-
PDF
- Split View
-
Views
-
Cite
Cite
Maximilian Y. Emmert, Sacha P. Salzberg, Hector Rodriguez Cetina Biefer, Simon H. Sündermann, Burkhardt Seifert, Jürg Grünenfelder, Stephan Jacobs, Volkmar Falk, Total arterial off-pump surgery provides excellent outcomes and does not compromise complete revascularization, European Journal of Cardio-Thoracic Surgery, Volume 41, Issue 4, April 2012, Pages e25–e31, https://doi.org/10.1093/ejcts/ezr225
Close - Share Icon Share
Abstract
The combination of aortic ‘no-touch’ off-pump surgery (OPCAB) and total arterial revascularization (TAR) can reduce peri-procedural morbidity and yields excellent long-term outcomes albeit at a reported risk of incomplete revascularization. The feasibility of OPCAB-TAR with specific regards to the complete revascularization (CR) in patients with multi-vessel disease was evaluated.
From 2003 to 2010, 712 patients underwent TAR including 526 patients who had OPCAB-TAR and 186 patients who received on-pump TAR [(ONCAB grafting (ONCABG)-TAR)]. Of these, 52% (n = 272; OPCAB) vs. 83% (n = 155; ONCABG) had triple-vessel disease (TVD). To balance patient characteristics, a non-parsimonious, propensity score (PS) model was applied. Endpoints evaluated were mortality, stroke, major adverse cardiac and cerebrovascular events (MACCE). To evaluate CR, an ‘Index of CR’ (ICOR) was calculated, defined as the number of distal anastomoses divided by the number of the diseased coronary vessels. CR was assumed when the following requirements were fulfilled: the number of distal anastomoses was equal to or higher than that of diseased vessels (ICOR ≥ 1), and all affected coronary territories (left anterior descending, circumflex artery and/or right coronary artery) were grafted.
Mortality was comparable between groups, whereas OPCAB patients suffered from significantly decreased rates of MACCE [3.0 vs. 7.0%; propensity-adjusted odd ratio (PAOR) = 0.24; confidence interval (CI) 95% 0.08–0.66; P = 0.006] including a clear trend towards reduced stroke and myocardial infarction. In the subgroup with TVD, OPCAB patients presented with significantly reduced rates for MACCE (1.8 vs. 5.8%; PAOR = 0.07; CI 95% 0.01–0.65; P = 0.02), including a significantly lower rate for stroke. For all-comers, the number of diseased vessels was lower after OPCAB (2.36 ± 0.73 vs. 2.87 ± 0.39; P < 0.001) and consequently, these patients received an overall lower number of distal anastomoses (2.42 ± 1.15 vs. 3.06 ± 0.98; P < 0.001). Although the ICOR was slightly lower (1.04 ± 0.37 vs. 1.07 ± 0.37; P = 0.02), CR was achieved more frequently in OPCAB patients (82.1 vs. 73.1%; P = 0.01). In the subgroup with TVD, the number of distal anastomoses (2.99 ± 1.14 vs. 3.10 ± 0.98; P = 0.19) and the ICOR (1.00 ± 0.38 vs. 1.03 ± 0.33; P = 0.19) was comparable between groups. The frequency of CR was slightly higher (75 vs. 67.7%; P = 0.11), and the proportion of complete in situ grafting was significantly higher after OPCAB (37.1 vs. 23.9%; P = 0.005).
Aortic ‘no-touch’ OPCAB-TAR leads to a significant reduction of MACCE. It does not compromise CR in patients with TVD and thus can be safely applied to these patients.
INTRODUCTION
Off-pump coronary artery bypass grafting (OPCAB) offers superior post-operative outcomes with regard to major cerebrovascular and cardiac complications [1–3] and disproportionally benefits high-risk patients [4]. The combination with an aortic, no-touch strategy and/or in situ total arterial revascularization (TAR) techniques can effectively reduce neurological complications while yielding excellent long-term outcomes [3, 5–8].
The so-called ‘aortic no-touch technique’ aims to avoid any aortic manipulation either by using in situ arterial conduits (double internal mammary artery and/or T- or Y-grafting) or by applying clamp-less anastomotic devices whenever free arterial grafts have to be used and proximal anastomoses become necessary.
Recent controlled randomized trials comparing off-pump and on-pump CABG in low-risk patients did not show a potential benefit for off-pump surgery [9–11]. Furthermore, off-pump surgery has been repeatedly criticized to come at a cost of less complete revascularization (CR), suggesting that it is not preferable in patients with multi-vessel disease and complex coronary lesions [9, 11, 12].
In this study, the safety and feasibility of combining OPCAB and TAR with specific regard to the completeness of revascularization (CR) in patients with multi-vessel disease, excluding single and double vessel disease was assessed.
MATERIALS AND METHODS
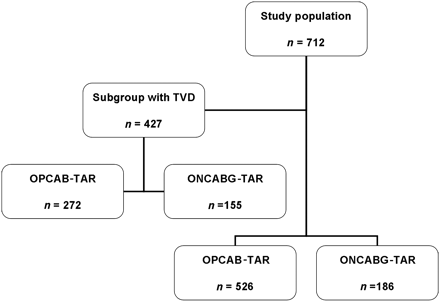
From 2003 to 2010, a total of 712 patients received total arterial grafting (TAR), including 526 patients who underwent off-pump TAR (OPCAB) and 186 patients who received on-pump TAR [ONCAB grafting (ONCABG)]. Of these, 52% (n = 272) in the OPCAB group vs. 83% (n = 155) in the ONCABG group suffered from triple-vessel disease (TVD) which were also evaluated in a separate subgroup analysis (Fig. 1). The study was approved by an institutional review board, including a waiver of informed consent. Tables 1 and 2 summarize demographics and preoperative variables.
