-
PDF
- Split View
-
Views
-
Cite
Cite
Heinz Deschka, Stefan Erler, Matthias Machner, Lémir El-Ayoubi, Aiman Alken, Gerhard Wimmer-Greinecker, Surgery of the ascending aorta, root remodelling and aortic arch surgery with circulatory arrest through partial upper sternotomy: results of 50 consecutive cases, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 3, March 2013, Pages 580–584, https://doi.org/10.1093/ejcts/ezs341
Close - Share Icon Share
Abstract
Partial upper sternotomy is a routine approach to aortic valve surgery. For surgery of the ascending aorta or the aortic arch, this method is not well established yet.
From October 2007 to October 2010, 50 consecutive patients underwent procedures of the ascending aorta and the aortic arch using partial upper sternotomy. Thirty-six patients underwent replacement or tightening of the ascending aorta, 11 patients received additional replacement of the proximal arch and in 3 cases, a complete replacement of the aortic arch was performed. Thirty-nine patients underwent additional aortic valve surgery.
Mean operation time was 249 ± 51 min. Mean aortic cross-clamp and cardiopulmonary bypass time were 95 ± 27 and 141 ± 35 min, respectively. No conversion to conventional sternotomy was performed. All valves appeared competent on postoperative echocardiography. Survival was 100%. One re-exploration for bleeding was necessary. One stroke (2%) occurred, one pacemaker was implanted due to third-degree AV block and 16 patients (32%) experienced atrial fibrillation. One patient suffered from sternal wound infection. One patient needed reoperation due to severe aortic insufficiency on postoperative day 13. Median postoperative ventilation time was 13 h, median intensive care unit (ICU) and hospital stay were 22 h and 7 days, respectively.
Results show that minimally invasive surgical procedures of the ascending aorta and the aortic arch may be performed safely, with an excellent clinical outcomes and superior cosmesis. Short ICU and hospital stay indicate the beneficial effects of reduced surgical trauma for patient recovery.
INTRODUCTION
In the late 1990s, different minimally invasive approaches were introduced for aortic valve surgery in order to reduce surgical trauma and to improve the postoperative course [1–3]. Among these approaches, partial upper sternotomy (Fig. 1) proved to be a safe and effective alternative to median sternotomy and was correlated with clinical benefits [4–9]. Based on these favourable results, upper ministernotomy has also been proposed as a surgical access for complex cardiac procedures including aortic root, ascending aorta and proximal aortic arch surgery [10–13]. However, experience is still limited to a few centres.
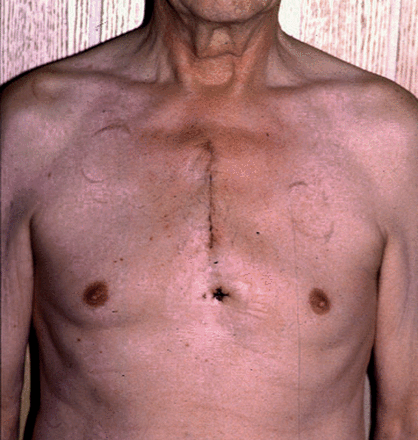
We report our experience and outcomes using ministernotomy on 50 patients in the surgery of pathologies of the ascending aorta and the aortic arch.
MATERIALS AND METHODS
Patient data and preoperative characteristics
We conducted a retrospective review of 50 consecutive patients who underwent surgical procedures of the ascending aorta and/or the aortic arch between October 2007 and October 2010. Demographic data of this patient cohort are presented in Table 1. Sixty-two percent of the patients were male (31 patients) and the mean age was 62.5 ± 8.9 years. Preoperatively calculated logistic EuroSCORE I was 10.0 ± 6.5.
