-
PDF
- Split View
-
Views
-
Cite
Cite
Michael Poullis, James McShane, Mathew Shaw, Michael Shackcloth, Richard Page, Neeraj Mediratta, John Gosney, Smoking status at diagnosis and histology type as determinants of long-term outcomes of lung cancer patients, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 5, May 2013, Pages 919–924, https://doi.org/10.1093/ejcts/ezs464
Close - Share Icon Share
Abstract
The study aimed to determine the importance of smoking status at operation and histology type with regard to long-term survival after potential curative surgery for lung cancer.
We analysed a prospectively validated thoracic surgery database (n = 2485). We benchmarked our 5-year survival against the International Association for the Study of Lung Cancer (IALSC) results. Univariate and Cox multivariate analyses were performed for the study group and for isolated adenocarcinoma and squamous carcinoma histological subtypes.
Benchmarking failed to reveal any differences in survival of our study cohort compared with the IALSC results, P = 0.16. Univariate analysis revealed that non-smokers have a statistically better long-term outcome, P < 0.0001, than ever smokers. Patients with adenocarcinoma, n = 1216, had a worse outcome in ever smokers, P = 0.006. In patients with squamous carcinoma, n = 1065, smoking status made no difference, P = 0.4. Long-term survival was not significantly different for adenocarcinoma or squamous carcinoma, P = 0.87. Cox multivariate analysis revealed that patients with adenocarcinoma who were current smokers had a significantly worse long-term survival compared with ex-smokers and non-smokers (hazard ratio: 1.26, 95 confidence interval: 1.01–1.56), P = 0.04. Age, body mass index, sex, T stage, N stage, predicted postoperative forced expiratory volume in one second (FEV1), residual disease, alcohol consumption and oral diabetes were additional significant factors affecting long-term survival. Pneumonectomy, pack years, bronchial resection margin, New York Heart Association class, hypertension, previous cerebrovascular event, diet or insulin-controlled diabetes and previous myocardial infarction were excluded by the analysis as significant risk factors. Smoking status did not affect long-term survival in patients with squamous cell carcinoma.
Smoking status at time of surgery does not effect long-term survival in patients with squamous cell carcinoma. Smoking status makes a significant difference to the long-term outcomes of patients with adenocarcinoma even after adjustment for their risk factors. This implies that a histological classification of adenocarcinoma may incorporate genetically diverse adenocarcinomas with regard to prognosis.
INTRODUCTION
Patients who continue to smoke after a diagnosis of lung cancer is made are known to have poor long-term survival compared with those who have stopped [1, 2]. Smoking status can be arbitrarily divided into three groups, non-smokers, ex-smokers and current smokes. Ex-smokers and current smokers can be grouped together and classified as ever smokers [3].
Different subtypes of non-small cell lung cancer histology type are known to be prognostically important with regard to long-term survival [4].
Epidermal growth factor receptor (EGFR) mutations are more common in non-smoker females with adneocarcinaoma [5, 6]. We speculated that ex-smokers have a different carcinogenic milieu from current smokers who develop adenocarcinoama. This led us to hypothesize that current smokers would have a different survival compared with ex- and non-smokers if they developed adenocarcinoma, but no difference would exist in patients who developed squamous carcinoma.
METHODS
Definitions for study [7]
Ex-smoker: A regular smoker who had stopped smoking prior to the initiating of their investigations into diagnosing their lung cancer by a period in excess of 60 days before surgery.
Current smoker: A patient who was smoking regularly within 60 days of surgery.
Ever smokers: A patient who is either an ex- or current smoker.
Non-smokers: Patients who had never smoked on a regular basis.
Local ethics
Local institutional review board approval was granted for this study.
Protocol
We analysed a prospective thoracic database of patients who had undergone potentially curative (R0) surgical resection for non-small cell lung cancer (n = 2485). The time period was October 2001 to September 2011. The database is independently validated by a dedicated team of data managers. All pathology specimens were reported by a specialist thoracic pathologist (author J.G.) working at a university teaching hospital. Subanalysis was performed in patients with adenocarcinoma or squamous carcinoma.
As we did not have cause of death during follow-up, a Framingham risk score was calculated for each patient, to try and adjust for differing risks of cardiovascular death over a 5-year follow-up period.
A power calculation was performed to ensure the avoidance of an underpowered study.
Staging
Staging was defined as pathological staging to eliminate bias by ‘better’ preoperative staging due to current multislice computed tomography (CT) and positron emission tomography (PET) scanning. Routine intraoperative mediastinal lymph node sampling has always been undertaken in our unit. PET scanning became routine for all patients 5 years ago in our unit. All patients with medisastinal lymph nodes enlarged by CT criteria or hot on PET were biopsied preoperatively via mediastinoscopy, mediastinotomy or endobronchial ultrasound.
Follow-up
Survival data for all patients were obtained through the National Strategic Tracing Service, as previously described [8].
Group characteristics
Study group preoperative, operative and pathological characteristics are shown in Table 1. Pack-years were determined by multiplying the average number of cigarette packs smoked daily by the number of years smoked [9].
