-
PDF
- Split View
-
Views
-
Cite
Cite
Claudia Schmidtke, Hans-Hinrich Sievers, Alex Frydrychowicz, Michael Petersen, Michael Scharfschwerdt, Antje Karluss, Ulrich Stierle, Doreen Richardt, First clinical results with the new sinus prosthesis used for valve-sparing aortic root replacement, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 3, March 2013, Pages 585–590, https://doi.org/10.1093/ejcts/ezs318
Close - Share Icon Share
Abstract
Sinuses of Valsalva are important in assuring the physiological function of the aortic valve. This study evaluates short-term clinical results of the reimplantation technique for aortic valve-sparing root replacement using a new prosthesis with three separate sinuses of Valsalva (sinus prosthesis).
Between February 2009 and February 2011, a total of 23 patients (20 m/3 f; mean age 52 ± 14.8 years; range 24–70 years) with aortic root aneurysm underwent aortic valve-sparing procedures according to the David reimplantation technique using the new sinus prosthesis. Eighteen patients had tricuspid and five patients bicuspid aortic valves. All patients received clinical as well as echocardiographic examinations postoperatively (mean 13 ± 9.3 months; 0.3–28 months).
There was no death and no reoperation of the aortic valve. At latest follow-up, most patients were in New York Heart Association class I (n = 22; 95.7%). In 95.7% aortic valve regurgitation (AR) was 0 or 1+; one patient had AR 2+. Pressure gradients were between the normal range (mean pressure gradient 4.7 ± 1.9 mmHg). Echocardiographic images demonstrate physiological aortic root dimensions and configuration with three separate sinuses of Valsalva without systolic contact of leaflets to the wall.
The new sinus prosthesis provides near normal root geometry and hemodynamics in valve-sparing aortic root replacement using the reimplantation technique, applicable for tricuspid and also bicuspid aortic valves.
INTRODUCTION
Aortic valve-sparing procedures were introduced in the 1990s by Sarsam and Yacoub as valve remodelling [1] and by David and Feindel [2] as valve reimplantation technique in patients with root aneurysm and macroscopically intact cusps. The results of both these techniques have been reported to be excellent, avoiding the shortcomings of the alternatives: the unfavourable early degeneration of bioprostheses, especially in young patients, and, on the other hand, the thromboembolism, bleeding and lifelong anticoagulation treatment in mechanical valves [3–6]. The original reimplantation technique was performed with a straight tube graft. From a physiological point of view, a prosthetic graft with integrated sinuses of Valsalva might lead to even more physiological results, because the sinuses of Valsalva have positive effects on aortic cusp stress reduction [7] and prevent the leaflets from contacting the prosthetic wall during systole, which is frequently seen with a straight tube graft without sinuses [8]. For imitating the sinuses, some sort of sinus grafts and modifications of the techniques using straight grafts have been developed [9, 10]. None of these grafts has separate sinuses. Therefore, a new prosthesis was developed, which includes separate sinuses between straight pillars resembling the physiological cylindrical shape of the root. The first encouraging in vitro results have been published elsewhere [11]. This prospective observational study aims to analyse first clinical results after valve-sparing root replacement according to David's reimplantation technique using the new sinus prosthesis in patients with tricuspid and bicuspid valve pathology.
MATERIALS AND METHODS
The study was approved by the Ethics Committee of the Medical Faculty of the University of Luebeck, Germany.
Patient population
Between February 2009 and February 2011, 23 consecutive patients underwent aortic valve reimplantation with the new sinus prosthesis. Criteria for the new sinus prosthesis was the agreement of patients after information. Demographics and clinical data are shown in Table 1.