| Sex (male) | 62.0% (31 patients) |
| Age | 62.5 ± 8.9 |
| EuroSCORE I (logistic) | 10.0 ± 6.5 |
| Ejection fraction (%) | 61.8 ± 12.0 |
| Raised body mass index | 36% (18 patients) |
| Arterial hypertension | 58% (29 patients) |
| COPD | 14% (7 patients) |
| Diabetes mellitus | 12% (6 patients) |
| Pulmonary hypertension | 6% (3 patients) |
| Peripheral arterial disease | 8% (4 patients) |
| Chronic renal insufficiency | 6% (3 patients) |
| Sex (male) | 62.0% (31 patients) |
| Age | 62.5 ± 8.9 |
| EuroSCORE I (logistic) | 10.0 ± 6.5 |
| Ejection fraction (%) | 61.8 ± 12.0 |
| Raised body mass index | 36% (18 patients) |
| Arterial hypertension | 58% (29 patients) |
| COPD | 14% (7 patients) |
| Diabetes mellitus | 12% (6 patients) |
| Pulmonary hypertension | 6% (3 patients) |
| Peripheral arterial disease | 8% (4 patients) |
| Chronic renal insufficiency | 6% (3 patients) |
COPD: chronic obstructive pulmonary disease.
| Sex (male) | 62.0% (31 patients) |
| Age | 62.5 ± 8.9 |
| EuroSCORE I (logistic) | 10.0 ± 6.5 |
| Ejection fraction (%) | 61.8 ± 12.0 |
| Raised body mass index | 36% (18 patients) |
| Arterial hypertension | 58% (29 patients) |
| COPD | 14% (7 patients) |
| Diabetes mellitus | 12% (6 patients) |
| Pulmonary hypertension | 6% (3 patients) |
| Peripheral arterial disease | 8% (4 patients) |
| Chronic renal insufficiency | 6% (3 patients) |
| Sex (male) | 62.0% (31 patients) |
| Age | 62.5 ± 8.9 |
| EuroSCORE I (logistic) | 10.0 ± 6.5 |
| Ejection fraction (%) | 61.8 ± 12.0 |
| Raised body mass index | 36% (18 patients) |
| Arterial hypertension | 58% (29 patients) |
| COPD | 14% (7 patients) |
| Diabetes mellitus | 12% (6 patients) |
| Pulmonary hypertension | 6% (3 patients) |
| Peripheral arterial disease | 8% (4 patients) |
| Chronic renal insufficiency | 6% (3 patients) |
COPD: chronic obstructive pulmonary disease.
Operative variables, operative morbidity and mortality, conversion rate, length of mechanical ventilation, ICU stay and hospital stay were evaluated. This study was approved by the local ethics committee (Medizinische Hochschule Hannover, Hannover, Germany).
Surgical procedure
We use ‘L’-shaped partial upper sternotomy as the standard approach for aortic valve, root, ascending aorta and proximal aortic arch operations. Exclusion criteria for a minimally invasive access at our institution are reoperations, aortic ruptures and acute type A dissections. Concomitant procedures may require a complete sternotomy as well.
Specific preoperative planning is not required to perform surgery of the ascending aorta and the aortic arch through upper ministernotomy. Routinely, all cardiac patients receive preoperative coronary angiography and echocardiography to assess cardiac status. A computed tomography scan of the chest is performed to determine exactly the dimension and location of the aortic aneurysm; unless it is gigantic, the operation can be performed in the routine fashion.
In our institution, partial upper sternotomy is performed in a standardized fashion. The 8-cm midline skin incision starts approximately two fingers below the sternal notch. Afterwards, the sternum is incised in an L-form manner from the sternal notch down to the left fourth intercostal space. The left internal mammary artery is not mobilized routinely. Only in cases of a tight and rigid rib cage, the left internal mammary artery is dissected from the surrounding fascia in the fourth intercostal space in order to spread the sternum more extensively. The surgical field is clearly exposed with pericardial traction sutures. For CBP in moderate hypothermia (32°C), the aorta is cannulated opposite to the origin of the brachiocephalic artery, and a two-stage cannula is placed in the right atrium. The left ventricle is vented through a small cannula placed in the right upper pulmonary vein or the pulmonary artery. The aortic cross-clamp is applied through the incision. Cold blood cardioplegia is administered in an antegrade fashion into the aortic root and the coronary ostia (Figs 2 and 3).