Preoperative, operative and pathological characteristics of the study group
| . | Current smokers (n = 812) . | Ex-smokers (n = 1522) . | Non-smokers (n = 151) . | P-value . |
|---|---|---|---|---|
| Preoperative | ||||
| Age (years) | 64.8 (64.2–65.4) | 68.9 (68.5–69.4) | 63.0 (60.5–65.4) | 0.002 |
| Female (%) | 438 (53.9) | 659 (43.3) | 99 (65.6) | <0.001 |
| Alcohol (units) | 7.2 (5.9–8.4) | 5.2 (4.5–5.9) | 2.7 (1.2–4.2) | 0.009 |
| BMI (kg/m2) | 25.0 (24.6–25.4) | 26.8 (26.4–27.1) | 26.8 (25.9–27.7) | 0.47 |
| Cardiac history (%) | 174 (21.4) | 492 (32.3) | 34 (22.5) | 0.0001 |
| Respiratory disease (%) | 235 (28.9) | 329 (21.6) | 9 (5.9) | <0.0001 |
| Diabetes (%) | 66 (8.2) | 174 (11.4) | 9 (6.12) | 0.02 |
| Hypertension (%) | 256 (31.5) | 638 (41.9) | 48 (31.8) | 0.79 |
| NYHA (%) | 0.001 | |||
| 1 | 512 (32.8) | 929 (59.5) | 120 (7.7) | |
| 2 | 276 (31.4) | 566 (64.4) | 37 (4.2) | |
| 3 | 61 (40.1) | 89 (58.6) | 2 (1.3) | |
| 4 | 2 (50.0) | 2 (50.0) | 0 (0.0) | |
| Pack years | 45.6 (43.6–47.7) | 36.2 (34.8–37.7) | 0.000 | <0.001 |
| Peripheral vascular disease (%) | 91 (11.2) | 189 (12.4) | 5 (3.6) | 0.008 |
| Renal failure (%) | 15 (1.9) | 39 (2.6) | 1 (0.7) | 0.23 |
| Predicted postoperative forced expiratory volume in one second (FEV1) (%) | 57.8 (56.6–58.9) | 59.0 (58.1–59.9) | 67.8 (64.5–71.1) | <0.001 |
| Framingham predicted risk of death over 5 years | 4.1 (3.8–4.4) | 6.2 (5.9–6.5) | 2.9 (2.3–3.4) | <0.001 |
| Intraoperative | ||||
| Pneumonectomy | 105 (12.9) | 196 (12.9) | 18 (11.9) | 0.51 |
| Lobectomy (%) | 606 (74.6) | 1136 (74.6) | 121 (80.7) | |
| Wedge (%) | 101 (12.5) | 190 (12.5) | 12 (7.4) | |
| Postoperative | ||||
| Adenocarcinoma (%) | 386 (47.5) | 717 (47.1) | 77 (51.0) | 0.01 |
| Squamous carcinoma (%) | 359 (44.2) | 667 (43.8) | 23 (15.3) | 0.08 |
| Tumour diameter (mm) | 37.5 (35.9–39.1) | 39.5 (38.2–40.9) | 36.1 (31.8–40.3) | <0.001 |
| Bronchial resection margin (mm) | 27.2 (25.8–28.6) | 29.7 (22.7–36.7) | 31.7 (27.8–35.7) | <0.001 |
| Stage | ||||
| Tumour | <0.001 | |||
| T1 | 333 (41.0) | 553 (36.3) | 57 (39.0) | |
| T2 | 437 (53.8) | 862 (56.6) | 83 (56.8) | |
| T3 | 42 (5.2) | 121 (7.1) | 6 (4.2) | |
| Node | 0.24 | |||
| N0 | 81 (10.0) | 197 (12.9) | 15 (9.9) | |
| N1 | 591 (72.8) | 1028 (67.5) | 119 (78.8) | |
| N2 | 141 (17.2) | 297 (19.6) | 17 (11.3) | |
| Adjuvant therapy | 69 (8.5) | 119 (7.8) | 17 (11.2) | 0.03 |
| In-hospital mortality (%) | 12 (1.5) | 47 (3.1) | 0 (0.0) | 0.009 |
| Median follow-up time (days) | 862 | 927 | 1029 | 0.48 |
| . | Current smokers (n = 812) . | Ex-smokers (n = 1522) . | Non-smokers (n = 151) . | P-value . |
|---|---|---|---|---|
| Preoperative | ||||
| Age (years) | 64.8 (64.2–65.4) | 68.9 (68.5–69.4) | 63.0 (60.5–65.4) | 0.002 |
| Female (%) | 438 (53.9) | 659 (43.3) | 99 (65.6) | <0.001 |
| Alcohol (units) | 7.2 (5.9–8.4) | 5.2 (4.5–5.9) | 2.7 (1.2–4.2) | 0.009 |
| BMI (kg/m2) | 25.0 (24.6–25.4) | 26.8 (26.4–27.1) | 26.8 (25.9–27.7) | 0.47 |
| Cardiac history (%) | 174 (21.4) | 492 (32.3) | 34 (22.5) | 0.0001 |
| Respiratory disease (%) | 235 (28.9) | 329 (21.6) | 9 (5.9) | <0.0001 |
| Diabetes (%) | 66 (8.2) | 174 (11.4) | 9 (6.12) | 0.02 |
| Hypertension (%) | 256 (31.5) | 638 (41.9) | 48 (31.8) | 0.79 |
| NYHA (%) | 0.001 | |||
| 1 | 512 (32.8) | 929 (59.5) | 120 (7.7) | |
| 2 | 276 (31.4) | 566 (64.4) | 37 (4.2) | |
| 3 | 61 (40.1) | 89 (58.6) | 2 (1.3) | |
| 4 | 2 (50.0) | 2 (50.0) | 0 (0.0) | |
| Pack years | 45.6 (43.6–47.7) | 36.2 (34.8–37.7) | 0.000 | <0.001 |
| Peripheral vascular disease (%) | 91 (11.2) | 189 (12.4) | 5 (3.6) | 0.008 |
| Renal failure (%) | 15 (1.9) | 39 (2.6) | 1 (0.7) | 0.23 |
| Predicted postoperative forced expiratory volume in one second (FEV1) (%) | 57.8 (56.6–58.9) | 59.0 (58.1–59.9) | 67.8 (64.5–71.1) | <0.001 |