| Patients (n) | 23 |
| Male/female (n) | 20/3 |
| Mean age ± SD (years) (min–max) | 52.0 ± 13.2 (24.2–70.5) |
| NYHA class | |
| I | 12 (52.2%) |
| II | 5 (21.7%) |
| III | 6 (26.1%) |
| IV | 0 |
| Marfan syndrome (n) | 3 (13.0%) |
| Valve pathology | |
| Tricuspid (n) | 18 (78.3%) |
| Bicuspid (n) | 5 (21.7%) |
| Takayasu arteritis (n) | 1 (4.3%) |
| Dimensions, maximal (mm) | |
| Aortic root | 51.4 ± 8.9 |
| Ascending aorta | 50.6 ± 10.0 |
| Aortic valve regurgitation (n) | |
| 0+ | 2 |
| 1+ | 4 |
| 2+ | 6 |
| 3+ | 11 |
| Mean ± SD | 2.1 ± 1.0 |
| Patients (n) | 23 |
| Male/female (n) | 20/3 |
| Mean age ± SD (years) (min–max) | 52.0 ± 13.2 (24.2–70.5) |
| NYHA class | |
| I | 12 (52.2%) |
| II | 5 (21.7%) |
| III | 6 (26.1%) |
| IV | 0 |
| Marfan syndrome (n) | 3 (13.0%) |
| Valve pathology | |
| Tricuspid (n) | 18 (78.3%) |
| Bicuspid (n) | 5 (21.7%) |
| Takayasu arteritis (n) | 1 (4.3%) |
| Dimensions, maximal (mm) | |
| Aortic root | 51.4 ± 8.9 |
| Ascending aorta | 50.6 ± 10.0 |
| Aortic valve regurgitation (n) | |
| 0+ | 2 |
| 1+ | 4 |
| 2+ | 6 |
| 3+ | 11 |
| Mean ± SD | 2.1 ± 1.0 |
| Patients (n) | 23 |
| Male/female (n) | 20/3 |
| Mean age ± SD (years) (min–max) | 52.0 ± 13.2 (24.2–70.5) |
| NYHA class | |
| I | 12 (52.2%) |
| II | 5 (21.7%) |
| III | 6 (26.1%) |
| IV | 0 |
| Marfan syndrome (n) | 3 (13.0%) |
| Valve pathology | |
| Tricuspid (n) | 18 (78.3%) |
| Bicuspid (n) | 5 (21.7%) |
| Takayasu arteritis (n) | 1 (4.3%) |
| Dimensions, maximal (mm) | |
| Aortic root | 51.4 ± 8.9 |
| Ascending aorta | 50.6 ± 10.0 |
| Aortic valve regurgitation (n) | |
| 0+ | 2 |
| 1+ | 4 |
| 2+ | 6 |
| 3+ | 11 |
| Mean ± SD | 2.1 ± 1.0 |
| Patients (n) | 23 |
| Male/female (n) | 20/3 |
| Mean age ± SD (years) (min–max) | 52.0 ± 13.2 (24.2–70.5) |
| NYHA class | |
| I | 12 (52.2%) |
| II | 5 (21.7%) |
| III | 6 (26.1%) |
| IV | 0 |
| Marfan syndrome (n) | 3 (13.0%) |
| Valve pathology | |
| Tricuspid (n) | 18 (78.3%) |
| Bicuspid (n) | 5 (21.7%) |
| Takayasu arteritis (n) | 1 (4.3%) |
| Dimensions, maximal (mm) | |
| Aortic root | 51.4 ± 8.9 |
| Ascending aorta | 50.6 ± 10.0 |
| Aortic valve regurgitation (n) | |
| 0+ | 2 |
| 1+ | 4 |
| 2+ | 6 |
| 3+ | 11 |
| Mean ± SD | 2.1 ± 1.0 |
Surgical technique
Cardiopulmonary bypass was performed in the standard fashion. Blood cardioplegia was applied antegrade for myocardial protection. In cases requiring circulatory arrest, deep hypothermia (15–18°C) was induced, along with cold antegrade cerebral perfusion. Prior to each valve-sparing procedure, the aortic cusps were inspected macroscopically to ascertain the absence of large fenestrations and degenerations such as calcification, thickening and fibrosis, which are exclusion criteria for the procedure. Detailed descriptions of the valve implantation technique can be found elsewhere [2]; in summary, dissection of the aortic root was performed as proximal as possible, the sinuses of Valsalva were excised leaving a rim of 3–4 mm to the attachment of the cusps followed by the excision of the coronary buttons. The commissures were straightened in a parallel fashion using a 2/0 Teflon pledged commissural suture to provide an appropriate leaflet coaptation. The distance between the straightened commissures (inner wall) was measured with a measuring tape. Usually, the distance between the three commissures equalled up to ±1 mm. The largest diameter was taken as the size of the sinus prosthesis (Uni-Graft® W SINUS, B. Braun Melsungen AG, Germany, Table 2). The graft was described elsewhere [11] in brief: the graft consists of three sinuses of Valsalva connected by straight pillars resembling the inter-leaflet triangles. These are marked by a black suture facilitating geometrical arrangement of the patient's commissures during implantation. The straight configuration of the pillars allows for fixing the commissures at any appropriate level (Fig. 1). The