| Parameter . | TVD . | P-value . | |
|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | ||
| Age (years) | 64 ± 11 | 62 ± 9 | 0.01 |
| Male (%) | 80.9 | 85.5 | 0.23 |
| EuroScore | 3.7 ± 1.0 | 4.0 ± 1.1 | 0.003 |
| EF (%) | 57 ± 15 | 55 ± 14 | 0.86 |
| Sinus rhythm (%) | 95.1 | 98.1 | 0.20 |
| Atrial fibrillation (%) | 4.2 | 1.3 | 0.16 |
| Triple-vessel disease (%) | 100 | 100 | 1.00 |
| Left main disease (%) | 32.4 | 29.7 | 0.59 |
| CCS 4 (%) | 11.1 | 12.3 | 0.75 |
| NYHA 4 (%) | 1.3 | 3.2 | 0.28 |
| Parameter . | TVD . | P-value . | |
|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | ||
| Age (years) | 64 ± 11 | 62 ± 9 | 0.01 |
| Male (%) | 80.9 | 85.5 | 0.23 |
| EuroScore | 3.7 ± 1.0 | 4.0 ± 1.1 | 0.003 |
| EF (%) | 57 ± 15 | 55 ± 14 | 0.86 |
| Sinus rhythm (%) | 95.1 | 98.1 | 0.20 |
| Atrial fibrillation (%) | 4.2 | 1.3 | 0.16 |
| Triple-vessel disease (%) | 100 | 100 | 1.00 |
| Left main disease (%) | 32.4 | 29.7 | 0.59 |
| CCS 4 (%) | 11.1 | 12.3 | 0.75 |
| NYHA 4 (%) | 1.3 | 3.2 | 0.28 |
EF: ejection fraction; BMI: body mass index; CCS: Canadian Cardiovascular Society Angina Classification; NYHA: New York Heart Association.
| Parameter . | TVD . | P-value . | |
|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | ||
| Age (years) | 64 ± 11 | 62 ± 9 | 0.01 |
| Male (%) | 80.9 | 85.5 | 0.23 |
| EuroScore | 3.7 ± 1.0 | 4.0 ± 1.1 | 0.003 |
| EF (%) | 57 ± 15 | 55 ± 14 | 0.86 |
| Sinus rhythm (%) | 95.1 | 98.1 | 0.20 |
| Atrial fibrillation (%) | 4.2 | 1.3 | 0.16 |
| Triple-vessel disease (%) | 100 | 100 | 1.00 |
| Left main disease (%) | 32.4 | 29.7 | 0.59 |
| CCS 4 (%) | 11.1 | 12.3 | 0.75 |
| NYHA 4 (%) | 1.3 | 3.2 | 0.28 |
| Parameter . | TVD . | P-value . | |
|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | ||
| Age (years) | 64 ± 11 | 62 ± 9 | 0.01 |
| Male (%) | 80.9 | 85.5 | 0.23 |
| EuroScore | 3.7 ± 1.0 | 4.0 ± 1.1 | 0.003 |
| EF (%) | 57 ± 15 | 55 ± 14 | 0.86 |
| Sinus rhythm (%) | 95.1 | 98.1 | 0.20 |
| Atrial fibrillation (%) | 4.2 | 1.3 | 0.16 |
| Triple-vessel disease (%) | 100 | 100 | 1.00 |
| Left main disease (%) | 32.4 | 29.7 | 0.59 |
| CCS 4 (%) | 11.1 | 12.3 | 0.75 |
| NYHA 4 (%) | 1.3 | 3.2 | 0.28 |
EF: ejection fraction; BMI: body mass index; CCS: Canadian Cardiovascular Society Angina Classification; NYHA: New York Heart Association.
| Parameter . | TVD . | P-value . | |
|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | ||
| Hyperlipidaemia (%) | 76.9 | 75.5 | 0.79 |
| Hypertension (%) | 48.2 | 61.3 | 0.01 |
| Positive family History (%) | 37.2 | 34.9 | 0.47 |
| Diabetes (%) | 25.7 | 20.6 | 0.28 |
| Smoking (%) | 58.6 | 65.6 | 0.19 |
| Adipositas (%) | 52.5 | 45.2 | 0.19 |
| PAD (%) | 16.0 | 9.7 | 0.08 |
| COPD (%) | 2.9 | 5.8 | 0.20 |
| Acute MI (<90 days) (%) | 15.4 | 20.0 | 0.23 |
| Previous MI (>90 days) (%) | 35.7 | 48.4 | 0.01 |
| Cerebrovascular disease (%) | 1.8 | 0.0 | 0.16 |
| Renal disease (%) | 0.7 | 1.9 | 0.62 |
| Parameter . | TVD . | P-value . | |
|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | ||
| Hyperlipidaemia (%) | 76.9 | 75.5 | 0.79 |
| Hypertension (%) | 48.2 | 61.3 | 0.01 |
| Positive family History (%) | 37.2 | 34.9 | 0.47 |
| Diabetes (%) | 25.7 | 20.6 | 0.28 |
| Smoking (%) | 58.6 | 65.6 | 0.19 |
| Adipositas (%) | 52.5 | 45.2 | 0.19 |
| PAD (%) | 16.0 | 9.7 | 0.08 |
| COPD (%) | 2.9 | 5.8 | 0.20 |
| Acute MI (<90 days) (%) | 15.4 | 20.0 | 0.23 |
| Previous MI (>90 days) (%) | 35.7 | 48.4 | 0.01 |
| Cerebrovascular disease (%) | 1.8 | 0.0 | 0.16 |
| Renal disease (%) | 0.7 | 1.9 | 0.62 |
PAD: peripheral artery disease; COPD: chronic obstructive pulmonary disease; MI: myocardial infarction.
