the GGO area alone showed little predictive power.
Sub-classification analysis
The tumours with similar solid component sizes and different M/L ratios provided evidence for almost identical recurrence-free survival rates (Fig. 3 and Table 3).
| M/L . | 0 mm < maxDinMW ≤ 10 mm . | 10 mm < maxDinMW ≤ 20 mm . | 20 mm < maxDinMW . |
|---|---|---|---|
| 0 < M/L ≤ 0.4 | 0.908 (0.772–0.965)a | 0.832 (0.609–0.934)a | 0.500 (0.006–0.910)a |
| 0.4 < M/L ≤ 0.8 | 1.0 (1.0–1.0)a | 0.715 (0.494–0.853)a | 0.715 (0.443–0.871)a |
| 0.8 < M/L | No relevant cases | 0.750 (0.128–0.961)a | 0.391 (0.127–0.652)a |
| M/L . | 0 mm < maxDinMW ≤ 10 mm . | 10 mm < maxDinMW ≤ 20 mm . | 20 mm < maxDinMW . |
|---|---|---|---|
| 0 < M/L ≤ 0.4 | 0.908 (0.772–0.965)a | 0.832 (0.609–0.934)a | 0.500 (0.006–0.910)a |
| 0.4 < M/L ≤ 0.8 | 1.0 (1.0–1.0)a | 0.715 (0.494–0.853)a | 0.715 (0.443–0.871)a |
| 0.8 < M/L | No relevant cases | 0.750 (0.128–0.961)a | 0.391 (0.127–0.652)a |
maxDinMW: maximum tumour diameter in the mediastinal window; M/L: (tumour area size in the mediastinal window)/(tumour area size in the lung window).
a95% CI.
| M/L . | 0 mm < maxDinMW ≤ 10 mm . | 10 mm < maxDinMW ≤ 20 mm . | 20 mm < maxDinMW . |
|---|---|---|---|
| 0 < M/L ≤ 0.4 | 0.908 (0.772–0.965)a | 0.832 (0.609–0.934)a | 0.500 (0.006–0.910)a |
| 0.4 < M/L ≤ 0.8 | 1.0 (1.0–1.0)a | 0.715 (0.494–0.853)a | 0.715 (0.443–0.871)a |
| 0.8 < M/L | No relevant cases | 0.750 (0.128–0.961)a | 0.391 (0.127–0.652)a |
| M/L . | 0 mm < maxDinMW ≤ 10 mm . | 10 mm < maxDinMW ≤ 20 mm . | 20 mm < maxDinMW . |
|---|---|---|---|
| 0 < M/L ≤ 0.4 | 0.908 (0.772–0.965)a | 0.832 (0.609–0.934)a | 0.500 (0.006–0.910)a |
| 0.4 < M/L ≤ 0.8 | 1.0 (1.0–1.0)a | 0.715 (0.494–0.853)a | 0.715 (0.443–0.871)a |
| 0.8 < M/L | No relevant cases | 0.750 (0.128–0.961)a | 0.391 (0.127–0.652)a |
maxDinMW: maximum tumour diameter in the mediastinal window; M/L: (tumour area size in the mediastinal window)/(tumour area size in the lung window).
a95% CI.
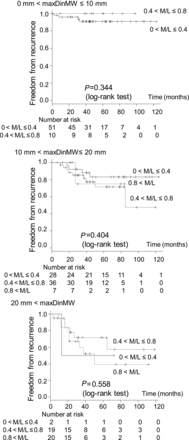
The sub-classified recurrence-free survival curves of patients with different M/L (tumour area size in the mediastinal window/tumour area size in the lung window) ratios. The tumours with similar solid component sizes and different M/L ratios showed almost identical recurrence-free survival rates.
Propensity score-matching analysis
For the propensity score-matching analysis, 34 patients in the GGO group were matched to 34 SLD patients. After propensity score matching, the distributions of sex, smoking history, CEA and maxDinMW were equivalent (Table 4). Additionally, the maxDinLW and the size of the lung window area were significantly larger in the GGO group than in the SLD group (Table 4). When the two groups were compared, selected procedures and the rates of positive vessel invasion, positive pleural invasion and positive pleural lavage cytology were similar. There was no significant difference in the recurrence-free survival rates between the two groups (Fig. 4). Five-year recurrence-free survival rates for the GGO group and the SLD group were 0.771 (95% CI 0.577–0.884) and 0.612 (95% CI 0.380–0.780), respectively.