| Framingham predicted risk of death over 5 years | 4.1 (3.8–4.4) | 6.2 (5.9–6.5) | 2.9 (2.3–3.4) | <0.001 |
| Intraoperative | ||||
| Pneumonectomy | 105 (12.9) | 196 (12.9) | 18 (11.9) | 0.51 |
| Lobectomy (%) | 606 (74.6) | 1136 (74.6) | 121 (80.7) | |
| Wedge (%) | 101 (12.5) | 190 (12.5) | 12 (7.4) | |
| Postoperative | ||||
| Adenocarcinoma (%) | 386 (47.5) | 717 (47.1) | 77 (51.0) | 0.01 |
| Squamous carcinoma (%) | 359 (44.2) | 667 (43.8) | 23 (15.3) | 0.08 |
| Tumour diameter (mm) | 37.5 (35.9–39.1) | 39.5 (38.2–40.9) | 36.1 (31.8–40.3) | <0.001 |
| Bronchial resection margin (mm) | 27.2 (25.8–28.6) | 29.7 (22.7–36.7) | 31.7 (27.8–35.7) | <0.001 |
| Stage | ||||
| Tumour | <0.001 | |||
| T1 | 333 (41.0) | 553 (36.3) | 57 (39.0) | |
| T2 | 437 (53.8) | 862 (56.6) | 83 (56.8) | |
| T3 | 42 (5.2) | 121 (7.1) | 6 (4.2) | |
| Node | 0.24 | |||
| N0 | 81 (10.0) | 197 (12.9) | 15 (9.9) | |
| N1 | 591 (72.8) | 1028 (67.5) | 119 (78.8) | |
| N2 | 141 (17.2) | 297 (19.6) | 17 (11.3) | |
| Adjuvant therapy | 69 (8.5) | 119 (7.8) | 17 (11.2) | 0.03 |
| In-hospital mortality (%) | 12 (1.5) | 47 (3.1) | 0 (0.0) | 0.009 |
| Median follow-up time (days) | 862 | 927 | 1029 | 0.48 |
Preoperative, operative and pathological characteristics of the study group
| . | Current smokers (n = 812) . | Ex-smokers (n = 1522) . | Non-smokers (n = 151) . | P-value . |
|---|---|---|---|---|
| Preoperative | ||||
| Age (years) | 64.8 (64.2–65.4) | 68.9 (68.5–69.4) | 63.0 (60.5–65.4) | 0.002 |
| Female (%) | 438 (53.9) | 659 (43.3) | 99 (65.6) | <0.001 |
| Alcohol (units) | 7.2 (5.9–8.4) | 5.2 (4.5–5.9) | 2.7 (1.2–4.2) | 0.009 |
| BMI (kg/m2) | 25.0 (24.6–25.4) | 26.8 (26.4–27.1) | 26.8 (25.9–27.7) | 0.47 |
| Cardiac history (%) | 174 (21.4) | 492 (32.3) | 34 (22.5) | 0.0001 |
| Respiratory disease (%) | 235 (28.9) | 329 (21.6) | 9 (5.9) | <0.0001 |
| Diabetes (%) | 66 (8.2) | 174 (11.4) | 9 (6.12) | 0.02 |
| Hypertension (%) | 256 (31.5) | 638 (41.9) | 48 (31.8) | 0.79 |
| NYHA (%) | 0.001 | |||
| 1 | 512 (32.8) | 929 (59.5) | 120 (7.7) | |
| 2 | 276 (31.4) | 566 (64.4) | 37 (4.2) | |
| 3 | 61 (40.1) | 89 (58.6) | 2 (1.3) | |
| 4 | 2 (50.0) | 2 (50.0) | 0 (0.0) | |
| Pack years | 45.6 (43.6–47.7) | 36.2 (34.8–37.7) | 0.000 | <0.001 |
| Peripheral vascular disease (%) | 91 (11.2) | 189 (12.4) | 5 (3.6) | 0.008 |
| Renal failure (%) | 15 (1.9) | 39 (2.6) | 1 (0.7) | 0.23 |
| Predicted postoperative forced expiratory volume in one second (FEV1) (%) | 57.8 (56.6–58.9) | 59.0 (58.1–59.9) | 67.8 (64.5–71.1) | <0.001 |
| Framingham predicted risk of death over 5 years | 4.1 (3.8–4.4) | 6.2 (5.9–6.5) | 2.9 (2.3–3.4) | <0.001 |
| Intraoperative | ||||
| Pneumonectomy | 105 (12.9) | 196 (12.9) | 18 (11.9) | 0.51 |
| Lobectomy (%) | 606 (74.6) | 1136 (74.6) | 121 (80.7) | |
| Wedge (%) | 101 (12.5) | 190 (12.5) | 12 (7.4) | |
| Postoperative | ||||
| Adenocarcinoma (%) | 386 (47.5) | 717 (47.1) | 77 (51.0) | 0.01 |
| Squamous carcinoma (%) | 359 (44.2) | 667 (43.8) | 23 (15.3) | 0.08 |
| Tumour diameter (mm) | 37.5 (35.9–39.1) | 39.5 (38.2–40.9) | 36.1 (31.8–40.3) | <0.001 |
| Bronchial resection margin (mm) | 27.2 (25.8–28.6) | 29.7 (22.7–36.7) | 31.7 (27.8–35.7) | <0.001 |
| Stage | ||||
| Tumour | <0.001 | |||
| T1 | 333 (41.0) | 553 (36.3) | 57 (39.0) | |
| T2 | 437 (53.8) | 862 (56.6) | 83 (56.8) | |
| T3 | 42 (5.2) | 121 (7.1) | 6 (4.2) | |
| Node | 0.24 | |||
| N0 | 81 (10.0) | 197 (12.9) | 15 (9.9) | |
| N1 | 591 (72.8) | 1028 (67.5) | 119 (78.8) | |
| N2 | 141 (17.2) | 297 (19.6) | 17 (11.3) | |
| Adjuvant therapy | 69 (8.5) | 119 (7.8) | 17 (11.2) | 0.03 |
| In-hospital mortality (%) | 12 (1.5) | 47 (3.1) | 0 (0.0) | 0.009 |