| Parameter . | TVD . | P-value . | |
|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | ||
| Hyperlipidaemia (%) | 76.9 | 75.5 | 0.79 |
| Hypertension (%) | 48.2 | 61.3 | 0.01 |
| Positive family History (%) | 37.2 | 34.9 | 0.47 |
| Diabetes (%) | 25.7 | 20.6 | 0.28 |
| Smoking (%) | 58.6 | 65.6 | 0.19 |
| Adipositas (%) | 52.5 | 45.2 | 0.19 |
| PAD (%) | 16.0 | 9.7 | 0.08 |
| COPD (%) | 2.9 | 5.8 | 0.20 |
| Acute MI (<90 days) (%) | 15.4 | 20.0 | 0.23 |
| Previous MI (>90 days) (%) | 35.7 | 48.4 | 0.01 |
| Cerebrovascular disease (%) | 1.8 | 0.0 | 0.16 |
| Renal disease (%) | 0.7 | 1.9 | 0.62 |
| Parameter . | TVD . | P-value . | |
|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | ||
| Hyperlipidaemia (%) | 76.9 | 75.5 | 0.79 |
| Hypertension (%) | 48.2 | 61.3 | 0.01 |
| Positive family History (%) | 37.2 | 34.9 | 0.47 |
| Diabetes (%) | 25.7 | 20.6 | 0.28 |
| Smoking (%) | 58.6 | 65.6 | 0.19 |
| Adipositas (%) | 52.5 | 45.2 | 0.19 |
| PAD (%) | 16.0 | 9.7 | 0.08 |
| COPD (%) | 2.9 | 5.8 | 0.20 |
| Acute MI (<90 days) (%) | 15.4 | 20.0 | 0.23 |
| Previous MI (>90 days) (%) | 35.7 | 48.4 | 0.01 |
| Cerebrovascular disease (%) | 1.8 | 0.0 | 0.16 |
| Renal disease (%) | 0.7 | 1.9 | 0.62 |
PAD: peripheral artery disease; COPD: chronic obstructive pulmonary disease; MI: myocardial infarction.
Surgical technique
ONCABG-TAR was carried out with standard cardiopulmonary bypass techniques and cross-clamping of the aorta. OPCAB was performed as previously described [3, 13]. In brief, a stabilizer (Octopus®4 Tissue Stabilizer, Medtronic, Minneapolis, MN, USA) was used to stabilize the heart and expose the target vessel. A shunt (ClearView® Intracoronary Shunt, Medtronic, Minneapolis, MN, USA) was routinely used to maintain distal perfusion. Routine ultrasound flow measurement (MediStim QuickFit®) was done in all cases. If insufficient flow was documented, the anastomosis was immediately repeated.
Revascularization strategy and aortic no-touch techniques
Prior to surgery, all vessels with significant lesions (>70%) were identified in the preoperative angiography and selected as target for revascularization. Left main coronary disease (LMD) was defined as stenosis of the left main stem >50%. Surgical revascularization was started with left internal mammary artery (LIMA) to LAD grafting, followed by the right coronary territory and finished with the circumflex coronary system. In patients suffering from LMD, the LAD and circumflex arteries were always grafted, regardless of the degree of stenosis. Total arterial grafting using in situ arterial grafts only [including T- or Y-grafting using the right internal mammary artery (RIMA) or the radial artery (RA)] was done whenever possible. If the RIMA and/or the RA had to be used as free arterial grafts requiring a proximal anastomosis, a less touch, clamp-less fashion (‘no-touch’ technique for proximal anastomosis) using the heartstring device (HEARTSTRING™ Proximal Seal System, Guidant, Indianapolis, IN, USA) was applied to decrease the risk for cerebral complications during clamping and de-clamping manoeuvres. Digital palpation was done to identify a healthy, non-calcified aortic segment. Thereafter an aortic-punch device was applied to create a circular aortotomy, before the coiled heartstring device was inserted to create a haemostatic seal against the aortic inner wall. The anastomosis was carried out using a continuous 6/0 Prolene suture and before tightening of the suture the device was removed.
Primary outcomes
To evaluate CR, an ‘Index of CR’ (ICOR) was calculated for each individual patient [13]. The ICOR was defined as the total number of distal anastomoses performed divided by the number of the diseased coronary vessels defined on the preoperative coronary angiography [14]. CR was assumed when the following two requirements were fulfilled: the number of distal anastomoses was equal to or higher than that of diseased vessels (ICOR ≥ ) and all affected coronary territories (LAD, CX and/or RCA) were grafted.
Secondary outcomes
Major outcome endpoints were mortality, stroke and major adverse cardiac and cerebrovascular events (MACCE) defined as composite of death, stroke and myocardial infarction (MI). In addition, major non-cardiac adverse events such as pulmonary, renal and bleeding complications were assessed. In patients who required intra-operative conversion, the ‘Intention-to-treat’ methodology was applied.