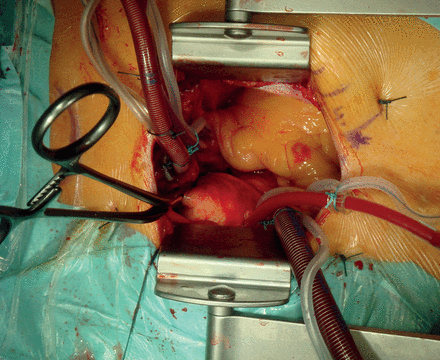
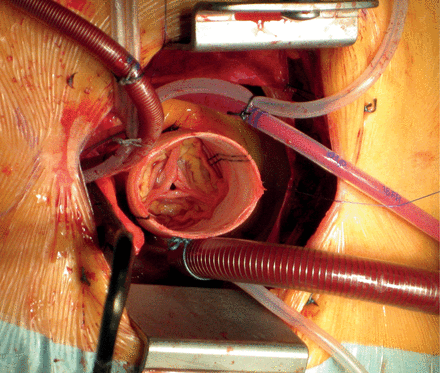
If circulatory arrest is required, hypothermia at 28°C is installed. For cerebral protection, selective antegrade cerebral perfusion is performed through the brachiocephalic trunk and the left common carotid artery. In 3 cases, deep hypothermic arrest (20°C) was required.
After closure of the aortotomy and filling of the heart, deairing is achieved through putting the patient in the Trendelenburg position and inflating the lungs. A suction line is placed in the highest point of the closed aorta to remove the remaining air. After insertion of temporary pacing wires and a chest tube, which is placed from a subxiphoidal position, the sternum is closed with 4–6 sternal wires in the conventional fashion.
Statistical analysis
Continuous variables are expressed as mean value ± standard deviation or as a median value. Categorical variables are expressed as percentages.
RESULTS
Surgical procedures and operative variables
The different surgical procedures and operative variables are summarized in Table 2. Aortic root replacement was performed with a stentless bioprosthesis implanted by the full root technique. Aortic valve reconstruction combined with replacement of the ascending aorta was performed using the ‘David procedure’. For aortoplasty, a longitudinal strip of the aorta, according to the size of the aortic ectasia, was resected and afterwards the longitudinal aortotomy was closed.
| . | EuroSCORE . | Operation time (min) . | CPB time (min) . | X-clamp time (min) . | Circulatory arrest/antegrade cerebral perfusion (min) . |
|---|---|---|---|---|---|
| Aortoplasty of ascending aorta (n = 20) | 10 ± 7.8 | 224.6 ± 42.1 | 128 ± 25.1 | 97.4 ± 21.2 | 0 |
| Plus aortic root replacement (n = 11) | 12.3 ± 9.7 | 224.6 ± 33.5 | 137.8 ± 21.1 | 108.6 ± 17.6 | 0 |
| Plus aortic valve replacement (n = 5) | 8.3 ± 4.9 | 234.8 ± 65.4 | 123.2 ± 33.4 | 88 ± 25.2 | 0 |
| Plus aortic valve reconstruction (n = 4) | 3.8 ± 2.3 | 219 ± 28 | 104.8 ± 18.3 | 71.8 ± 16.3 | 0 |
| Replacement of ascending aorta (n = 16) | 10.1 ± 5 | 258.7 ± 45.8 | 140.3 ± 43.7 | 97 ± 33.9 | 0 |
| Alone (n = 3) | 7.4 ± 2.1 | 203.3 ± 42.1 | 74.3 ± 7.1 | 52.7 ± 8.9 | 0 |
| Plus aortic valve replacement (n = 9) | 9.4 ± 4.8 | 247.8 ± 34.6 | 140.9 ± 30.4 | 94.5 ± 27.1 | 0 |
