-
PDF
- Split View
-
Views
-
Cite
Cite
Kazuo Yamanaka, Yuki Hori, Jin Ikarashi, Takayoshi Kusuhara, Daisuke Nakatsuka, Keiichi Hirose, Takeshi Nishina, Masatoshi Fujita, Durability of aortic valve preservation with root reconstruction for acute type A aortic dissection , European Journal of Cardio-Thoracic Surgery, Volume 41, Issue 4, April 2012, Pages e32–e36, https://doi.org/10.1093/ejcts/ezr292
Close - Share Icon Share
Abstract
We evaluated the durability of aortic valve preservation with root reconstruction for acute type A aortic dissection (AAAD).
From January 2002 to March 2011, 140 patients [70 males, 68 ± 12 (SD) years] underwent emergency operation for AAAD. The aortic valve was preserved and one or more Valsalva sinuses were reconstructed. Techniques used for reconstruction were valve resuspension and additional reinforcement of the aortic root with Teflon felt patches, and gelatin-resorcinol-formaldehyde-glue (GRF-glue) was used for mending the dissection. The mean follow-up period was 44.0 ± 26.2 months. We classified the degree of aortic regurgitation (AR) into four grades (0, 1+, 2+ and 3+) using echocardiography. Based on a retrospective analysis of pre-operative echocardiographic findings, the 127 survivors were divided into two groups: group 1 (G1) included 98 patients with 0 or 1+ AR, and group 2 (G2) 29 patients with 2+ or 3+ AR. In addition, we measured the post-operative native aortic root dimension of AAAD patients with use of echocardiography or CT scan.
The operative mortality rate was 9.3% (13/140). Freedom from aortic root re-operation was 100%. Aortic root pseudoaneurysm formation and severe AR requiring aortic valve replacement did not occur. Pre-operative AR of 0.2 ± 0.4 in G1 did not deteriorate (0.5 ± 0.5 at discharge, 0.4 ± 0.4 at follow-up). Meanwhile, pre-operative AR of 2.4 ± 0.5 in G2 improved to 0.6 ± 0.5 (P < 0.05) at discharge and 1.0 ± 0.6 (P < 0.05) at follow-up. The native aortic root dimension in G2 at follow-up was significantly larger than G1 (36.0 ± 4.7 vs. 33.9 ± 5.0 mm).
Aortic valve preservation and root reconstruction appear to be an appropriate surgical approach to AAAD.
INTRODUCTION
Surgical management of patients with acute Stanford type A aortic dissection (AAAD) has substantially improved in recent years [1, 2]. Although operative survival at an acute stage still remains the main priority, recent technical approaches have been directed towards reducing the incidence of late complications to increase long-term survival and reduce the necessity of late re-operations. An aggressive surgical approach with total root replacement has been demonstrated to improve long-term prognosis in some subsets of patients, such as those with Marfan syndrome or dilated aortic root [3]. However, while preservation of native aortic valve and root is recommended in the majority of patients with no or mild aortic regurgitation (AR), the best surgical method for those presenting with significant AR is still being debated [4, 5]. The present study was designed to summarize our results after repair of AAAD focusing on the efficacy of a conservative approach to those cases complicated by significant AR.
PATIENTS AND METHODS
Patient characteristics
From January 2002 to March 2011, 142 consecutive patients underwent emergency operation for AAAD. Two patients with annulo-aortic ectasia (1.4%), who were indicated for a composite replacement of the aortic valve and root, were excluded from subsequent analysis. The remaining 140 patients [70 males, 68.0 ± 9.5 (SD) years], who were treated with a replacement of the ascending aorta and varying portions of the aortic arch with a Dacron prosthesis, were included in this study. Two patients had previously undergone thoracic aortic endovascular repair, two patients graft replacement for thoracoabdominal aortic aneurysm, and another patient coronary fistula operation. One-third of the patients were operated on under the condition of cardiogenic shock. Associated procedures included partial or total aortic arch replacement in 25 patients, CABG in two patients and exploratory laparotomy in two patients. Further pre-operative patient characteristics are shown in Table 1.