Statistical analysis
The collection of data was carried out prospectively. Continuous data are presented as mean ± SD and are compared using the Mann–Whitney test. Categorical data are shown as numbers and percentage, and are compared using the χ2 test and Fisher's exact test. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using univariate logistic regression. A propensity score (PS) was calculated using logistic regression and a non-parsimonious model with numerous preoperative variables to balance characteristics between off-pump and on-pump groups. The c-statistic was 0.86 and 0.82 in the subgroup analysis for patients with TVD. Missing values in preoperative variables were replaced using multiple imputation regression methods. Thereafter, the PS was divided into quintiles and analysed as a categorical variable. PS-adjusted logistic regression analysis was performed to assess binary endpoints and two-way analysis of variance for continuous endpoints. All analyses were performed using SPSS 18 (SPSS Inc., Chicago, IL, USA), and P < 0.05 was assumed to be statistically significant.
Besides size, weight, gender and body mass index, the preoperative variables for the non-parsimonious PS model included patient characteristics and demographics such as cardiovascular risk factors and comorbidities, including peripheral artery disease (PAD), chronic obstructive pulmonary disease (COPD) and renal disease. Cerebrovascular disease was defined as a brain dysfunction due to the known history of a transient ischaemic attack or stroke. Patients who presented with a medical history of cerebrovascular disease were susceptive for it or were of advanced age (>65 years) routinely underwent preoperative Doppler carotid ultrasound assessment. Preoperative conditions included were: preceding MI, MI within 3 months prior to surgery, preceding cardiogenic-shock, congestive heart-failure, arrhythmias, number of diseased coronary vessels, previous CABG, elective, urgent/or emergent presentation, previous percutaneous coronary intervention (PCI), previous stent implantation, New York Heart Association class, Canadian Cardiovascular Society Angina Classification, logistic EuroSCORE and others.
RESULTS
Patient demographics
Patient demographics and preoperative characteristics including EuroSCORE, cardiovascular risk factors and co-morbidities are summarized in Tables 1 and 2.
Crude outcome and propensity-adjusted analysis for all-comers
When compared with patients who underwent the on-pump approach, OPCAB patients suffered from significantly decreased rates of MACCE (3.0 vs. 7.0%; OR = 0.41; CI 95% 0.19–0.88; P = 0.02), including a clear trend towards reduced stroke (1.0 vs. 2.7%; OR = 0.34; CI 95% 0.09–1.2; P = 0.09) and MI (1.4 vs. 2.2%; OR = 0.63; CI 95% 0.16–2.32; P = 0.50; Table 3; Fig. 2). There were no significant differences with regard to non-cardiac complications and mortality was comparable for both groups (1.5 vs. 2.2%; OR = 0.70; CI 95% 0.20–2.36; P = 0.56) in the crude outcome, but appeared to be even significantly lower after adjustment [propensity-adjusted odd ratio (PAOR) = 0.16; CI 95% 0.03–0.78; P = 0.02]. After PS adjustment, the significant benefit for OPCAB patients with regard to the occurrence of MACCE (PAOR = 0.24; CI 95% 0.08–0.66; P = 0.006) remained clearly visible.
| Parameter . | OPCAB (n = 526) . | ONCABG (n = 186) . | OR . | CI 95% . | P-value . | PA OR . | PA CI 95% . | PA P-value . |
|---|---|---|---|---|---|---|---|---|
| Mortality (%) | 1.5 | 2.2 | 0.70 | 0.20–2.36 | 0.56 | 0.16 | 0.03–0.78 | 0.02 |
| Cerebrovascular accident (CVA) (%) | 1.0 | 2.7 | 0.34 | 0.09–1.21 | 0.09 | 0.22 | 0.04–1.05 | 0.06 |
| Myocardial infarction (%) | 1.4 | 2.2 | 0.63 | 0.16–2.38 | 0.50 | 0.77 | 0.14–4.14 | 0.76 |
| Bleeding (%) | 5.5 | 3.8 | 1.49 | 0.64–3.42 | 0.35 | 0.76 | 0.28–2.30 | 0.66 |
| Renal dysfunction (%) | 3.4 | 2.7 | 1.28 | 0.46–3.50 | 0.62 | 0.78 | 0.23–2.61 | 0.69 |
| Respiratory failure (%) | 1.9 | 3.4 | 0.54 | 0.16–1.82 | 0.33 | 0.34 | 0.07–1.67 | 0.18 |
| Pleural effusions/pneumothorax (%) | 1.9 | 4.6 | 0.40 | 0.14–1.14 | 0.09 | 0.36 | 0.10–1.22 | 0.10 |
| Sinus rhythm (%) | 94.2 | 96.2 | 0.63 | 0.26–1.53 | 0.31 | 1.25 | 0.44–3.54 | 0.66 |
| Atrial fibrillation (%) | 4.4 | 3.8 | 1.17 | 0.47–2.91 | 0.72 | 0.67 | 0.22–1.98 | 0.47 |
| MACCE (CVA/MI/mortality) (%) | 3.0 | 7.0 | 0.41 | 0.19–0.88 | 0.02 | 0.24 | 0.08–0.66 | 0.006 |
| Parameter . | OPCAB (n = 526) . | ONCABG (n = 186) . | OR . | CI 95% . | P-value . | PA OR . | PA CI 95% . | PA P-value . |
|---|---|---|---|---|---|---|---|---|
| Mortality (%) | 1.5 | 2.2 | 0.70 | 0.20–2.36 | 0.56 | 0.16 | 0.03–0.78 | 0.02 |
| Cerebrovascular accident (CVA) (%) | 1.0 | 2.7 | 0.34 | 0.09–1.21 | 0.09 | 0.22 | 0.04–1.05 | 0.06 |
| Myocardial infarction (%) | 1.4 | 2.2 | 0.63 | 0.16–2.38 | 0.50 | 0.77 | 0.14–4.14 | 0.76 |
| Bleeding (%) | 5.5 | 3.8 | 1.49 | 0.64–3.42 | 0.35 | 0.76 | 0.28–2.30 | 0.66 |
| Renal dysfunction (%) | 3.4 | 2.7 | 1.28 | 0.46–3.50 | 0.62 | 0.78 | 0.23–2.61 | 0.69 |
| Respiratory failure (%) | 1.9 | 3.4 | 0.54 | 0.16–1.82 | 0.33 | 0.34 | 0.07–1.67 | 0.18 |
| Pleural effusions/pneumothorax (%) | 1.9 | 4.6 | 0.40 | 0.14–1.14 | 0.09 | 0.36 | 0.10–1.22 | 0.10 |
| Sinus rhythm (%) | 94.2 | 96.2 | 0.63 | 0.26–1.53 | 0.31 | 1.25 | 0.44–3.54 | 0.66 |
| Atrial fibrillation (%) | 4.4 | 3.8 | 1.17 | 0.47–2.91 | 0.72 | 0.67 | 0.22–1.98 | 0.47 |
| MACCE (CVA/MI/mortality) (%) | 3.0 | 7.0 | 0.41 | 0.19–0.88 | 0.02 | 0.24 | 0.08–0.66 | 0.006 |
OR: odds ratio; CI 95%: confidence interval 95%; PAOR: propensity-adjusted OR; PA CI 95%: propensity-adjusted CI 95%; PA P-value: propensity-adjusted P-value; IABP: intra-aortic balloon pump; MACCE: major adverse cardiac and cerebrovascular events.