Patient demographics for propensity score-matched cases from the GGO-predominant group and the solid component-predominant group
| . | GGO group . | SLD group . | eQQ mean . | P-value . |
|---|---|---|---|---|
| Number of patients | 34 | 34 | ||
| Age (mean ± SD) | 68.0 ± 9.7 | 67.8 ± 11.4 | 2.9706 | 0.9266a |
| Sex (male) | 21 | 18 | 0.0882 | 0.624b |
| Smoking history (no) | 10 | 9 | 0.0294 | 1b |
| CEA (>5) | 10 | 15 | 0.1471 | 0.314b |
| Maximum tumour diameter measured in the lung window (mm) (mean ± SD) | 27.81 ± 7.04 | 22.76 ± 5.93 | 0.0047a | |
| Maximum tumour diameter measured in the mediastinal window (mm) (mean ± SD) | 19.20 ± 5.07 | 20.92 ± 5.52 | 1.9118 | 0.2177a |
| Tumor area size in the lung window (mm2) (mean ± SD) | 423.1 ± 207.3 | 318.1 ± 166.1 | 0.0244a | |
| Tumour area size in the mediastinal window (mm2) (mean ± SD) | 202.5 ± 116.4 | 254.4 ± 146.4 | 0.2087a | |
| GGO area size = (tumour area size in the lung window)– (tumour area size in the mediastinal window) (mm2) (mean ± SD) | 220.6 ± 173.2 | 63.6 ± 57.7 | 1.575e-07a | |
| Anatomical resectionc (yes) | 31 | 26 | 0.186b | |
| Vessel invasion (yes) | 12 | 19 | 0.144b | |
| Pleural invasion (yes) | 12 | 12 | 1b | |
| Pleural lavage cytology (positive) | 2 | 1 | 1b | |
| Recurrence | 8 | 11 | 0.59b |
| . | GGO group . | SLD group . | eQQ mean . | P-value . |
|---|---|---|---|---|
| Number of patients | 34 | 34 | ||
| Age (mean ± SD) | 68.0 ± 9.7 | 67.8 ± 11.4 | 2.9706 | 0.9266a |
| Sex (male) | 21 | 18 | 0.0882 | 0.624b |
| Smoking history (no) | 10 | 9 | 0.0294 | 1b |
| CEA (>5) | 10 | 15 | 0.1471 | 0.314b |
| Maximum tumour diameter measured in the lung window (mm) (mean ± SD) | 27.81 ± 7.04 | 22.76 ± 5.93 | 0.0047a | |
| Maximum tumour diameter measured in the mediastinal window (mm) (mean ± SD) | 19.20 ± 5.07 | 20.92 ± 5.52 | 1.9118 | 0.2177a |
| Tumor area size in the lung window (mm2) (mean ± SD) | 423.1 ± 207.3 | 318.1 ± 166.1 | 0.0244a | |
| Tumour area size in the mediastinal window (mm2) (mean ± SD) | 202.5 ± 116.4 | 254.4 ± 146.4 | 0.2087a | |
| GGO area size = (tumour area size in the lung window)– (tumour area size in the mediastinal window) (mm2) (mean ± SD) | 220.6 ± 173.2 | 63.6 ± 57.7 | 1.575e-07a | |
| Anatomical resectionc (yes) | 31 | 26 | 0.186b | |
| Vessel invasion (yes) | 12 | 19 | 0.144b | |
| Pleural invasion (yes) | 12 | 12 | 1b | |
| Pleural lavage cytology (positive) | 2 | 1 | 1b | |
| Recurrence | 8 | 11 | 0.59b |
eQQ mean: the mean distance between the two empirical quantile functions; SD: standard deviation; GGO: ground glass opacity; GGO group: ground glass opacity-predominant group; SLD group: solid component-predominant group; CEA: carcinoembryonic antigen.
aWilcoxon rank sum test.
bFisher's exact test.
cAnatomical resection: segmentectomy or lobectomy.