| Median follow-up time (days) | 862 | 927 | 1029 | 0.48 |
| . | Current smokers (n = 812) . | Ex-smokers (n = 1522) . | Non-smokers (n = 151) . | P-value . |
|---|---|---|---|---|
| Preoperative | ||||
| Age (years) | 64.8 (64.2–65.4) | 68.9 (68.5–69.4) | 63.0 (60.5–65.4) | 0.002 |
| Female (%) | 438 (53.9) | 659 (43.3) | 99 (65.6) | <0.001 |
| Alcohol (units) | 7.2 (5.9–8.4) | 5.2 (4.5–5.9) | 2.7 (1.2–4.2) | 0.009 |
| BMI (kg/m2) | 25.0 (24.6–25.4) | 26.8 (26.4–27.1) | 26.8 (25.9–27.7) | 0.47 |
| Cardiac history (%) | 174 (21.4) | 492 (32.3) | 34 (22.5) | 0.0001 |
| Respiratory disease (%) | 235 (28.9) | 329 (21.6) | 9 (5.9) | <0.0001 |
| Diabetes (%) | 66 (8.2) | 174 (11.4) | 9 (6.12) | 0.02 |
| Hypertension (%) | 256 (31.5) | 638 (41.9) | 48 (31.8) | 0.79 |
| NYHA (%) | 0.001 | |||
| 1 | 512 (32.8) | 929 (59.5) | 120 (7.7) | |
| 2 | 276 (31.4) | 566 (64.4) | 37 (4.2) | |
| 3 | 61 (40.1) | 89 (58.6) | 2 (1.3) | |
| 4 | 2 (50.0) | 2 (50.0) | 0 (0.0) | |
| Pack years | 45.6 (43.6–47.7) | 36.2 (34.8–37.7) | 0.000 | <0.001 |
| Peripheral vascular disease (%) | 91 (11.2) | 189 (12.4) | 5 (3.6) | 0.008 |
| Renal failure (%) | 15 (1.9) | 39 (2.6) | 1 (0.7) | 0.23 |
| Predicted postoperative forced expiratory volume in one second (FEV1) (%) | 57.8 (56.6–58.9) | 59.0 (58.1–59.9) | 67.8 (64.5–71.1) | <0.001 |
| Framingham predicted risk of death over 5 years | 4.1 (3.8–4.4) | 6.2 (5.9–6.5) | 2.9 (2.3–3.4) | <0.001 |
| Intraoperative | ||||
| Pneumonectomy | 105 (12.9) | 196 (12.9) | 18 (11.9) | 0.51 |
| Lobectomy (%) | 606 (74.6) | 1136 (74.6) | 121 (80.7) | |
| Wedge (%) | 101 (12.5) | 190 (12.5) | 12 (7.4) | |
| Postoperative | ||||
| Adenocarcinoma (%) | 386 (47.5) | 717 (47.1) | 77 (51.0) | 0.01 |
| Squamous carcinoma (%) | 359 (44.2) | 667 (43.8) | 23 (15.3) | 0.08 |
| Tumour diameter (mm) | 37.5 (35.9–39.1) | 39.5 (38.2–40.9) | 36.1 (31.8–40.3) | <0.001 |
| Bronchial resection margin (mm) | 27.2 (25.8–28.6) | 29.7 (22.7–36.7) | 31.7 (27.8–35.7) | <0.001 |
| Stage | ||||
| Tumour | <0.001 | |||
| T1 | 333 (41.0) | 553 (36.3) | 57 (39.0) | |
| T2 | 437 (53.8) | 862 (56.6) | 83 (56.8) | |
| T3 | 42 (5.2) | 121 (7.1) | 6 (4.2) | |
| Node | 0.24 | |||
| N0 | 81 (10.0) | 197 (12.9) | 15 (9.9) | |
| N1 | 591 (72.8) | 1028 (67.5) | 119 (78.8) | |
| N2 | 141 (17.2) | 297 (19.6) | 17 (11.3) | |
| Adjuvant therapy | 69 (8.5) | 119 (7.8) | 17 (11.2) | 0.03 |
| In-hospital mortality (%) | 12 (1.5) | 47 (3.1) | 0 (0.0) | 0.009 |
| Median follow-up time (days) | 862 | 927 | 1029 | 0.48 |
Benchmarking
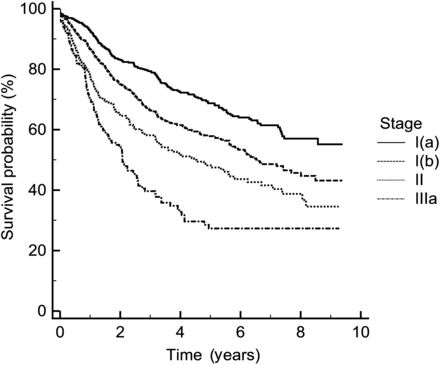
We benchmarked our 5-year survival against the International Association for the Study of Lung Cancer (IALSC) results [10], Fig. 1, to ensure the validity of our conclusions.
Univariate analysis
The Kaplan–Meier survival for patients who are non-smokers, ex-smokers and current smokers, and outcomes for the study group, and by histological type are shown in Fig. 2.
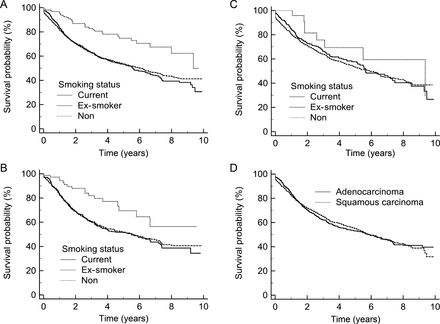
Smoking status and long-term survival. (A) Study group, current smokers n = 828, ex-smokers n = 1525, non-smokers n = 157. (B) Adenocarcinoma, current smokers n = 383, ex-smokers n = 714, non-smokers n = 75. (C) Squamous carcinoma, current smokers n = 358, ex-smokers n = 643, non-smokers n = 25. (D) Adenocarcinoma (n = 1216) vs squamous carcinoma (n = 1065), P = 0.87.
Multivariate analysis
Stepwise Cox multivariate regression analysis was utilised to determine the significant factors determining long-term survival. Entry criterion was P < 0.1, and removal criterion was P > 0.05. Multivariate Cox risk adjusted survival curves were created to demonstrate the effect of smoking status on long-term survival, Fig. 3. The covariates were plotted at their mean.