| Parameter . | OPCAB (n = 526) . | ONCABG (n = 186) . | OR . | CI 95% . | P-value . | PA OR . | PA CI 95% . | PA P-value . |
|---|---|---|---|---|---|---|---|---|
| Mortality (%) | 1.5 | 2.2 | 0.70 | 0.20–2.36 | 0.56 | 0.16 | 0.03–0.78 | 0.02 |
| Cerebrovascular accident (CVA) (%) | 1.0 | 2.7 | 0.34 | 0.09–1.21 | 0.09 | 0.22 | 0.04–1.05 | 0.06 |
| Myocardial infarction (%) | 1.4 | 2.2 | 0.63 | 0.16–2.38 | 0.50 | 0.77 | 0.14–4.14 | 0.76 |
| Bleeding (%) | 5.5 | 3.8 | 1.49 | 0.64–3.42 | 0.35 | 0.76 | 0.28–2.30 | 0.66 |
| Renal dysfunction (%) | 3.4 | 2.7 | 1.28 | 0.46–3.50 | 0.62 | 0.78 | 0.23–2.61 | 0.69 |
| Respiratory failure (%) | 1.9 | 3.4 | 0.54 | 0.16–1.82 | 0.33 | 0.34 | 0.07–1.67 | 0.18 |
| Pleural effusions/pneumothorax (%) | 1.9 | 4.6 | 0.40 | 0.14–1.14 | 0.09 | 0.36 | 0.10–1.22 | 0.10 |
| Sinus rhythm (%) | 94.2 | 96.2 | 0.63 | 0.26–1.53 | 0.31 | 1.25 | 0.44–3.54 | 0.66 |
| Atrial fibrillation (%) | 4.4 | 3.8 | 1.17 | 0.47–2.91 | 0.72 | 0.67 | 0.22–1.98 | 0.47 |
| MACCE (CVA/MI/mortality) (%) | 3.0 | 7.0 | 0.41 | 0.19–0.88 | 0.02 | 0.24 | 0.08–0.66 | 0.006 |
| Parameter . | OPCAB (n = 526) . | ONCABG (n = 186) . | OR . | CI 95% . | P-value . | PA OR . | PA CI 95% . | PA P-value . |
|---|---|---|---|---|---|---|---|---|
| Mortality (%) | 1.5 | 2.2 | 0.70 | 0.20–2.36 | 0.56 | 0.16 | 0.03–0.78 | 0.02 |
| Cerebrovascular accident (CVA) (%) | 1.0 | 2.7 | 0.34 | 0.09–1.21 | 0.09 | 0.22 | 0.04–1.05 | 0.06 |
| Myocardial infarction (%) | 1.4 | 2.2 | 0.63 | 0.16–2.38 | 0.50 | 0.77 | 0.14–4.14 | 0.76 |
| Bleeding (%) | 5.5 | 3.8 | 1.49 | 0.64–3.42 | 0.35 | 0.76 | 0.28–2.30 | 0.66 |
| Renal dysfunction (%) | 3.4 | 2.7 | 1.28 | 0.46–3.50 | 0.62 | 0.78 | 0.23–2.61 | 0.69 |
| Respiratory failure (%) | 1.9 | 3.4 | 0.54 | 0.16–1.82 | 0.33 | 0.34 | 0.07–1.67 | 0.18 |
| Pleural effusions/pneumothorax (%) | 1.9 | 4.6 | 0.40 | 0.14–1.14 | 0.09 | 0.36 | 0.10–1.22 | 0.10 |
| Sinus rhythm (%) | 94.2 | 96.2 | 0.63 | 0.26–1.53 | 0.31 | 1.25 | 0.44–3.54 | 0.66 |
| Atrial fibrillation (%) | 4.4 | 3.8 | 1.17 | 0.47–2.91 | 0.72 | 0.67 | 0.22–1.98 | 0.47 |
| MACCE (CVA/MI/mortality) (%) | 3.0 | 7.0 | 0.41 | 0.19–0.88 | 0.02 | 0.24 | 0.08–0.66 | 0.006 |
OR: odds ratio; CI 95%: confidence interval 95%; PAOR: propensity-adjusted OR; PA CI 95%: propensity-adjusted CI 95%; PA P-value: propensity-adjusted P-value; IABP: intra-aortic balloon pump; MACCE: major adverse cardiac and cerebrovascular events.