base and the sinotubular junction are circular in shape (Fig. 1). The graft was trimmed to leave a 3–4 mm proximal shirt, which was fixed to the subannular position using 12–14 interrupted horizontal 2-0-Teflon felt pledgeted mattress sutures to get a suture line free of blood. The suture line in the graft follows the anatomic conditions, which is a small scalloped course in the inter-leaflet triangle between the left- and right-coronary sinus and a pronounced scalloped course between the right- and non-coronary sinus to prevent injury to the conduction system and the membranous septum (Fig. 1). At the base of the inter-leaflet triangle between the left- and non-coronary sinus, the suture line runs circular without scalloping. The proximal part of the commissural pillars at the inter-leaflet triangles was only slightly incised between the left- and right-coronary sinus, if the patient's triangle was prominent to avoid distortion of the prosthesis, but we did not incise the prosthesis between the right an non-coronary sinus and the non- and left-coronary sinus to prevent the dilatation of the annulus. The knots of the proximal suture line were tied carefully having a Hegar dilatator of 24–26 mm in diameter (related to body size) placed in the left ventricular outflow tract. This manoeuvre warrants a fine circular shape of the annulus without kinking in the sinus rim and without stenosis postoperatively. Care must be taken not to knot these proximal sutures too strongly to prevent injury to the often fragile tissue in the subannular position. The commissures were stretched softly and secured with the formerly placed 2/0-Teflon commissural sutures as U-stitches to the appropriate position in the straight pillars of the graft. This was followed by the implantation of the native valve into the prostheses, stitching the resection lines of the sinuses by a running 5-0 Prolene suture from inside the prosthesis [12]. The coronary ostia were reimplanted into the conduit by using the button technique. Once the root reconstruction was accomplished, the cusps were finally inspected. If any prolapse was detected, a central plication at the free edge of the cusps was performed with 6-0 Prolene sutures, reinforced with small pericardial pledgets. Thereafter, the distal anastomosis was performed.
| Patients (n) | 23 |
| Bypass time (min) | 211 ± 29 |
| Cross clamp time (min) | 177 ± 28 |
| Size of prosthesis (mm) | |
| Mean ± SD | 30 ± 1.2 |
| 28/30/32 mm (n) | 5/15/3 |
| Concomitant procedures (n) | |
| Mitral valve repair | 1 (4.3%) |
| Cusp intervention | 5 (21.7%) |
| Plication | 4 |
| Pericardial patch | 1 |
| CABG | 6 (26.1%) |
| Maze procedure | 2 (8.7%) |
| Patients (n) | 23 |
| Bypass time (min) | 211 ± 29 |
| Cross clamp time (min) | 177 ± 28 |
| Size of prosthesis (mm) | |
| Mean ± SD | 30 ± 1.2 |
| 28/30/32 mm (n) | 5/15/3 |
| Concomitant procedures (n) | |
| Mitral valve repair | 1 (4.3%) |
| Cusp intervention | 5 (21.7%) |
| Plication | 4 |
| Pericardial patch | 1 |
| CABG | 6 (26.1%) |
| Maze procedure | 2 (8.7%) |
CABG: coronary artery bypass graft surgery.
| Patients (n) | 23 |
| Bypass time (min) | 211 ± 29 |
| Cross clamp time (min) | 177 ± 28 |
| Size of prosthesis (mm) | |
| Mean ± SD | 30 ± 1.2 |
| 28/30/32 mm (n) | 5/15/3 |
| Concomitant procedures (n) | |
| Mitral valve repair | 1 (4.3%) |
| Cusp intervention | 5 (21.7%) |
| Plication | 4 |
| Pericardial patch | 1 |
| CABG | 6 (26.1%) |
| Maze procedure | 2 (8.7%) |
| Patients (n) | 23 |
| Bypass time (min) | 211 ± 29 |
| Cross clamp time (min) | 177 ± 28 |
| Size of prosthesis (mm) | |
| Mean ± SD | 30 ± 1.2 |
| 28/30/32 mm (n) | 5/15/3 |
| Concomitant procedures (n) | |
| Mitral valve repair | 1 (4.3%) |
| Cusp intervention | 5 (21.7%) |
| Plication | 4 |
| Pericardial patch | 1 |
| CABG | 6 (26.1%) |
| Maze procedure | 2 (8.7%) |
CABG: coronary artery bypass graft surgery.