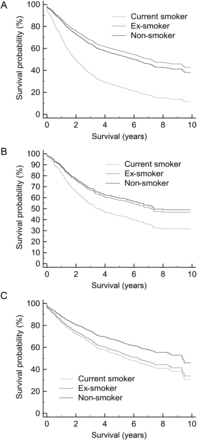
Cox risk adjusted survival of smoking status and long-term survival, plotted at the mean of the covariates. (A) Study group; (B) adenocarcinoma; (C) squamous carcinoma.
Statistical software
All statistical analysis other than the neuronal network was performed with MedCalc for Windows, (version 12.1.4, MedCalc Software, Mariakerke, Belgium).
RESULTS
One hundred per cent long-term follow-up was achieved.
Power calculation
With a median survival time of 866 days, a hazard ratio of 0.87, accrual time of 4000 days, additional follow-up time after recruitment of 4000 days, an alpha of 0.05 and a power of 0.8, we estimated that we needed 1532 patients, implying our analysis was not underpowered.
Study group
Study group preoperative, operative and pathological characteristics for current, ex-, and non-smokers are shown in Table 1. Univariate analysis revealed significant differences in age, P = 0.002, sex, P < 0.001, alcohol consumption, P = 0.009, cardiac history, P = 0.0001, respiratory disease, P < 0.0001, New York Heart Association (NYHA), P = 0.001, pack years, P < 0.001, peripheral vascular disease, P = 0.008, predicted postoperative forced expiratory volume in 1 s, P < 0.001, histology type, P = 0.01, tumour diameter (mm), P < 0.001, bronchial resection margin (mm), P < 0.001, T stage, P < 0.001 and in-hospital mortality, P = 0.009, between the three groups.
Too few patients had a mixed adenosquamous histology subtype to allow a meaningful analysis to be performed.
Benchmarking
Benchmarking at 5 years failed to reveal any significant differences between our institutions stage survival and the IALSC results, Fig. 1, P = 0.16.
Univariate survival analysis
Univariate analysis, Fig. 2, revealed a significant difference in long-term survival by smoking status, with non-smokers having a statistically better long-term outcome, P < 0.0001, than ever smokers [odds ratio: 0.45, 95% confidence interval (CI): 0.32–0.63]. Patients with adenocarcinoma also had a worse outcome in ever smokers, P = 0.006 (odds ratio: 0.47, 95% CI: 0.29–0.75). In patients with squamous carcinoma, smoking status made no difference, P = 0.4 (odds ratio: 0.94, 95% CI: 0.77–1.14).
The long-term survival was not significantly different for adenocarcinoma or squamous carcinoma, P = 0.87 (odds ratio: 0.99, 95% CI: 0.87–1.12).
Multivariate analysis
Cox multivariate regression analysis, Table 2 and Fig. 3, was performed on the whole group and then for just patients who had adenocarcinoma or squamous carcinoma.
Cox multivariate analysis for whole group, adenocarcinoma only and squamous carcinoma only
| Covariate . | Relative risk . | 95% CI of relative risk . | P-value . |
|---|---|---|---|
| Whole study group | |||
| Age | 1.0332 | 1.0245–1.0419 | <0.0001 |
| BMI | 1.0008 | 1.0001–1.0015 | 0.0358 |
| Female | 0.8229 | 0.7088–0.9554 | 0.0109 |
| T stage | |||
| 1 | 0.6616 | 0.5566–0.7865 | <0.0001 |
| 3 | 1.8653 | 1.4478–2.4031 | <0.0001 |
| 4 | 1.6968 | 1.1911–2.4172 | 0.0036 |
| N stage | |||
| 1 | 1.5205 | 1.2797–1.8066 | <0.0001 |
| 2 | 2.4497 | 1.9737–3.0406 | <0.0001 |
| Predicted postop FEV1 | 0.9873 | 0.9827–0.9920 | <0.0001 |
| Current smoker | 1.3283 | 1.1436–1.5428 | 0.0002 |
| Residual disease | 1.6093 | 1.1992–2.1596 | 0.0016 |
| Alcohol | 1.0050 | 1.0009–1.0091 | 0.0172 |
| Diabetes | 2.1096 | 1.2122–3.6713 | 0.0086 |
| Insulin | |||
| No history | 1.3704 | 1.1686–1.6070 | 0.0001 |
| Histology | |||
| Adenosquamous | 2.4476 | 1.0095–5.9344 | 0.0487 |
| Carcinoid | 0.1888 | 0.0705–0.5052 | 0.0010 |
| Squamous | 0.8353 | 0.7218–0.9666 | 0.0162 |
| Adenocarcinoma | |||
| Age | 1.0251 | 1.0129–1.0374 | 0.0001 |
| BMI | 1.0009 | 1.0002–1.0017 | 0.0160 |
| Female | 1.4256 | 1.1521–1.7639 | 0.0012 |
| T stage | |||
| T1 | 0.6469 | 0.5135–0.8150 | 0.0002 |
| N stage | |||
| N1 | 1.9139 | 1.4904–2.4577 | <0.0001 |
| N2 | 3.0033 | 2.2303–4.0442 | <0.0001 |
| N3 | 15.2426 | 2.0173–115.1696 | 0.0086 |
| Predicted postop FEV1 | 0.9828 | 0.9761–0.9896 | <0.0001 |
| Current smoker | 1.2576 | 1.0106–1.5649 | 0.0409 |