Patient demographics for propensity score-matched cases from the GGO-predominant group and the solid component-predominant group
| . | GGO group . | SLD group . | eQQ mean . | P-value . |
|---|---|---|---|---|
| Number of patients | 34 | 34 | ||
| Age (mean ± SD) | 68.0 ± 9.7 | 67.8 ± 11.4 | 2.9706 | 0.9266a |
| Sex (male) | 21 | 18 | 0.0882 | 0.624b |
| Smoking history (no) | 10 | 9 | 0.0294 | 1b |
| CEA (>5) | 10 | 15 | 0.1471 | 0.314b |
| Maximum tumour diameter measured in the lung window (mm) (mean ± SD) | 27.81 ± 7.04 | 22.76 ± 5.93 | 0.0047a | |
| Maximum tumour diameter measured in the mediastinal window (mm) (mean ± SD) | 19.20 ± 5.07 | 20.92 ± 5.52 | 1.9118 | 0.2177a |
| Tumor area size in the lung window (mm2) (mean ± SD) | 423.1 ± 207.3 | 318.1 ± 166.1 | 0.0244a | |
| Tumour area size in the mediastinal window (mm2) (mean ± SD) | 202.5 ± 116.4 | 254.4 ± 146.4 | 0.2087a | |
| GGO area size = (tumour area size in the lung window)– (tumour area size in the mediastinal window) (mm2) (mean ± SD) | 220.6 ± 173.2 | 63.6 ± 57.7 | 1.575e-07a | |
| Anatomical resectionc (yes) | 31 | 26 | 0.186b | |
| Vessel invasion (yes) | 12 | 19 | 0.144b | |
| Pleural invasion (yes) | 12 | 12 | 1b | |
| Pleural lavage cytology (positive) | 2 | 1 | 1b | |
| Recurrence | 8 | 11 | 0.59b |
| . | GGO group . | SLD group . | eQQ mean . | P-value . |
|---|---|---|---|---|
| Number of patients | 34 | 34 | ||
| Age (mean ± SD) | 68.0 ± 9.7 | 67.8 ± 11.4 | 2.9706 | 0.9266a |
| Sex (male) | 21 | 18 | 0.0882 | 0.624b |
| Smoking history (no) | 10 | 9 | 0.0294 | 1b |
| CEA (>5) | 10 | 15 | 0.1471 | 0.314b |
| Maximum tumour diameter measured in the lung window (mm) (mean ± SD) | 27.81 ± 7.04 | 22.76 ± 5.93 | 0.0047a | |
| Maximum tumour diameter measured in the mediastinal window (mm) (mean ± SD) | 19.20 ± 5.07 | 20.92 ± 5.52 | 1.9118 | 0.2177a |
| Tumor area size in the lung window (mm2) (mean ± SD) | 423.1 ± 207.3 | 318.1 ± 166.1 | 0.0244a | |
| Tumour area size in the mediastinal window (mm2) (mean ± SD) | 202.5 ± 116.4 | 254.4 ± 146.4 | 0.2087a | |
| GGO area size = (tumour area size in the lung window)– (tumour area size in the mediastinal window) (mm2) (mean ± SD) | 220.6 ± 173.2 | 63.6 ± 57.7 | 1.575e-07a | |
| Anatomical resectionc (yes) | 31 | 26 | 0.186b | |
| Vessel invasion (yes) | 12 | 19 | 0.144b | |
| Pleural invasion (yes) | 12 | 12 | 1b | |
| Pleural lavage cytology (positive) | 2 | 1 | 1b | |
| Recurrence | 8 | 11 | 0.59b |
eQQ mean: the mean distance between the two empirical quantile functions; SD: standard deviation; GGO: ground glass opacity; GGO group: ground glass opacity-predominant group; SLD group: solid component-predominant group; CEA: carcinoembryonic antigen.
aWilcoxon rank sum test.
bFisher's exact test.
cAnatomical resection: segmentectomy or lobectomy.