| Age (years) | 68.0 ± 9.5 | |
| BSA (m2) | 1.58 ± 0.18 | |
| Male:female | 70:70 | |
| Hypertension [n (%)] | 83 (59.3) | |
| Coronary artery disease [n (%)] | 6 (4.2) | |
| Diabetes mellitus [n (%)] | 3 (2.1) | |
| Current smoker [n (%)] | 56 (40.0) | |
| Chronic renal insufficiency [n (%)] | 6 (4.2) | |
| Pulmonary disease [n (%)] | 2 (1.4) | |
| Marfan syndrome [n (%)] | 2 (1.4) | |
| Cardiogenic shock [n (%)] | 45 (32.1) | |
| Malperfusion [n (%)] | 11 (7.9) | |
| History of cerebrovascular accident [n (%)] | 15 (10.7) | |
| Previous cardiac operation [n (%)] | 1 (0.7) |
| Age (years) | 68.0 ± 9.5 | |
| BSA (m2) | 1.58 ± 0.18 | |
| Male:female | 70:70 | |
| Hypertension [n (%)] | 83 (59.3) | |
| Coronary artery disease [n (%)] | 6 (4.2) | |
| Diabetes mellitus [n (%)] | 3 (2.1) | |
| Current smoker [n (%)] | 56 (40.0) | |
| Chronic renal insufficiency [n (%)] | 6 (4.2) | |
| Pulmonary disease [n (%)] | 2 (1.4) | |
| Marfan syndrome [n (%)] | 2 (1.4) | |
| Cardiogenic shock [n (%)] | 45 (32.1) | |
| Malperfusion [n (%)] | 11 (7.9) | |
| History of cerebrovascular accident [n (%)] | 15 (10.7) | |
| Previous cardiac operation [n (%)] | 1 (0.7) |
Values are presented as the mean ± SD.
BSA: body surface area.
| Age (years) | 68.0 ± 9.5 | |
| BSA (m2) | 1.58 ± 0.18 | |
| Male:female | 70:70 | |
| Hypertension [n (%)] | 83 (59.3) | |
| Coronary artery disease [n (%)] | 6 (4.2) | |
| Diabetes mellitus [n (%)] | 3 (2.1) | |
| Current smoker [n (%)] | 56 (40.0) | |
| Chronic renal insufficiency [n (%)] | 6 (4.2) | |
| Pulmonary disease [n (%)] | 2 (1.4) | |
| Marfan syndrome [n (%)] | 2 (1.4) | |
| Cardiogenic shock [n (%)] | 45 (32.1) | |
| Malperfusion [n (%)] | 11 (7.9) | |
| History of cerebrovascular accident [n (%)] | 15 (10.7) | |
| Previous cardiac operation [n (%)] | 1 (0.7) |
| Age (years) | 68.0 ± 9.5 | |
| BSA (m2) | 1.58 ± 0.18 | |
| Male:female | 70:70 | |
| Hypertension [n (%)] | 83 (59.3) | |
| Coronary artery disease [n (%)] | 6 (4.2) | |
| Diabetes mellitus [n (%)] | 3 (2.1) | |
| Current smoker [n (%)] | 56 (40.0) | |
| Chronic renal insufficiency [n (%)] | 6 (4.2) | |
| Pulmonary disease [n (%)] | 2 (1.4) | |
| Marfan syndrome [n (%)] | 2 (1.4) | |
| Cardiogenic shock [n (%)] | 45 (32.1) | |
| Malperfusion [n (%)] | 11 (7.9) | |
| History of cerebrovascular accident [n (%)] | 15 (10.7) | |
| Previous cardiac operation [n (%)] | 1 (0.7) |
Values are presented as the mean ± SD.
BSA: body surface area.