| Residual disease | 1.7298 | 1.1255–2.6585 | 0.0129 |
| Alcohol (units/week) | 1.0076 | 1.0022–1.0129 | 0.0058 |
| Diabetes | |||
| None | 1.5791 | 1.2588–1.9809 | 0.0001 |
| Diabetes | |||
| Oral | 1.8474 | 1.1394–2.9953 | 0.0133 |
| Squamous carcinoma | |||
| Age | 1.0364 | 1.0227–1.0503 | <0.0001 |
| T stage | |||
| T1 | 0.6827 | 0.5080–0.9176 | 0.0118 |
| T3 | 2.2029 | 1.5584–3.1138 | <0.0001 |
| T4 | 2.2505 | 1.3245–3.8239 | 0.0028 |
| N stage | |||
| N1 | 1.3248 | 1.0252–1.7120 | 0.0324 |
| N2 | 1.9391 | 1.3547–2.7755 | 0.0003 |
| Pneumonectomy | 1.5096 | 1.1229–2.0293 | 0.0066 |
| Dyspnoea NYHA | |||
| 0 | 0.7333 | 0.5561–0.9671 | 0.0288 |
| Dyspnoea NYHA | |||
| 1 | 0.7015 | 0.5497–0.8954 | 0.0046 |
| Diabetes | |||
| Insulin | 3.4653 | 1.5416–7.7896 | 0.0028 |
| Covariate . | Relative risk . | 95% CI of relative risk . | P-value . |
|---|---|---|---|
| Whole study group | |||
| Age | 1.0332 | 1.0245–1.0419 | <0.0001 |
| BMI | 1.0008 | 1.0001–1.0015 | 0.0358 |
| Female | 0.8229 | 0.7088–0.9554 | 0.0109 |
| T stage | |||
| 1 | 0.6616 | 0.5566–0.7865 | <0.0001 |
| 3 | 1.8653 | 1.4478–2.4031 | <0.0001 |
| 4 | 1.6968 | 1.1911–2.4172 | 0.0036 |
| N stage | |||
| 1 | 1.5205 | 1.2797–1.8066 | <0.0001 |
| 2 | 2.4497 | 1.9737–3.0406 | <0.0001 |
| Predicted postop FEV1 | 0.9873 | 0.9827–0.9920 | <0.0001 |
| Current smoker | 1.3283 | 1.1436–1.5428 | 0.0002 |
| Residual disease | 1.6093 | 1.1992–2.1596 | 0.0016 |
| Alcohol | 1.0050 | 1.0009–1.0091 | 0.0172 |
| Diabetes | 2.1096 | 1.2122–3.6713 | 0.0086 |
| Insulin | |||
| No history | 1.3704 | 1.1686–1.6070 | 0.0001 |
| Histology | |||
| Adenosquamous | 2.4476 | 1.0095–5.9344 | 0.0487 |
| Carcinoid | 0.1888 | 0.0705–0.5052 | 0.0010 |
| Squamous | 0.8353 | 0.7218–0.9666 | 0.0162 |
| Adenocarcinoma | |||
| Age | 1.0251 | 1.0129–1.0374 | 0.0001 |
| BMI | 1.0009 | 1.0002–1.0017 | 0.0160 |
| Female | 1.4256 | 1.1521–1.7639 | 0.0012 |
| T stage | |||
| T1 | 0.6469 | 0.5135–0.8150 | 0.0002 |
| N stage | |||
| N1 | 1.9139 | 1.4904–2.4577 | <0.0001 |
| N2 | 3.0033 | 2.2303–4.0442 | <0.0001 |
| N3 | 15.2426 | 2.0173–115.1696 | 0.0086 |
| Predicted postop FEV1 | 0.9828 | 0.9761–0.9896 | <0.0001 |
| Current smoker | 1.2576 | 1.0106–1.5649 | 0.0409 |
| Residual disease | 1.7298 | 1.1255–2.6585 | 0.0129 |
| Alcohol (units/week) | 1.0076 | 1.0022–1.0129 | 0.0058 |
| Diabetes | |||
| None | 1.5791 | 1.2588–1.9809 | 0.0001 |
| Diabetes | |||
| Oral | 1.8474 | 1.1394–2.9953 | 0.0133 |
| Squamous carcinoma | |||
| Age | 1.0364 | 1.0227–1.0503 | <0.0001 |
| T stage | |||
| T1 | 0.6827 | 0.5080–0.9176 | 0.0118 |
| T3 | 2.2029 | 1.5584–3.1138 | <0.0001 |
| T4 | 2.2505 | 1.3245–3.8239 | 0.0028 |
| N stage | |||
| N1 | 1.3248 | 1.0252–1.7120 | 0.0324 |
| N2 | 1.9391 | 1.3547–2.7755 | 0.0003 |
| Pneumonectomy | 1.5096 | 1.1229–2.0293 | 0.0066 |
| Dyspnoea NYHA | |||
| 0 | 0.7333 | 0.5561–0.9671 | 0.0288 |
| Dyspnoea NYHA | |||
| 1 | 0.7015 | 0.5497–0.8954 | 0.0046 |
| Diabetes | |||
| Insulin | 3.4653 | 1.5416–7.7896 | 0.0028 |
Cox multivariate analysis for whole group, adenocarcinoma only and squamous carcinoma only
| Covariate . | Relative risk . | 95% CI of relative risk . | P-value . |
|---|---|---|---|
| Whole study group | |||
| Age | 1.0332 | 1.0245–1.0419 | <0.0001 |
| BMI | 1.0008 | 1.0001–1.0015 | 0.0358 |
| Female | 0.8229 | 0.7088–0.9554 | 0.0109 |
| T stage | |||
| 1 | 0.6616 | 0.5566–0.7865 | <0.0001 |
| 3 | 1.8653 | 1.4478–2.4031 | <0.0001 |
| 4 | 1.6968 | 1.1911–2.4172 | 0.0036 |
| N stage | |||
| 1 | 1.5205 | 1.2797–1.8066 | <0.0001 |
| 2 | 2.4497 | 1.9737–3.0406 | <0.0001 |
| Predicted postop FEV1 | 0.9873 | 0.9827–0.9920 | <0.0001 |
| Current smoker | 1.3283 | 1.1436–1.5428 | 0.0002 |
| Residual disease | 1.6093 | 1.1992–2.1596 | 0.0016 |
| Alcohol | 1.0050 | 1.0009–1.0091 | 0.0172 |
| Diabetes | 2.1096 | 1.2122–3.6713 | 0.0086 |
| Insulin | |||
| No history | 1.3704 | 1.1686–1.6070 | 0.0001 |
| Histology | |||
| Adenosquamous | 2.4476 | 1.0095–5.9344 | 0.0487 |
| Carcinoid | 0.1888 | 0.0705–0.5052 | 0.0010 |
| Squamous | 0.8353 | 0.7218–0.9666 | 0.0162 |
| Adenocarcinoma | |||
| Age | 1.0251 | 1.0129–1.0374 | 0.0001 |