| Plus aortic valve reconstruction (n = 4) | 13.4 ± 6.8 | 322.3 ± 35.3 | 188.8 ± 36.8 | 135.3 ± 17.8 | 0 |
| Replacement of ascending aorta and proximal aortic arch (n = 11) | 10.3 ± 7.4 | 273.7 ± 63.2 | 161.8 ± 51.4 | 94.4 ± 38.4 | 14.8 ± 9.7 |
| Alone (n = 5) | 4.3 ± 2.1 | 215.5 ± 29.8 | 124.3 ± 19.8 | 60 ± 8 | 21.5 ± 5.8 |
| Plus aortic valve replacement (n = 1) | 11.1 | 258 | 142 | 76 | 10 |
| Plus aortic valve reconstruction (n = 5) | 13.3 ± 6.6 | 304.8 ± 39.9 | 183.8 ± 42.8 | 112.7 ± 28.1 | 10.2 ± 9.4 |
| Replacement of ascending aorta and complete aortic arch (n = 3) | 17.1 ± 14.8 | 295 ± 35 | 100.4 ± 59.6 | 83 ± 4 | 43.5 ± 3.5 |
| All procedures (n = 50) | 10.0 ± 6.5 | 249.5 ± 50.7 | 140.9 ± 35.3 | 95.5 ± 27.5 | 24.3 ± 9.6 |
| . | EuroSCORE . | Operation time (min) . | CPB time (min) . | X-clamp time (min) . | Circulatory arrest/antegrade cerebral perfusion (min) . |
|---|---|---|---|---|---|
| Aortoplasty of ascending aorta (n = 20) | 10 ± 7.8 | 224.6 ± 42.1 | 128 ± 25.1 | 97.4 ± 21.2 | 0 |
| Plus aortic root replacement (n = 11) | 12.3 ± 9.7 | 224.6 ± 33.5 | 137.8 ± 21.1 | 108.6 ± 17.6 | 0 |
| Plus aortic valve replacement (n = 5) | 8.3 ± 4.9 | 234.8 ± 65.4 | 123.2 ± 33.4 | 88 ± 25.2 | 0 |
| Plus aortic valve reconstruction (n = 4) | 3.8 ± 2.3 | 219 ± 28 | 104.8 ± 18.3 | 71.8 ± 16.3 | 0 |
| Replacement of ascending aorta (n = 16) | 10.1 ± 5 | 258.7 ± 45.8 | 140.3 ± 43.7 | 97 ± 33.9 | 0 |
| Alone (n = 3) | 7.4 ± 2.1 | 203.3 ± 42.1 | 74.3 ± 7.1 | 52.7 ± 8.9 | 0 |
| Plus aortic valve replacement (n = 9) | 9.4 ± 4.8 | 247.8 ± 34.6 | 140.9 ± 30.4 | 94.5 ± 27.1 | 0 |
| Plus aortic valve reconstruction (n = 4) | 13.4 ± 6.8 | 322.3 ± 35.3 | 188.8 ± 36.8 | 135.3 ± 17.8 | 0 |
| Replacement of ascending aorta and proximal aortic arch (n = 11) | 10.3 ± 7.4 | 273.7 ± 63.2 | 161.8 ± 51.4 | 94.4 ± 38.4 | 14.8 ± 9.7 |
| Alone (n = 5) | 4.3 ± 2.1 | 215.5 ± 29.8 | 124.3 ± 19.8 | 60 ± 8 | 21.5 ± 5.8 |
| Plus aortic valve replacement (n = 1) | 11.1 | 258 | 142 | 76 | 10 |
| Plus aortic valve reconstruction (n = 5) | 13.3 ± 6.6 | 304.8 ± 39.9 | 183.8 ± 42.8 | 112.7 ± 28.1 | 10.2 ± 9.4 |
| Replacement of ascending aorta and complete aortic arch (n = 3) | 17.1 ± 14.8 | 295 ± 35 | 100.4 ± 59.6 | 83 ± 4 | 43.5 ± 3.5 |
| All procedures (n = 50) | 10.0 ± 6.5 | 249.5 ± 50.7 | 140.9 ± 35.3 | 95.5 ± 27.5 | 24.3 ± 9.6 |
CPB time: cardiopulmonary bypass time.