Surgical technique
The procedure started with a median sternotomy, followed by cannulation through the right atrial appendage and the ascending aorta under epiaortic echo guiding with the Seldinger method. After the patient was on extracorporeal circulation (ECC), a left ventricular decompression line was inserted through the right superior pulmonary vein. The patient was then cooled down to a bladder temperature of 25–30°C at which point the ECC was discontinued. We then installed the antegrade selective cerebral perfusion (ASCP) and subsequently protected the heart with cold blood antegrade and retrograde cardioplegia. The eventual arch procedure and distal ascending aortic anastomosis were performed under total circulatory arrest. Once this was completed, ASCP was stopped and antegrade perfusion was resumed through a side arm of the prosthesis. Then air was removed from the prosthesis and the prosthesis was clamped. The proximal ascending aortic procedure was performed during the subsequent rewarming.
All of the patients included in this study had a reconstruction of one or more of the Valsalva sinuses that were affected by the dissection. The aortic root was reconstructed with Teflon felt and gelatin–resorcinol–formaldehyde-glue (GRF-glue, Fii, Saint-Just Malmont, France). GRF-glue was inserted between the dissected aortic layers.
The aortic valve was resuspended by a commissuroplasty, using a U-stitch pledget in all patients. The ascending aorta was replaced with a prosthesis starting at the level of the sinotubular junction in all but three patients who underwent a local repair of the intimal tear or partial remodelling method [6], and aortic valve repair for cusp prolapse was performed in two patients.
Operation time, ECC time, aortic cross-clamp time and ASCP time were 323 ± 85, 171 ± 46, 103 ± 32, 53 ± 33 min, respectively. Minimum bladder temperature during ECC was 27.3 ± 2.8°C.
Classification of AR and measurement of aortic root dimension
The 127 survivors, 64 males and 63 females with a mean age of 68 ± 12 years, were divided into two groups based on the degree of AR present at the time of operation. Data were obtained by retrospective analysis of the pre-operative transthoracic echocardiographic (TTE) findings or the intra-operative transesophageal echocardiographic findings. Because all pre-operative examinations were done on an emergency basis with the main purpose of confirming the diagnosis of aortic dissection, the degree of AR could only be classified as absent (0), trivial-mild (1+), moderate (2+) or severe (3+). In addition, we measured the post-operative native root dimensions of AAAD patients by TTE and/or CT scan.
Post-operative follow-up
Follow-up was achieved either by yearly outpatient visit or by phone and/or letter to the patient and the referring physician. A file on the current status, medication, morbidity and mortality was completed for each patient. Follow-up was closed on 31 August 2011. Follow-up of the 127 discharged patients was completed in 112 patients (88%) with 487 patient-years (mean 3.8 ± 2.2 years, ranging from 6 months to 9.9 years).
Statistical analysis
All values are expressed as the mean ± SD. Comparative analysis of parametric parameters was performed with Student's t-test, and the χ2 tests were used to analyse differences among the categorical data. Actual survival rates were determined with the Kaplan–Meier method. Statview for Windows, version 5.0 (SAS Institute Inc., Cary, NC, USA), was used for the statistical analyses, and a P-value of <0.05 was considered statistically significant.
RESULTS
The operative mortality rate and late deaths were 9.3% (13/140) and 6.3% (8/127), respectively, with an actual survival rate of 85.0% (119/140) at 5 years. There were 7 deaths in the operating room and 6 deaths during the admission due to heart failure (n = 3), renal failure (n = 1), multiorgan failure (n = 1) and pneumonia (n = 1). Causes of late deaths were stroke (n = 1), pneumonia (n = 3), malignancy (n = 1), sepsis (n = 2) and unknown one (n = 1). No patient died of congestive heart failure due to AR. Actual survival rates in both groups were not statistically different (Fig. 1).