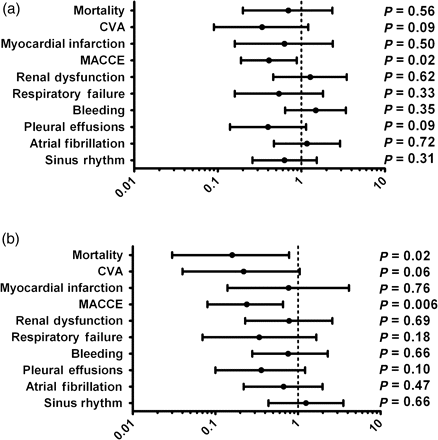
Forrest plots with ORs and 95% CIs showing crude (a) and propensity-adjusted outcomes (b) for all-comers.
Crude outcome and propensity-adjusted analysis for patients with TVD
In the subgroup analysis, OPCAB patients with TVD presented with significantly reduced rates for MACCE (1.8 vs. 5.8%; OR = 0.30; CI 95% 0.10–0.92; P = 0.04) including a significantly lower rate for stroke (0.4 vs. 3.2%; OR = 0.11; CI 95% 0.01–0.95; P = 0.04) and a lower rate for MI (0.7 vs. 1.9%; OR = 0.35; CI 95% 0.03–3.47; P = 0.37; Table 4; Fig. 3).
Following PS adjustment, MACCE was still significantly lower after PS adjustment (PAOR = 0.07; CI 95% 0.01–0.65; P = 0.02). Overall mortality was comparable between both groups and did not display any significant differences neither before (1.5 vs. 0.6%; OR = 2.29; CI 95% 0.25–20.74; P = 0.45), nor after PS adjustment (PAOR = 0.30; CI 95% 0.01–7.35; P = 0.46). Similarly the overall occurrence of non-cardiac complications was comparable between groups with a trend to reduced pulmonary complications among OPCAB patients.
| Parameter . | TVD . | OR . | CI 95% . | P-value . | PA OR . | PA CI 95% . | PA P-value . | |
|---|---|---|---|---|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | |||||||
| Mortality (%) | 1.5 | 0.6 | 2.29 | 0.25–20.74 | 0.45 | 0.30 | 0.01–7.35 | 0.46 |
| Cerebrovascular accident (CVA) (%) | 0.4 | 3.2 | 0.11 | 0.01–0.95 | 0.04 | n/a | n/a | n/a |
| Myocardial infarction (%) | 0.7 | 1.9 | 0.35 | 0.03–3.47 | 0.37 | 0.30 | 0.02–3.94 | 0.36 |
| Bleeding (%) | 6.3 | 3.9 | 1.65 | 0.63–4.29 | 0.30 | 0.76 | 0.21–2.74 | 0.67 |
| Renal dysfunction (%) | 3.7 | 3.2 | 1.14 | 0.38–3.41 | 0.80 | 0.60 | 0.15–2.34 | 0.46 |
| Respiratory failure (%) | 1.1 | 3.9 | 0.27 | 0.03–2.30 | 0.23 | 0.22 | 0.02–2.09 | 0.19 |
| Pleural effusions/pneumothorax (%) | 0.7 | 5.2 | 0.12 | 0.01–1.04 | 0.06 | 0.11 | 0.01–0.94 | 0.04 |
| Sinus rhythm (%) | 95.1 | 95.5 | 0.91 | 0.31–2.68 | 0.87 | 1.76 | 0.51–6.10 | 0.37 |
| Atrial fibrillation (%) | 4.2 | 4.5 | 0.92 | 0.30–2.82 | 0.89 | 0.50 | 0.14–1.81 | 0.30 |
| MACCE (CVA/MI/mortality) (%) | 1.8 | 5.8 | 0.30 | 0.10–0.92 | 0.04 | 0.07 | 0.01–0.65 | 0.02 |
| Parameter . | TVD . | OR . | CI 95% . | P-value . | PA OR . | PA CI 95% . | PA P-value . | |
|---|---|---|---|---|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | |||||||
| Mortality (%) | 1.5 | 0.6 | 2.29 | 0.25–20.74 | 0.45 | 0.30 | 0.01–7.35 | 0.46 |
| Cerebrovascular accident (CVA) (%) | 0.4 | 3.2 | 0.11 | 0.01–0.95 | 0.04 | n/a | n/a | n/a |
| Myocardial infarction (%) | 0.7 | 1.9 | 0.35 | 0.03–3.47 | 0.37 | 0.30 | 0.02–3.94 | 0.36 |
| Bleeding (%) | 6.3 | 3.9 | 1.65 | 0.63–4.29 | 0.30 | 0.76 | 0.21–2.74 | 0.67 |
| Renal dysfunction (%) | 3.7 | 3.2 | 1.14 | 0.38–3.41 | 0.80 | 0.60 | 0.15–2.34 | 0.46 |
| Respiratory failure (%) | 1.1 | 3.9 | 0.27 | 0.03–2.30 | 0.23 | 0.22 | 0.02–2.09 | 0.19 |
| Pleural effusions/pneumothorax (%) | 0.7 | 5.2 | 0.12 | 0.01–1.04 | 0.06 | 0.11 | 0.01–0.94 | 0.04 |
| Sinus rhythm (%) | 95.1 | 95.5 | 0.91 | 0.31–2.68 | 0.87 | 1.76 | 0.51–6.10 | 0.37 |
| Atrial fibrillation (%) | 4.2 | 4.5 | 0.92 | 0.30–2.82 | 0.89 | 0.50 | 0.14–1.81 | 0.30 |
| MACCE (CVA/MI/mortality) (%) | 1.8 | 5.8 | 0.30 | 0.10–0.92 | 0.04 | 0.07 | 0.01–0.65 | 0.02 |
OR: odds ratio; CI 95%: confidence interval 95%; PA OR: propensity-adjusted OR; PA CI 95%: propensity-adjusted CI 95%; PA P-value: propensity-adjusted P-value; IABP: intra-aortic balloon pump; MACCE: major adverse cardiac and cerebrovascular events; n/a: not calculable (too small number of events).