X-clamp time: aortic cross-clamping time.
| . | EuroSCORE . | Operation time (min) . | CPB time (min) . | X-clamp time (min) . | Circulatory arrest/antegrade cerebral perfusion (min) . |
|---|---|---|---|---|---|
| Aortoplasty of ascending aorta (n = 20) | 10 ± 7.8 | 224.6 ± 42.1 | 128 ± 25.1 | 97.4 ± 21.2 | 0 |
| Plus aortic root replacement (n = 11) | 12.3 ± 9.7 | 224.6 ± 33.5 | 137.8 ± 21.1 | 108.6 ± 17.6 | 0 |
| Plus aortic valve replacement (n = 5) | 8.3 ± 4.9 | 234.8 ± 65.4 | 123.2 ± 33.4 | 88 ± 25.2 | 0 |
| Plus aortic valve reconstruction (n = 4) | 3.8 ± 2.3 | 219 ± 28 | 104.8 ± 18.3 | 71.8 ± 16.3 | 0 |
| Replacement of ascending aorta (n = 16) | 10.1 ± 5 | 258.7 ± 45.8 | 140.3 ± 43.7 | 97 ± 33.9 | 0 |
| Alone (n = 3) | 7.4 ± 2.1 | 203.3 ± 42.1 | 74.3 ± 7.1 | 52.7 ± 8.9 | 0 |
| Plus aortic valve replacement (n = 9) | 9.4 ± 4.8 | 247.8 ± 34.6 | 140.9 ± 30.4 | 94.5 ± 27.1 | 0 |
| Plus aortic valve reconstruction (n = 4) | 13.4 ± 6.8 | 322.3 ± 35.3 | 188.8 ± 36.8 | 135.3 ± 17.8 | 0 |
| Replacement of ascending aorta and proximal aortic arch (n = 11) | 10.3 ± 7.4 | 273.7 ± 63.2 | 161.8 ± 51.4 | 94.4 ± 38.4 | 14.8 ± 9.7 |
| Alone (n = 5) | 4.3 ± 2.1 | 215.5 ± 29.8 | 124.3 ± 19.8 | 60 ± 8 | 21.5 ± 5.8 |
| Plus aortic valve replacement (n = 1) | 11.1 | 258 | 142 | 76 | 10 |
| Plus aortic valve reconstruction (n = 5) | 13.3 ± 6.6 | 304.8 ± 39.9 | 183.8 ± 42.8 | 112.7 ± 28.1 | 10.2 ± 9.4 |
| Replacement of ascending aorta and complete aortic arch (n = 3) | 17.1 ± 14.8 | 295 ± 35 | 100.4 ± 59.6 | 83 ± 4 | 43.5 ± 3.5 |
| All procedures (n = 50) | 10.0 ± 6.5 | 249.5 ± 50.7 | 140.9 ± 35.3 | 95.5 ± 27.5 | 24.3 ± 9.6 |
| . | EuroSCORE . | Operation time (min) . | CPB time (min) . | X-clamp time (min) . | Circulatory arrest/antegrade cerebral perfusion (min) . |
|---|---|---|---|---|---|
| Aortoplasty of ascending aorta (n = 20) | 10 ± 7.8 | 224.6 ± 42.1 | 128 ± 25.1 | 97.4 ± 21.2 | 0 |
| Plus aortic root replacement (n = 11) | 12.3 ± 9.7 | 224.6 ± 33.5 | 137.8 ± 21.1 | 108.6 ± 17.6 | 0 |
| Plus aortic valve replacement (n = 5) | 8.3 ± 4.9 | 234.8 ± 65.4 | 123.2 ± 33.4 | 88 ± 25.2 | 0 |
| Plus aortic valve reconstruction (n = 4) | 3.8 ± 2.3 | 219 ± 28 | 104.8 ± 18.3 | 71.8 ± 16.3 | 0 |
| Replacement of ascending aorta (n = 16) | 10.1 ± 5 | 258.7 ± 45.8 | 140.3 ± 43.7 | 97 ± 33.9 | 0 |
| Alone (n = 3) | 7.4 ± 2.1 | 203.3 ± 42.1 | 74.3 ± 7.1 | 52.7 ± 8.9 | 0 |
| Plus aortic valve replacement (n = 9) | 9.4 ± 4.8 | 247.8 ± 34.6 | 140.9 ± 30.4 | 94.5 ± 27.1 | 0 |
| Plus aortic valve reconstruction (n = 4) | 13.4 ± 6.8 | 322.3 ± 35.3 | 188.8 ± 36.8 | 135.3 ± 17.8 | 0 |