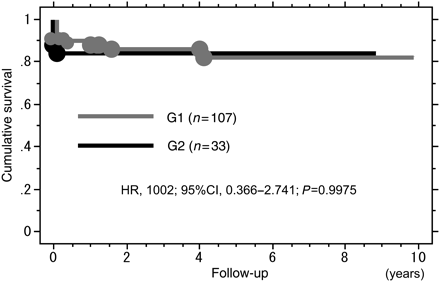
Kaplan–Meier curves demonstrating cumulative survival rates in G1 (group 1:0 or 1+ AR, grey) and G2 (group 2: 2+ or 3+ AR, black). HR: hazard ratio; CI: confidence interval.
Freedom from aortic root re-operation was 100%. Aortic root pseudoaneurysm formation and severe AR requiring aortic valve replacement did not occur. Group 1 (G1) included 98 patients (77%) with either absent (0) or trivial-mild (1+) AR, group 2 (G2) included 29 patients (23%) with either moderate (2+) or severe (3+) (Table 2). Follow-up clinical and echocardiographic data comprising 68 patients revealed that 55 patients had absent or grade 1+ AR (81%), 13 had grade 2+ AR (19%) and none developed grade 3+ AR (Table 3). Pre-operative AR of 0.2 ± 0.4 in G1 did not deteriorate (0.5 ± 0.5 at discharge, 0.4 ± 0.4 at follow-up). Notably, pre-operative AR of 2.4 ± 0.5 in G2 improved to 0.6 ± 0.5 (P < 0.05) at discharge and 1.0 ± 0.6 (P < 0.05) at follow-up (Fig. 2). Table 4 summarizes details of the follow-up aortic root dimensions at the level of Valsalva sinuses measured with the CT scan. Twelve of 117 patients (10%) had an aortic root with >41 mm dimension. However, none had a diameter exceeding 50 mm. The native aortic root dimension at follow-up in G2 was significantly larger than G1 (36.0 ± 4.7 vs. 33.9 ± 5.0 mm, P < 0.05).
| Degree of AR . | Pre-operative (n = 127) (%) . | Post-operative (n = 123) (%) . | Follow-up (n = 68) (%) . |
|---|---|---|---|
| 0 | 84 (66.1) | 78 (63.4) | 31 (45.5) |
| 1+ | 14 (11.0) | 41 (33.3) | 24 (35.2) |
| 2+ | 17 (13.3) | 4 (3.2) | 13 (19.1) |
| 3+ | 12 (9.4) | 0 | 0 |
| Degree of AR . | Pre-operative (n = 127) (%) . | Post-operative (n = 123) (%) . | Follow-up (n = 68) (%) . |
|---|---|---|---|
| 0 | 84 (66.1) | 78 (63.4) | 31 (45.5) |
| 1+ | 14 (11.0) | 41 (33.3) | 24 (35.2) |
| 2+ | 17 (13.3) | 4 (3.2) | 13 (19.1) |
| 3+ | 12 (9.4) | 0 | 0 |
TEE: transthoracic echocardiography; AR: aortic regurgitation; n: number of patients who had echocardiography.
| Degree of AR . | Pre-operative (n = 127) (%) . | Post-operative (n = 123) (%) . | Follow-up (n = 68) (%) . |
|---|---|---|---|
| 0 | 84 (66.1) | 78 (63.4) | 31 (45.5) |
| 1+ | 14 (11.0) | 41 (33.3) | 24 (35.2) |
| 2+ | 17 (13.3) | 4 (3.2) | 13 (19.1) |
| 3+ | 12 (9.4) | 0 | 0 |
| Degree of AR . | Pre-operative (n = 127) (%) . | Post-operative (n = 123) (%) . | Follow-up (n = 68) (%) . |
|---|---|---|---|
| 0 | 84 (66.1) | 78 (63.4) | 31 (45.5) |
| 1+ | 14 (11.0) | 41 (33.3) | 24 (35.2) |
| 2+ | 17 (13.3) | 4 (3.2) | 13 (19.1) |
| 3+ | 12 (9.4) | 0 | 0 |
TEE: transthoracic echocardiography; AR: aortic regurgitation; n: number of patients who had echocardiography.