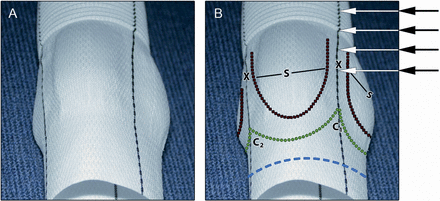
New sinus prosthesis (Uni-Graft® W SINUS, B. Braun Melsungen AG, Germany). (A) Original and (B) prepared as follows: Blue dotted line: resection line of the proximal prosthesis 3–4 mm apart from the nadir of the sinuses. Green dotted line: proximal suture line, in the inter-leaflet triangle between the right and non-coronary sinus (C1), the proximal suture line has a more scalloped shape than between the right- and left-coronary sinus (C2); C2 is slightly incised if the patient's inter-leaflet triangle is prominent. S: sinuses of Valsalva. X: commissural pillar between sinuses is straight in line with the proximal and distal circular tube and allows for the attachment of the patient's commissures at any height of the graft (arrows), which may even be extended into the tube graft if necessary. Black dotted line: suture line for reimplantation of the aortic valve.
Five of the patients had a bicuspid aortic valve type 1, L/R [13]. In these cases, the inter-leaflet triangle and commissure between the left- and right-coronary sinus are underdeveloped. The shorter than normal distance between this area and the fully developed commissures between the right- and non-coronary sinus and the left- and non-coronary sinus could be easily adapted to the prosthesis because the commissural pillars of the prosthesis have a certain width allowing the accommodation for asymmetry in commissural distance (Fig. 2). Another advantage of this prosthesis is the fact that the inter-leaflet triangles or pillars are straight, and run in parallel to the straight tube graft. Thus, any height of the patient's commissures can be adapted to the prosthesis, even if these commissures reach out of the sinus area into the tube graft.
![Adaptability of different commissural orientations to the sinus prosthesis. Cross section of the schematic new sinus prosthesis (A), this area as a top view (B) shows the width of the commissural pillars (X) of 45° facilitating adaptation to commissural orientations between 75° and 165°. This allows for an adaptation to most valve geometries, also for most bicuspid aortic valves, especially type 1 [13].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/43/3/10.1093_ejcts_ezs318/1/m_ezs31802.gif?Expires=1748105431&Signature=SlM3Efx9LH~zLh67V9MFH2LA0n4OPy5DLf5nYy96ZyavpC7syXrCMPrBpEI3CZ9BFY0jk6cyFE-pf9NxcCVWmuEJp8EfdIJGlCA2Fuzfbh7RzEXAgdhat2VA12XfTd1ePqIASDims~AOyQ70P15qYA1NeWYhR0v7BWl-AjrkNHkSaHJLnLlIvpntoCe3xmfIVb4e6N337hJBSWMLDLOla2fHiblfZ2LPGj07BhFK3m0ZxG~koKoiGT~TGZL2G48SfPvtXLIDRqrWHYVpzwIeRV2~1pSl4oDKobW9ycYs~91VtxIzZ8iFVFYSDwaWrF0p2TcyUaGIb4IUSCnySOHR2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Adaptability of different commissural orientations to the sinus prosthesis. Cross section of the schematic new sinus prosthesis (A), this area as a top view (B) shows the width of the commissural pillars (X) of 45° facilitating adaptation to commissural orientations between 75° and 165°. This allows for an adaptation to most valve geometries, also for most bicuspid aortic valves, especially type 1 [13].
In one patient with a tricuspid valve, magnetic resonance imaging (MRI) studies were carried out as follows: 3D cine phase contrast imaging with velocity sensitivity in all three spatial directions (so-called ‘4D flow MRI’) was performed on a clinical 3T scanner (Achieva, Philips, The Best, Netherlands) with a 16-channel phased-array body coil after written informed consent of the patients. Data acquisition was retrospectively electrocardiography-triggered and used a navigator approach to compensate for breathing-motion-related artefacts. Visualization, using 3D streamlines, time-resolved 3D particle paths and vector graph display, was achieved using the commercially available software tool GTFlow v1.6.6 (GyroTools, Zurich, Switzerland) on a standard PC. The tool allows for comprehensive visualization of data and a stepwise or animated image reading exploiting the three-dimensionality and temporal resolution of the data. Operative data are outlined in Table 2.