| Replacement of ascending aorta and proximal aortic arch (n = 11) | 10.3 ± 7.4 | 273.7 ± 63.2 | 161.8 ± 51.4 | 94.4 ± 38.4 | 14.8 ± 9.7 |
| Alone (n = 5) | 4.3 ± 2.1 | 215.5 ± 29.8 | 124.3 ± 19.8 | 60 ± 8 | 21.5 ± 5.8 |
| Plus aortic valve replacement (n = 1) | 11.1 | 258 | 142 | 76 | 10 |
| Plus aortic valve reconstruction (n = 5) | 13.3 ± 6.6 | 304.8 ± 39.9 | 183.8 ± 42.8 | 112.7 ± 28.1 | 10.2 ± 9.4 |
| Replacement of ascending aorta and complete aortic arch (n = 3) | 17.1 ± 14.8 | 295 ± 35 | 100.4 ± 59.6 | 83 ± 4 | 43.5 ± 3.5 |
| All procedures (n = 50) | 10.0 ± 6.5 | 249.5 ± 50.7 | 140.9 ± 35.3 | 95.5 ± 27.5 | 24.3 ± 9.6 |
CPB time: cardiopulmonary bypass time.
X-clamp time: aortic cross-clamping time.
Total operation time was 249 ± 51 min. Mean aortic cross-clamp and cardiopulmonary bypass time were 95 ± 27 min and 141 ± 35 min, respectively. Twenty-two percent of all operations (11 patients) were performed in moderate hypothermia with circulatory arrest and antegrade cerebral perfusion. Mean cerebral perfusion time was 24 ± 10 min. In 3 cases (6%), circulatory arrest in deep hypothermia was necessary.
No conversion to full sternotomy was observed and all reconstructed (n = 13) or prosthetic aortic valves (n = 26) were competent in the echocardiography at the end of the surgery.
Early postoperative outcome
Early outcomes are presented in Table 3. Hospital survival was 100%. One patient needed a reoperation due to severe aortic valve insufficiency after primary successful aortic valve reconstruction after 13 days. In one case, a re-exploration due to bleeding was necessary. One subxiphoidal pericardiotomy (2%) and one pericardial puncture were performed to treat late pericardial effusions. Postoperative atrial fibrillation occurred in 32% and one pacemaker (2%) had to be implanted postoperatively due to third-degree AV block. The incidences of stroke, renal failure requiring dialysis and sternal infection were 2% each.
| . | Bleeding (ml) . | Ventilation time (h) . | Ventilation time (>24 h) . | ICU stay (h) . | ICU stay (>24 h) . | Hospital stay (days) . |
|---|---|---|---|---|---|---|
| Mean | 397.2 ± 246.6 | 28.6 ± 30.9 | 50 ± 50.9 | 11.0 ± 6.5 | ||
| Median | 300 (125–1200) | 12.7 (3–717) | 22 (8–1248) | 7 (5–81) | ||
| Percentage | 10.4 | 4.2 |
| . | Bleeding (ml) . | Ventilation time (h) . | Ventilation time (>24 h) . | ICU stay (h) . | ICU stay (>24 h) . | Hospital stay (days) . |
|---|---|---|---|---|---|---|
| Mean | 397.2 ± 246.6 | 28.6 ± 30.9 | 50 ± 50.9 | 11.0 ± 6.5 | ||
| Median | 300 (125–1200) | 12.7 (3–717) | 22 (8–1248) | 7 (5–81) | ||
| Percentage | 10.4 | 4.2 |
ICU: intensive care unit.