| Degree of AR . | G1 (n = 42) (%) . | G2 (n = 26) (%) . |
|---|---|---|
| 0 | 26 (62.0) | 5 (19.2) |
| 1+ | 13 (31.0) | 11 (42.3) |
| 2+ | 3 (7.1) | 10 (38.0) |
| 3+ | 0 | 0 |
| Degree of AR . | G1 (n = 42) (%) . | G2 (n = 26) (%) . |
|---|---|---|
| 0 | 26 (62.0) | 5 (19.2) |
| 1+ | 13 (31.0) | 11 (42.3) |
| 2+ | 3 (7.1) | 10 (38.0) |
| 3+ | 0 | 0 |
AR: aortic regurgitation; n: number of survived patients who had echocardiography; TEE: transthoracic echocardiogram.
| Degree of AR . | G1 (n = 42) (%) . | G2 (n = 26) (%) . |
|---|---|---|
| 0 | 26 (62.0) | 5 (19.2) |
| 1+ | 13 (31.0) | 11 (42.3) |
| 2+ | 3 (7.1) | 10 (38.0) |
| 3+ | 0 | 0 |
| Degree of AR . | G1 (n = 42) (%) . | G2 (n = 26) (%) . |
|---|---|---|
| 0 | 26 (62.0) | 5 (19.2) |
| 1+ | 13 (31.0) | 11 (42.3) |
| 2+ | 3 (7.1) | 10 (38.0) |
| 3+ | 0 | 0 |
AR: aortic regurgitation; n: number of survived patients who had echocardiography; TEE: transthoracic echocardiogram.
Follow-up TTE and/or CT measurements of the aortic root dimensions at the level of Valsalva sinuses
| . | G1 (n = 89) . | G2 (n = 28) . | Total (n = 117) . |
|---|---|---|---|
| Aortic root dimension (cm) | |||
| <36 | 57 | 13 | 70 |
| 36–40 | 25 | 10 | 35 |
| 41–45 | 5 | 4 | 9 |
| 46–50 | 2 | 1 | 3 |
| >50 | 0 | 0 | 0 |
| Mean dimension | 33.9 ± 5.0 | 36.0 ± 4.7* | 34.4 ± 3.9 |
| . | G1 (n = 89) . | G2 (n = 28) . | Total (n = 117) . |
|---|---|---|---|
| Aortic root dimension (cm) | |||
| <36 | 57 | 13 | 70 |
| 36–40 | 25 | 10 | 35 |
| 41–45 | 5 | 4 | 9 |
| 46–50 | 2 | 1 | 3 |
| >50 | 0 | 0 | 0 |
| Mean dimension | 33.9 ± 5.0 | 36.0 ± 4.7* | 34.4 ± 3.9 |
Values are presented as the mean ± SD.
TTE: transthoracic echocardiography; CT: computed tomography.
*P < 0.05, vs. G1.
Follow-up TTE and/or CT measurements of the aortic root dimensions at the level of Valsalva sinuses
| . | G1 (n = 89) . | G2 (n = 28) . | Total (n = 117) . |
|---|---|---|---|
| Aortic root dimension (cm) | |||
| <36 | 57 | 13 | 70 |
| 36–40 | 25 | 10 | 35 |
| 41–45 | 5 | 4 | 9 |
| 46–50 | 2 | 1 | 3 |
| >50 | 0 | 0 | 0 |
| Mean dimension | 33.9 ± 5.0 | 36.0 ± 4.7* | 34.4 ± 3.9 |
| . | G1 (n = 89) . | G2 (n = 28) . | Total (n = 117) . |
|---|---|---|---|
| Aortic root dimension (cm) | |||
| <36 | 57 | 13 | 70 |
| 36–40 | 25 | 10 | 35 |
| 41–45 | 5 | 4 | 9 |
| 46–50 | 2 | 1 | 3 |
| >50 | 0 | 0 | 0 |
| Mean dimension | 33.9 ± 5.0 | 36.0 ± 4.7* | 34.4 ± 3.9 |
Values are presented as the mean ± SD.