| BMI | 1.0009 | 1.0002–1.0017 | 0.0160 |
| Female | 1.4256 | 1.1521–1.7639 | 0.0012 |
| T stage | |||
| T1 | 0.6469 | 0.5135–0.8150 | 0.0002 |
| N stage | |||
| N1 | 1.9139 | 1.4904–2.4577 | <0.0001 |
| N2 | 3.0033 | 2.2303–4.0442 | <0.0001 |
| N3 | 15.2426 | 2.0173–115.1696 | 0.0086 |
| Predicted postop FEV1 | 0.9828 | 0.9761–0.9896 | <0.0001 |
| Current smoker | 1.2576 | 1.0106–1.5649 | 0.0409 |
| Residual disease | 1.7298 | 1.1255–2.6585 | 0.0129 |
| Alcohol (units/week) | 1.0076 | 1.0022–1.0129 | 0.0058 |
| Diabetes | |||
| None | 1.5791 | 1.2588–1.9809 | 0.0001 |
| Diabetes | |||
| Oral | 1.8474 | 1.1394–2.9953 | 0.0133 |
| Squamous carcinoma | |||
| Age | 1.0364 | 1.0227–1.0503 | <0.0001 |
| T stage | |||
| T1 | 0.6827 | 0.5080–0.9176 | 0.0118 |
| T3 | 2.2029 | 1.5584–3.1138 | <0.0001 |
| T4 | 2.2505 | 1.3245–3.8239 | 0.0028 |
| N stage | |||
| N1 | 1.3248 | 1.0252–1.7120 | 0.0324 |
| N2 | 1.9391 | 1.3547–2.7755 | 0.0003 |
| Pneumonectomy | 1.5096 | 1.1229–2.0293 | 0.0066 |
| Dyspnoea NYHA | |||
| 0 | 0.7333 | 0.5561–0.9671 | 0.0288 |
| Dyspnoea NYHA | |||
| 1 | 0.7015 | 0.5497–0.8954 | 0.0046 |
| Diabetes | |||
| Insulin | 3.4653 | 1.5416–7.7896 | 0.0028 |
| Covariate . | Relative risk . | 95% CI of relative risk . | P-value . |
|---|---|---|---|
| Whole study group | |||
| Age | 1.0332 | 1.0245–1.0419 | <0.0001 |
| BMI | 1.0008 | 1.0001–1.0015 | 0.0358 |
| Female | 0.8229 | 0.7088–0.9554 | 0.0109 |
| T stage | |||
| 1 | 0.6616 | 0.5566–0.7865 | <0.0001 |
| 3 | 1.8653 | 1.4478–2.4031 | <0.0001 |
| 4 | 1.6968 | 1.1911–2.4172 | 0.0036 |
| N stage | |||
| 1 | 1.5205 | 1.2797–1.8066 | <0.0001 |
| 2 | 2.4497 | 1.9737–3.0406 | <0.0001 |
| Predicted postop FEV1 | 0.9873 | 0.9827–0.9920 | <0.0001 |
| Current smoker | 1.3283 | 1.1436–1.5428 | 0.0002 |
| Residual disease | 1.6093 | 1.1992–2.1596 | 0.0016 |
| Alcohol | 1.0050 | 1.0009–1.0091 | 0.0172 |
| Diabetes | 2.1096 | 1.2122–3.6713 | 0.0086 |
| Insulin | |||
| No history | 1.3704 | 1.1686–1.6070 | 0.0001 |
| Histology | |||
| Adenosquamous | 2.4476 | 1.0095–5.9344 | 0.0487 |
| Carcinoid | 0.1888 | 0.0705–0.5052 | 0.0010 |
| Squamous | 0.8353 | 0.7218–0.9666 | 0.0162 |
| Adenocarcinoma | |||
| Age | 1.0251 | 1.0129–1.0374 | 0.0001 |
| BMI | 1.0009 | 1.0002–1.0017 | 0.0160 |
| Female | 1.4256 | 1.1521–1.7639 | 0.0012 |
| T stage | |||
| T1 | 0.6469 | 0.5135–0.8150 | 0.0002 |
| N stage | |||
| N1 | 1.9139 | 1.4904–2.4577 | <0.0001 |
| N2 | 3.0033 | 2.2303–4.0442 | <0.0001 |
| N3 | 15.2426 | 2.0173–115.1696 | 0.0086 |
| Predicted postop FEV1 | 0.9828 | 0.9761–0.9896 | <0.0001 |
| Current smoker | 1.2576 | 1.0106–1.5649 | 0.0409 |
| Residual disease | 1.7298 | 1.1255–2.6585 | 0.0129 |
| Alcohol (units/week) | 1.0076 | 1.0022–1.0129 | 0.0058 |
| Diabetes | |||
| None | 1.5791 | 1.2588–1.9809 | 0.0001 |
| Diabetes | |||
| Oral | 1.8474 | 1.1394–2.9953 | 0.0133 |
| Squamous carcinoma | |||
| Age | 1.0364 | 1.0227–1.0503 | <0.0001 |
| T stage | |||
| T1 | 0.6827 | 0.5080–0.9176 | 0.0118 |
| T3 | 2.2029 | 1.5584–3.1138 | <0.0001 |
| T4 | 2.2505 | 1.3245–3.8239 | 0.0028 |
| N stage | |||
| N1 | 1.3248 | 1.0252–1.7120 | 0.0324 |
| N2 | 1.9391 | 1.3547–2.7755 | 0.0003 |
| Pneumonectomy | 1.5096 | 1.1229–2.0293 | 0.0066 |
| Dyspnoea NYHA | |||
| 0 | 0.7333 | 0.5561–0.9671 | 0.0288 |
| Dyspnoea NYHA | |||
| 1 | 0.7015 | 0.5497–0.8954 | 0.0046 |
| Diabetes | |||
| Insulin | 3.4653 | 1.5416–7.7896 | 0.0028 |
After adjusting for risk factors, Cox regression analysis of the whole group demonstrated that current smokers (hazard ratio: 1.32, 95 CI: 1.14–1.54), P = 0.0002, had a significantly worse long-term survival, Fig. 3A. Age, body mass index (BMI), sex, T stage, N stage, predicted postoperative FEV1, residual disease, alcohol consumption, insulin-controlled diabetes and histology type were additional significant factors affecting long-term survival, Table 2. Pneumonectomy, pack years, bronchial resection margin, NYHA dyspnoea status, previous cerebrovascular event, hypertension, oral or diet-controlled diabetes and previous myocardial infarction were excluded by the analysis as significant risk factors.