| . | Bleeding (ml) . | Ventilation time (h) . | Ventilation time (>24 h) . | ICU stay (h) . | ICU stay (>24 h) . | Hospital stay (days) . |
|---|---|---|---|---|---|---|
| Mean | 397.2 ± 246.6 | 28.6 ± 30.9 | 50 ± 50.9 | 11.0 ± 6.5 | ||
| Median | 300 (125–1200) | 12.7 (3–717) | 22 (8–1248) | 7 (5–81) | ||
| Percentage | 10.4 | 4.2 |
| . | Bleeding (ml) . | Ventilation time (h) . | Ventilation time (>24 h) . | ICU stay (h) . | ICU stay (>24 h) . | Hospital stay (days) . |
|---|---|---|---|---|---|---|
| Mean | 397.2 ± 246.6 | 28.6 ± 30.9 | 50 ± 50.9 | 11.0 ± 6.5 | ||
| Median | 300 (125–1200) | 12.7 (3–717) | 22 (8–1248) | 7 (5–81) | ||
| Percentage | 10.4 | 4.2 |
ICU: intensive care unit.
Mean postoperative blood loss was 397 ± 247 ml within 24 h. Fifty-two percent of the patients required blood transfusions and 2.3 ± 4.5 units (median 1.5) of red blood cells were administered. Mean ventilation time was 28.6 ± 30.9 h (median 12.7 h). 10.4% of the patients required mechanical ventilation support longer than 24 h. Mean ICU stay was 50 ± 51 h (median 22 h) and the percentage of patients with prolonged (>48 h) ICU treatment was 4.2%. Patients were discharged after 11.0 ± 6.5 days (median 7).
DISCUSSION
Positive experiences with upper hemisternotomy as an approach to aortic valve replacement are extensively published [4–9]. As a general consensus, results are favourable for minimally invasive aortic valve replacement regarding perioperative blood loss, ventilation time, ICU and general hospital stay. Beneficial effects on perioperative mortality were not demonstrated.
After a change of the department's chairman, the strategy to adopt this minimally invasive access as the standard approach for all isolated aortic valve operations was implemented in October 2007.
To meet the continuously growing demand of our patients and referring cardiologist for minimally invasive solutions for even complex surgical procedures, we have extended the indications for ministernotomy to any kind of elective procedure of the ascending aorta and the aortic arch.
For upper ministernotomy, various incisions have been proposed. The most frequently performed approaches are the ‘J’, ‘L’ or ‘T’ incision [7–9]. Our standard approach to aortic valve replacement is the ‘L’ hemisternotomy because of a, in our opinion, superior exposition of the valve compared with the ‘J’ incision. We have adopted this approach also for surgery of the ascending aorta and especially the aortic arch, where exposition of these anatomical structures is even more crucial. From our point of view, the ‘T’-shaped ministernotomy is not favourable because it bears an increased risk of sternal instability due to the bilateral osteotomy, which creates three sternal segments that have to be refixed.
Although ministernotomy has proven to be a feasible and safe approach to aortic valve surgery, the practicality and safety of this access for complex surgical procedures are not well documented. The largest cohorts reported in the literature are published by Totaro et al. [13], Tabata et al. [11] and Svenson et al. [10].
Totaro et al. [13] report on their experience with upper ministernotomy in 1126 cases. A cohort of 354 patients underwent complex surgical procedures, including 241 cases of ascending aorta and aortic arch replacements. The heterogeneity of surgical techniques and operative procedures as well as the lack of clear inclusion or exclusion criteria makes it very difficult to interpret reported results. But despite these serious limitations, the authors were able to show that complex surgical procedures can be performed through ministernotomy without compromising the postoperative outcomes.