| Parameter . | TVD . | OR . | CI 95% . | P-value . | PA OR . | PA CI 95% . | PA P-value . | |
|---|---|---|---|---|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | |||||||
| Mortality (%) | 1.5 | 0.6 | 2.29 | 0.25–20.74 | 0.45 | 0.30 | 0.01–7.35 | 0.46 |
| Cerebrovascular accident (CVA) (%) | 0.4 | 3.2 | 0.11 | 0.01–0.95 | 0.04 | n/a | n/a | n/a |
| Myocardial infarction (%) | 0.7 | 1.9 | 0.35 | 0.03–3.47 | 0.37 | 0.30 | 0.02–3.94 | 0.36 |
| Bleeding (%) | 6.3 | 3.9 | 1.65 | 0.63–4.29 | 0.30 | 0.76 | 0.21–2.74 | 0.67 |
| Renal dysfunction (%) | 3.7 | 3.2 | 1.14 | 0.38–3.41 | 0.80 | 0.60 | 0.15–2.34 | 0.46 |
| Respiratory failure (%) | 1.1 | 3.9 | 0.27 | 0.03–2.30 | 0.23 | 0.22 | 0.02–2.09 | 0.19 |
| Pleural effusions/pneumothorax (%) | 0.7 | 5.2 | 0.12 | 0.01–1.04 | 0.06 | 0.11 | 0.01–0.94 | 0.04 |
| Sinus rhythm (%) | 95.1 | 95.5 | 0.91 | 0.31–2.68 | 0.87 | 1.76 | 0.51–6.10 | 0.37 |
| Atrial fibrillation (%) | 4.2 | 4.5 | 0.92 | 0.30–2.82 | 0.89 | 0.50 | 0.14–1.81 | 0.30 |
| MACCE (CVA/MI/mortality) (%) | 1.8 | 5.8 | 0.30 | 0.10–0.92 | 0.04 | 0.07 | 0.01–0.65 | 0.02 |
| Parameter . | TVD . | OR . | CI 95% . | P-value . | PA OR . | PA CI 95% . | PA P-value . | |
|---|---|---|---|---|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | |||||||
| Mortality (%) | 1.5 | 0.6 | 2.29 | 0.25–20.74 | 0.45 | 0.30 | 0.01–7.35 | 0.46 |
| Cerebrovascular accident (CVA) (%) | 0.4 | 3.2 | 0.11 | 0.01–0.95 | 0.04 | n/a | n/a | n/a |
| Myocardial infarction (%) | 0.7 | 1.9 | 0.35 | 0.03–3.47 | 0.37 | 0.30 | 0.02–3.94 | 0.36 |
| Bleeding (%) | 6.3 | 3.9 | 1.65 | 0.63–4.29 | 0.30 | 0.76 | 0.21–2.74 | 0.67 |
| Renal dysfunction (%) | 3.7 | 3.2 | 1.14 | 0.38–3.41 | 0.80 | 0.60 | 0.15–2.34 | 0.46 |
| Respiratory failure (%) | 1.1 | 3.9 | 0.27 | 0.03–2.30 | 0.23 | 0.22 | 0.02–2.09 | 0.19 |
| Pleural effusions/pneumothorax (%) | 0.7 | 5.2 | 0.12 | 0.01–1.04 | 0.06 | 0.11 | 0.01–0.94 | 0.04 |
| Sinus rhythm (%) | 95.1 | 95.5 | 0.91 | 0.31–2.68 | 0.87 | 1.76 | 0.51–6.10 | 0.37 |
| Atrial fibrillation (%) | 4.2 | 4.5 | 0.92 | 0.30–2.82 | 0.89 | 0.50 | 0.14–1.81 | 0.30 |
| MACCE (CVA/MI/mortality) (%) | 1.8 | 5.8 | 0.30 | 0.10–0.92 | 0.04 | 0.07 | 0.01–0.65 | 0.02 |
OR: odds ratio; CI 95%: confidence interval 95%; PA OR: propensity-adjusted OR; PA CI 95%: propensity-adjusted CI 95%; PA P-value: propensity-adjusted P-value; IABP: intra-aortic balloon pump; MACCE: major adverse cardiac and cerebrovascular events; n/a: not calculable (too small number of events).
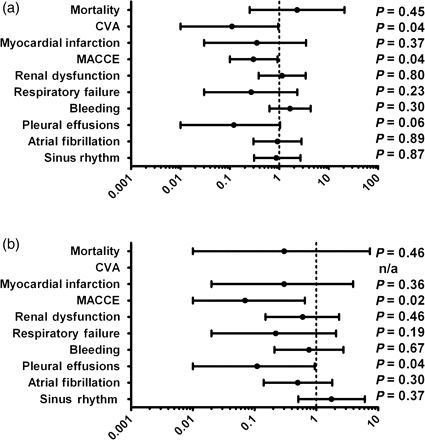
Forrest plots with ORs and 95% CIs showing crude (a) and propensity-adjusted outcomes (b) for patients with TVD; n/a, not calculable (too small number of events).