Follow-up
Clinical and echocardiographic follow-up was accomplished at discharge as well as on a regular basis via the outpatient clinic. Latest results were used for data analysis. During examination, patients were assessed according to the New York Heart Association (NYHA) functional classification. In addition, valve-related complications, performance and outcome analysis were reported according to the guidelines of the American Association for Thoracic Surgery and the Society of Thoracic Surgeons [14]. Additional follow-up data were obtained through a detailed review of medical records. Follow-up was complete, with a mean follow-up period 13 ± 9.3 months (maximum 28 months).
Echocardiographic examinations
The echocardiograms were performed on a Vivid 7 (GE). Dimensions for the aortic annulus, native and neoaortic sinuses and sinotubular junction were all measured offline during diastole and are presented as the mean value of at least two consecutive cardiac cycles. Aortic regurgitation was graded as none (0), mild (grade 1+), moderate (grade 2+), moderately severe (grade 3+) and severe (grade 4+) based on both colour flow and continuous-wave Doppler data acquisition according Hatle and Perry [15, 16].
RESULTS
There was no death and no reoperation on the aortic valve. Most patients were NYHA functional status I (n = 22; 95.7%; n = 1 NYHA II). No neurological, thromboembolic or bleeding events occurred. One patient required a pacemaker implantation during the follow-up period.
Echocardiographic imaging showed a physiological geometry of the aortic root with normal diameters (Fig. 3); mean diameters in detail were annulus 22.7 ± 2.6 mm, sinus of Valsalva 31.1 ± 3.6 mm and sinotubular junction 28.0 ± 2.9 mm. No contact of the leaflets to the wall of the prosthesis was seen (Fig. 4). Left ventricular function was normal with an ejection fraction of 66 ± 9.0%.
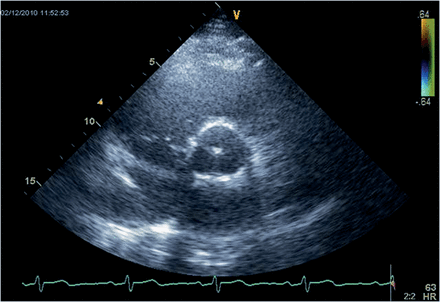
Transthoracic echocardiography of the new sinus prosthesis. Short axis view (B-mode) of the neoaortic root 22 months after implantation (same patient as in Figure 4) showing the normal configuration of the root.
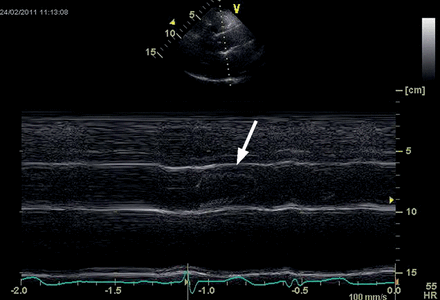
Transthoracic echocardiography of the new sinus prosthesis. Long axis view (M-mode) of the neoaortic root 22 months after implantation showing no contact of the aortic cusps with the aortic wall (arrow).
Aortic valve function
Maximum and mean pressure gradients were 8.1 ± 3.6 and 4.7 ± 1.9 mmHg, respectively. Aortic regurgitation was 0: n = 11, 1+: n = 11, 2+: n = 1. In one patient with tricuspid aortic valve flow, studies showed an almost normal central flow and vortices in one sinus and physiological right-handed helicity throughout the ascending aorta and aortic arch. Also, early diastolic retrograde flow was confirmed by vessel segmentation and flow quantification (Fig. 5).
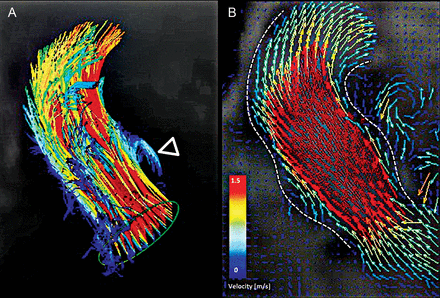
4D flow of new sinus prosthesis. Flow-sensitive time-resolved ‘4D’ magnetic resonance imaging with flow sensitivity in all three directions performed in one patient with a tricuspid valve. Colour-coding was performed with respect to the absolute measured velocities. 3D particle traces, resembling virtual massless particles emitted from the left ventricular outflow tract (A), and vector graphs (B) at a systolic time frame, depict the acquired flow patterns. Dashed white lines enhance the outline of the vessel. Four main features are represented: a systolic vortex that is filling the left-coronary sinus in A (open arrowhead) and laminar-like flow gently filling the root (B). Visualization performed with GTFlow v1.6.6, GyroTools, Zurich, Switzerland.