Tabata et al. [11] retrospectively compared two matched cohorts of 79 patients each. In Group A, surgery of the ascending aorta and proximal aortic arch was performed minimally invasively, whereas in Group B, conventional sternotomy was performed. Total aortic arch replacement was not done. The decision for minimally invasive surgery was not based on clear criteria and was very much dependent on the surgeon's choice. The authors did not find significant differences in operative times, mortality and morbidity, but length of hospital stay and transfusion requirements were significantly reduced.
Svenson et al. [10] retrospectively analysed a series of 54 patients receiving either ascending aorta or combined ascending aorta and aortic arch repairs. In 41 patients, concomitant aortic valve replacement was performed. Thirty-three percent of these cases were reoperations. Results showed that minimal access aortic surgery can be done safely and has positive effects on postoperative recovery.
Our results support these findings. Early mortality is 0 and no conversion to complete sternotomy was required. Postoperative bleeding is within the range of previously reported values. The incidence of re-exploration due to postoperative bleeding and wound infection was minimal. Furthermore, the low percentage of prolonged ventilation time and ICU stay as well as a relatively short hospital stay indicate less respiratory compromise and a faster mobilization after minimally invasive surgery.
In a meta-analysis of studies using ministernotomy as an approach for surgery of the aortic root, the ascending aorta and aortic arch, Perrotta et al. [12] conclude that it is still difficult to evaluate the efficiency of partial upper sternotomy as a surgical approach to complex aortic procedures. The main concerns were the limited patient numbers in the few existing studies and the fact that these procedures were performed by only a few surgeons. This was justified by an assumed long learning curve. This assumed long learning curve and the supposed dependency on specialized surgeons are frequent points of criticism.
Our presented results clearly disprove these prejudices. Since October 2007, all our elective patients with any aortic disease have been consecutively treated through ministernotomy, and no selection has taken place. The operative treatment of these patients was not restricted to certain surgeons. Therefore, the presented data reflect early outcomes of our standard surgical treatment since October 2007, including the learning curve of all six cardiac staff surgeons of our department. To shorten this learning curve and accelerate the implementation of partial upper sternotomy as the standard surgical approach, all surgeons familiar with this technique assisted their less-experienced colleagues in several surgical cases until they felt comfortable with this new technique.
Compared with the preoperatively calculated logistic EuroSCORE I, an early outcome is excellent and proves that appropriate training and a team approach facilitate a fast introduction of this new technique with accurate surgical quality.
In the above-cited meta-analysis of Perrotta et al. [12], it is pointed out that so far there exist no reports about root remodelling or root reimplantation using ministernotomy. Our series includes 13 patients (26%) who received aortic root reconstruction and ascending aorta replacement or aortic valve reconstruction combined with aortoplasty. Another 11 patients (22%) received aortic root replacement together with aortoplasty.
This proves the high complexity of the surgical procedures in our series and shows that the indications for ministernotomy can be extended to reconstructive procedures of the aortic root combined with ascending aorta/aortic arch replacements.
Of course, this study suffers from limitations. Due to the retrospective study design and the absence of a control group, the statistical power is limited. The creation of a control group would not have been meaningful, because since 2007, all elective surgical procedures of the aortic root, ascending aorta and aortic arch have been performed through partial upper ministernotomy. Historical collectives are not comparable because, parallel to the adjustment of the surgical approach, significant changes in the surgical team took place. Although the statistical evidence suffers, this fact proves on the other hand that ministernotomy is feasible in daily routine even for complex cardiac procedures.
Regarding our results and despite the limitations of our study, we conclude that surgery of the ascending aorta and the aortic arch, performed through partial upper hemisternotomy, is safe, effective and offers excellent cosmesis. Operative times are comparable with conventional full sternotomy. Short ventilation times as well as short ICU and hospital stay indicate that patients benefit from reduced surgical trauma.
Conflict of interest: none declared.