Patients with adenocarcinoma who were current smokers had a significantly worse long-term survival compared with ex-smokers and non-smokers, even after adjustment for their risk factors, Fig. 3B (hazard ratio: 1.26, 95 CI: 1.01–1.56), P = 0.04. Age, BMI, sex, T stage, N stage, predicted postoperative FEV1, residual disease, alcohol consumption and oral diabetes were additional significant factors affecting long-term survival, Table 2. Pneumonectomy, pack years, bronchial resection margin, NYHA class, hypertension, previous cerebrovascular event, diet or insulin-controlled diabetes and previous myocardial infarction were excluded by the analysis as significant risk factors. The ex-smokers and non-smokers had very similar long-term survivals.
Smoking status did not affect long-term survival in patients with squamous cell carcinoma, Fig. 3C. Age, T Stage, N Stage, pneumonectomy, HYHA class 0 and 1 and insulin-controlled diabetes were significant factors affecting long-term survival, Table 2. BMI, sex, predicted postoperative FEV1, pack years, alcohol consumption (units/week), bronchial resection margin, hypertension, previous cerebrovascular event, diet or oral controlled diabetes and previous myocardial infarction were excluded by the analysis as significant risk factors.
DISCUSSION
Smoking status has a significant impact on long-term survival in patients undergoing potentially curative surgical resection of adenocarcinoma of the lung. As smoking status, after Cox risk adjustment, showed little difference in patients with squamous carcinoma of the lung, the biology of adenocarcinomas in current smokers, ex-smokers and non-smokers may be different.
EGFR status is a known prognostic indictor in lung cancer patients. The biology of adenocarcinomas is known to be dependent on the EGFR status, which is partly determined by smoking status. The EGFR status of squamous carcinomas is not related to smoking status. Thus, smoking status may determine EGFR status in adenocarcinomas, but not squamous carcinomas, and hence infer differing prognosis depending on histological type.
Never smokers have recently been described as having a better long-term survival than ever smokers [11]; however, this was in a predominantly non-surgical series. Our univariate and multivariate analyses confirmed this finding.
The finding that the risk factors we identified, Table 2, are known to affect long-term survival post lung cancer resectional surgery confirms the validity of our model [12].
Non-smokers who develop lung cancer are more likely to develop an adenocarcinoma than a squamous carcinoma and to have an EGFR mutation [5, 11]. The presence of this mutation has been shown to affect survival with adjuvant chemotherapy [13–15]. The relatively low incidence of adenocarcinoma in the non-smokers in our study may reflect the high incidence of partners who smoke in Liverpool, potentially exposing non-smokers to a significant smoking exposure, but we do not have the data to confirm or refute this postulate. It should be noted that we only had 151 non-smokers in our study, see limitations below, and the potential for a type I error exists.
Previous work has examined the outcome of patients with adenocarcinoma or squamous carcinoma of the lung; however, they failed to examine the effects of smoking status on long-term outcomes [16]. Previous work has also analysed the effect of smoking history prior to resection of Stage I lung cancer [17]. This demonstrated that pack years was a significant factor, however, histology type and predicted pulmonary function post surgery were not analysed.
We were unable to identify any risk factors that could differentiate the survival of ex- and current smokers. Adjusting for pack years failed to identify the increased smoking exposure as a risk factor [18], however, predicted postoperative FEV1 was a significant determinant of long-term survival in our study cohort.
The Framingham risk score has previously been shown to be an independent significant risk factor for long-term survival post-lung resectional surgery [19]. Smokers, non-smokers and ex-smokers had significantly different Framingham risk scores predicting death over 5 years on univariate analysis, however, after Cox multivariate analysis, smoking status was still a significant factor determining long-term survival in patients with adenocarcinoma, but not squamous carcinomas. The Framingham risk score was not significantly different between patients with adenocarcinoma and those with squamous carcinoma.
Adjuvant chemotherapy studies have failed to include smoking status, ex- or current, as a potential confounding variable in their analysis [20]. We have not analysed our adjuvant data as patients are partially selected on the postoperative performance status, introducing an error in the analysis due to selection bias.
Non-smokers, ex-smokers and current smokers may represent distinct biologically different patient populations, with regard to long-term survival after a potentially curative resection of non-small cell lung cancer. Current adjuvant studies do not adequately adjust for these biological survival differences in their study designs or analysis.
LIMITATIONS
We do not have post-discharge pack history information of current smokers and their post-discharge smoking habits. Data related to passive exposure due to environmental tobacco smoke and fumes were not available.
Causes of lung cancer in non-smokers are many and include: radon, second-hand smoke, asbestos exposure, aerosolized oils caused by cooking, other environmental and occupational exposures, family history of lung cancer, genetic predisposition, and potentially, human papillomavirus [21]. We do not have any of these variables recorded in our database.
PET scanning was introduced in the middle of this retrospective study period. PET scanning was a variable in the Cox regression analysis; however, it was not shown to be a significant factor determining long-term survival, as previously shown [22].
Our study was adequately powered with regard to smokers and ex-smokers; however, we are concerned that it is underpowered with regard to non-smokers. Our conclusion that ex-smokers have different long-term outcomes if they develop adenocarcinoma as opposed to squamous carcinoma, compared with current smokers, however, remains valid.
We do not have cause of death for our analysis. We have tried to adjust for cardiovascular risk of death via the use of the Framingham risk-prediction model for estimating the risk of death at 5 years, however, this has limitations.
We do not have any EGFR status data available.
Conflict of interest: none declared.