TTE: transthoracic echocardiography; CT: computed tomography.
*P < 0.05, vs. G1.
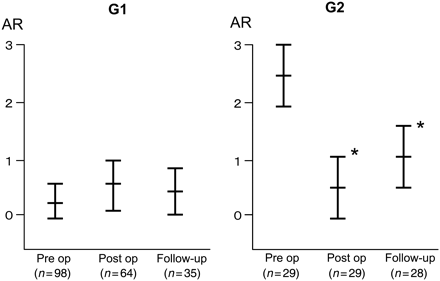
Changes in the grade of aortic regurgitation (AR) in G1 (group 1: 0 or 1+ AR) and G2 (group 2: 2+ or 3+ AR). Values are presented as the mean ± SD. *P < 0.05 vs. Pre op.
Twelve additional aortic re-operations were performed during follow-up. These included three false aneurysms at the distal prosthesis-aortic arch anastomosis, one prosthesis infection, two descending aortic aneurysm replacements, two thoracoabdominal aortic aneurysm replacements, three abdominal aortic aneurysm replacements and one iliac artery reconstruction. There were no deaths due to these additional re-operations.
DISCUSSION
AR complicates 40–60% of patients with AAAD [4]. Excluding 20–30% of patients with Marfan syndrome [7] or a dilated aortic root in whom total root replacement is recommended, the others can be treated with preserving the native aortic valve [3, 5, 8].
Similar to the experiences reported by Pessoto [9] and Casselman [10], retrospective analysis of our series revealed that approximately one-quarter of our patients who survived were complicated by moderate-to-severe AR and a non-dilated aortic root, pre-operatively [8]. These patients developed acute AR in a previously normally functioning aortic valve as a consequence of the proximal extension of the dissecting process with loss of commissural support and prolapse of the valve leaflets [4]. Based on the pathophysiological mechanisms, we treated AR by simple reconstruction of the native aortic root and sino- tubular junction, thus resupporting the valve commissures.
The freedom from re-operations after AV resuspension at 5 years was reported to be 82% (Kirsch et al. [11]), 96% (Sabik et al. [12]), 89% (Casselman et al. [10]), 81% (Von Sesser et al. [13]) and 95% (Geirssen et al. [14]). Fortunately, the freedom from aortic root re-operation in our series was 100%.
The common causes of proximal re-operation are severe AR, psudoanuerysm dissection and infectious endocarditis at the proximal suture line. Pre-operative moderate–severe AR was present in 23% of patients in our series. However, in contrast to other reports [6, 8], pre-operative AR grade exceeding 2+ in patients who underwent AV resuspension was not a predictor for proximal re-operation. Thus, AV resuspension results in an acceptable long-term durability. Grade of AR after repair was <3+ in all our patients.
The usage of the GRF-glue for aortic root repair in patients with AAAD remains controversial. The usefulness of the GRF-glue for acute aortic dissection has been reported, for the first time, by Bachet et al. [15]. Subsequently, other surgeons reported that the use of the GRF-glue decreased the incidence of re-operation and increased the event-free survival rate as a result of the persistent occlusion of the repaired false lumen [4, 10]. In contrast, Pessotto et al. reported that the use of the GRF-glue had no impact on the incidence of re-operation, particularly in patients with a significant pre-operative AR [9]. Similarly, Fukunaga et al. observed necrotic changes and media regeneration in the redissected area of the aortic root [16]. In the present study, however, tissue necrosis related to the GRF-glue was not observed until now, which may be explained by our ideas to improve the efficacy of the GRF-glue. We first injected gelatin-resorcin mixture into the false lumen, then mixed 1 ml formaldehyde–glutalaldehyde with a ratio of 1–40. Meanwhile, after the approval of bioglue in 2011, we utilize bioglue more frequently because of the easy mixing of the new material.