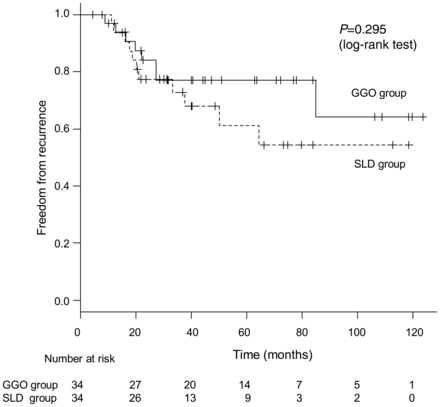
The recurrence-free survival curves of propensity score-matched patients from the GGO-predominant and solid component-predominant groups. No significant difference was observed.
DISCUSSION
In this study, we conducted four different types of analyses (multivariate analysis, ROC analysis, survival analysis according to subcategories and survival analysis of propensity score-matched pairs) to evaluate the impacts of GGO and the solid component on recurrence. The reason we did four kinds of analyses was to overcome a possible weakness of a single method. The retrieved results indicate that the GGO component had little influence on recurrence and could be regarded as biologically in situ. The prognosis was solely dependent on the solid component, which is consistent with previous reports [12, 13]. To elucidate the biological behaviour of the GGO component, we included the whole spectrum of lung adenocarcinomas with GGO components composing 0–100% of the tumour.
Previous radiological and pathological assessments of tumours of the same size successfully demonstrated that the ratio of the solid (invasive) component to the GGO (non-invasive) component is a good indicator of prognosis [3–5, 14] and that the predictive power could be maximized using a volumetric approach [15]. In this study, we evaluated TDR and reconfirmed that TDR is a good indicator of prognosis (AUC: 0.808). However, it is still unclear how these findings can be incorporated into a T assessment in an uncomplicated manner. The current study supports the idea that a T factor could be assessed simply by measuring the solid component size of the tumour.
We did not include F-18 fluorodeoxyglucose-positron emission tomography (FDG-PET) data in this study, because the current TNM classification does not require FDG-PET data. As noted by recent multi-center studies, predictive performance could become powerful by incorporating the FDG-PET maximum standardized uptake values [13, 16]. Because we did not include preoperative FDG-PET assessment data, invasive mucinous adenocarcinomas were measured as radiologically solid components, which may have attenuated the statistical power of this study. The heterogeneity of PET techniques and performance and the difficulty of inter-institutional standardization are challenges in need of solutions [16].
In this study, an ROC analysis revealed that GGO component exclusion from the measurement of tumour size could yield better prognostic performance for recurrence and pathological vessel invasion, but this was not true for pathological pleural invasion (Fig. 2). We identified a discrepancy between previous studies and ours for the predictive performance of pleural indentation [13, 16]. The tumour morphologies were evaluated only by tumour dimension in this study, and pleural indentation and spiculation around the tumour (observed using HRCT) were not evaluated, although they were previously assessed in several studies [3, 14, 15]. This may explain why the predictive performance of pleural invasion did not change by excluding the GGO component from the study measurements. The tumour location in the study population may also have played a role. If centrally located tumours had been dominant in the study population, the incidence of pleural involvement would have been less frequent.
The use of the optimal surgical procedure was not analysed in detail in this study. Because AISs/MIAs displayed 100% recurrence-free survival (Fig. 1), sublobar resection may be a possible option for peripheral AISs/MIAs if a sufficient surgical margin is guaranteed. Several recurrences were observed even in tumours with a subcentimeter solid component size (Fig. 3a), and the prognosis became worse as the solid component size increased (Fig. 3). A delayed cut-end recurrence after a limited resection for small GGO adenocarcinoma is possible [17]. Lobectomy and lymphadenectomy are the gold standard procedures for the surgical treatment of lung cancer. A sublobar resection of a tumour with a solid component is controversial and should be cautiously performed [18].
CONCLUSION
The current study may support the hypothesis that the GGO component can be excluded from the T-factor assessment of lung adenocarcinomas. GGO components even in part-solid tumours could be regarded as carcinomas in situ (Tis). Because this retrospective study consisted of a small number of patients with a relatively small events number (34 recurrence), and data collection was limited to those patients whose preoperative HRCT was available, the power of the retrieved results could be limited. Further assessment in a larger cohort will be necessary.
Conflict of interest: None declared.
REFERENCES
Author notes
Presented at the 20th European Conference on General Thoracic Surgery, Essen, Germany, 10-13 June 2012.