DISCUSSION
First surgical repair of aortic root aneurysms with a valve-bearing conduit was described in 1968 by Bentall and De Bono [17]. In case of mechanical valve, lifelong anticoagulation is necessary with the well-known risks of thromboembolism and bleeding, potentially causing extracardiac disastrous morbidity or mortality [6]. Also in bioprostheses, there are shortcomings such as limited durability, especially in the younger age patient group [5]. Thus, the development of reconstructive techniques to spare the patients’ own aortic valve is an attractive alternative to avoid the limitations of bioprostheses and mechanical valves.
Two principally different aortic valve-sparing procedures were introduced in the 1990s: the remodelling technique by Sarsam and Yacoub [1] and the reimplantation technique by David and Feindel [2]. Refinements and modifications of the reimplantation technique were carried out over time, especially to mimic the sinuses of Valsalva.
Some kind of neoaortic sinuses can be created by the plication of the cylindrical graft or by using commercially available grafts like the Vascutek® Gelweave Valsalva™ graft (Terumo Cardiovascular Systems Corp., Ann Arbor, MI) [9, 18]. In these grafts, the aortic sinuses, however, are not separate, as is the case in normal anatomy [3]. Whether the straight commissural pillars and the separate sinuses of Valsalva of the sinus prosthesis represent an advantage with regard to an increase coaptation area and thus less recurrence of aortic regurgitation over time compared with the Vascutek® Gelweave Valsalva™ graft without separate sinuses remains to be evaluated.
The presented new sinus prosthesis imitates the cylindrical shape of the native root with three separate sinuses of Valsalva and straight commissural pillars resembling the inter-leaflet triangles and commissures in between. Echocardiographically, the postoperative aortic root cannot be distinguished from a native aortic root configuration (Fig. 3). It remains stable in diameters over the follow-up period and pressure gradients over the valve are normal. Interestingly, the annulus diameter at follow-up was 22.7 ± 2.4 mm, although the proximal suture line stitches were tied on a 24 or 26 Hegar dilatator in place. This reduction in annulus diameter may be related to a release of the tension in the tied single sutures after the extraction of the dilatator. Nevertheless, this reduction did not lead to a pressure gradient at rest and probably even not at exercise as described by D'Ancona et al. [19]. There was only one patient with aortic regurgitation (AR) 1+ at discharge who had AR 2+ at follow-up, while all other patients had none or mild regurgitation, supporting the concept of the cylindrical graft with separate sinuses that may increase the coaptation area because the inter-leaflet triangles are not displaced outward. Also, the different heights of patients' commissures can be accomplished adequately because the commissural pillars of the graft are straight allowing for adequately fixing the commissures at any level.
Although the prosthesis may best fit to symmetrical tricuspid aortic valves, it is possible to use this graft for bicuspid valves [20]. In patients with bicuspid aortic valve type 1 [13] and preoperatively aortic valve regurgitation (AR) 3+ (n = 4), the valve could be implanted into the graft with excellent results (no AR in two patients, and AR 1+ in the other two patients). The width of the commissural pillars of the graft allows for the adaptation of commissural orientation between 75° and 165° (Fig. 2), which seems appropriate for the most commissural orientations in bicuspid aortic valves type 1, the most common form of bicuspid aortic valves [13, 21].
The flow in the patient with a tricuspid aortic valve showed the physiological characteristics of central flow, right-handed helicity and vortices in the sinuses (Fig. 5). Whether this flow pattern has any influence on long-term valve function and/or ascending aorta pathology remains speculative.
As already shown in in vitro tests [11], there was no contact of the cusps with the prosthesis (Fig. 4), which may favourably influence leaflet durability.
In conclusion, the new sinus prosthesis used for aortic valve-sparing root replacement can yield excellent short-term results in patients with tricuspid and bicuspid aortic valves and aortic root aneurysm. Longer term follow-up studies are necessary for the further judgment of this new prosthesis.
Conflict of interest: Hans-Hinrich Sievers discloses that he has a financial relationship with B. Braun Melsungen AG, Germany.