LIMITATIONS
There are several limitations to the present study. First, the current report is a retrospective review. Accordingly, three different chief surgeons have operated on the patients included in this study. Although each surgeon might have a personal approach, the technique used to reconstruct the aortic root was relatively uniform with the use of Teflon, GRF-glue to approximate the dissected layers. In addition, the decision to perform a root reconstruction was made intra-operatively and was entirely dependent on the surgeon's preference and estimation of the feasibility. This might have contributed to a selection bias of patients undergoing root reconstruction. Second, the current study has another inherent limitation, because echocardiographic examination was conducted only in patients associated with heart failure or valvular disease. Although we followed up all patients by CT scan once or twice a year, it seems necessary to accurately assess the post-operative aortic root dimension and the extent of AR using echocardiography in all patients in the future studies. Third, the mean follow-up period of 44 months was relatively short because a large proportion of patients underwent surgery recently. In this meaning, a longer time of the follow-up period would be needed to clarity the prognosis of operated patients.
In conclusion, our data support that aortic valve preservation with valve resuspension and additional reinforcement of the aortic root (Teflon felt patches and GRF-glue) is durable and has the same long-term survival as the root replacement. Although the selection of the type of proximal operation during initial repair should depend on the condition of the valve leaflets and the extent of root involvement, this technique appears to be applicable to most of cases.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr B. Pfannmueller (Leipzig, Germany): It's always a challenging problem to decide what do to for these patients with aortic dissection, normal leaflets and no dilated sinuses. Because of that, your strategy is very interesting. I have two questions.
The first question refers to the cannulation. You cannulated all patients through the ascending aorta using the Seldinger technique and epiaortic echo. Could you explain how you managed to reach the true lumen and whether you had problems?
Your data showed an increase in the percentage of patients with moderate aortic regurgitation, from 3% post-operatively to 19% at follow-up. Thirty-eight percent of the patients with pre-operative moderate or severe aortic regurgitation had moderate aortic regurgitation again at follow-up, which reflects the data in the literature for such patients.
The average age of the patients was 68 years. And because of that, I would like to ask whether it would be an option for you, looking at your data now, to replace these valves intraoperatively?
Dr Yamanaka: I would like to answer the first question. In our series, we used central cannulation in more than 80% of patients; the other sites for cannulation were the right axillary artery or the femoral artery. We usually perform central cannulation guided by epiaortic and transoesophageal echo. We initially check the ascending aorta by epiaortic echo and decide the site of puncture and we insert a guidewire and check whether it is located in the true lumen using both epiaortic and transoesophageal echo. Finally we insert a cannula using Seldinger's method.
We try central cannulation wherever possible in all patients. If the patients' haemodynamics are unstable, we alternatively use apex cannulation. And once again the second question?
Dr Pfannmueller: Would you replace the valves now if you know that 38% of the patients with previous moderate or severe aortic regurgitation have moderate aortic regurgitation again and the average age of the patients is 68 years?
Dr Yamanaka: Usually, we don't replace the aortic valve in cases of moderate aortic regurgitation. I think most of the patients had normal valvular function previously, but the proximal extension of the dissection process caused acute aortic regurgitation as a result of the loss of the commissure support, so I think it's not related to the severity of the aortic regurgitation perioperatively. Accordingly, we try to save the aortic valve whenever possible except for pathological dilated aorta.
Author notes
Presented at the 25th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Lisbon, Portugal, 1–5 October 2011.
- aortic valve
- aortic valve insufficiency
- pseudoaneurysm
- echocardiography
- computed tomography
- proximal aortic dissection
- aortic valve replacement
- tissue dissection
- follow-up
- gelatin
- objective (goal)
- paranasal sinuses
- polytetrafluoroethylene
- reconstructive surgical procedures
- repeat surgery
- surgical procedures, operative
- survivors
- formaldehyde
- supraaortic valve area
- surgical mortality