Intra-operative data and CR
In the total cohort, significantly less OPCAB patients suffered from TVD (51.8 vs. 83.3%; P = 0.001) and the mean number of diseased vessels was lower among these patients when compared with the ONCABG group (2.36 ± 0.73 vs. 2.87 ± 0.39; P < 0.001; Table 5). Consequently, OPCAB patients received an overall lower number of distal anastomoses (2.42 ± 1.15 vs. 3.06 ± 0.98; P < 0.001). Although the mean ‘ICOR’ appeared to be slightly lower (1.04 ± 0.37 vs. 1.07 ± 0.37; P = 0.02) after OPCAB, CR was achieved more frequently in these patients (82.1 vs. 73.1%; P = 0.01).
| Parameter . | OPCAB, (n = 526) . | ONCABG, (n = 186) . | P-value . | TVD . | P-value . | |
|---|---|---|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | |||||
| CPB conversion (%) | 6.5 | — | — | 6.3 | — | |
| CPB time (min) | — | 95 ± 37 | — | — | 96 ± 37 | — |
| Aortic X-clamp time (min) | — | 42 ± 29 | — | — | 43 ± 29 | — |
| IABP intraoperative (%) | 2.9 | 6.5 | 0.04 | 3.3 | 7.1 | 0.09 |
| Number of diseased vessels | 2.36 ± 0.73 | 2.87 ± 0.39 | <0.001 | 3.00 | 3.00 | 1.00 |
| Total number of anastomoses per patient | 2.42 ± 1.15 | 3.06 ± 0.98 | <0.001 | 2.99 ± 1.14 | 3.10 ± 0.98 | 0.19 |
| Complete in situ grafting (%) | 73.4 | 76.9 | 0.38 | 37.1 | 23.9 | 0.005 |
| LIMA (%) | 93.7 | 94.1 | 1.00 | 93.0 | 94.2 | 0.69 |
| RIMA (%) | 50.0 | 81.7 | <0.001 | 58.8 | 83.2 | <0.001 |
| Radial artery (%) | 44.3 | 50.0 | 0.19 | 66.9 | 50.3 | <0.001 |
| Completeness of revascularization (%) | 82.1 | 73.1 | 0.01 | 75.0 | 67.7 | 0.11 |
| ICOR | 1.04 ± 0.37 | 1.09 ± 0.37 | 0.02 | 1.00 ± 0.38 | 1.03 ± 0.33 | 0.19 |
| Parameter . | OPCAB, (n = 526) . | ONCABG, (n = 186) . | P-value . | TVD . | P-value . | |
|---|---|---|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | |||||
| CPB conversion (%) | 6.5 | — | — | 6.3 | — | |
| CPB time (min) | — | 95 ± 37 | — | — | 96 ± 37 | — |
| Aortic X-clamp time (min) | — | 42 ± 29 | — | — | 43 ± 29 | — |
| IABP intraoperative (%) | 2.9 | 6.5 | 0.04 | 3.3 | 7.1 | 0.09 |
| Number of diseased vessels | 2.36 ± 0.73 | 2.87 ± 0.39 | <0.001 | 3.00 | 3.00 | 1.00 |
| Total number of anastomoses per patient | 2.42 ± 1.15 | 3.06 ± 0.98 | <0.001 | 2.99 ± 1.14 | 3.10 ± 0.98 | 0.19 |
| Complete in situ grafting (%) | 73.4 | 76.9 | 0.38 | 37.1 | 23.9 | 0.005 |
| LIMA (%) | 93.7 | 94.1 | 1.00 | 93.0 | 94.2 | 0.69 |
| RIMA (%) | 50.0 | 81.7 | <0.001 | 58.8 | 83.2 | <0.001 |
| Radial artery (%) | 44.3 | 50.0 | 0.19 | 66.9 | 50.3 | <0.001 |
| Completeness of revascularization (%) | 82.1 | 73.1 | 0.01 | 75.0 | 67.7 | 0.11 |
| ICOR | 1.04 ± 0.37 | 1.09 ± 0.37 | 0.02 | 1.00 ± 0.38 | 1.03 ± 0.33 | 0.19 |
CPB: cardiopulmonary bypass; LIMA: left internal mammary artery; RIMA: right internal mammary artery; ICOR: index of complete revascularization; IABP: intra aortic balloon pump.
| Parameter . | OPCAB, (n = 526) . | ONCABG, (n = 186) . | P-value . | TVD . | P-value . | |
|---|---|---|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | |||||
| CPB conversion (%) | 6.5 | — | — | 6.3 | — | |
| CPB time (min) | — | 95 ± 37 | — | — | 96 ± 37 | — |
| Aortic X-clamp time (min) | — | 42 ± 29 | — | — | 43 ± 29 | — |
| IABP intraoperative (%) | 2.9 | 6.5 | 0.04 | 3.3 | 7.1 | 0.09 |
| Number of diseased vessels | 2.36 ± 0.73 | 2.87 ± 0.39 | <0.001 | 3.00 | 3.00 | 1.00 |
| Total number of anastomoses per patient | 2.42 ± 1.15 | 3.06 ± 0.98 | <0.001 | 2.99 ± 1.14 | 3.10 ± 0.98 | 0.19 |
| Complete in situ grafting (%) | 73.4 | 76.9 | 0.38 | 37.1 | 23.9 | 0.005 |
| LIMA (%) | 93.7 | 94.1 | 1.00 | 93.0 | 94.2 | 0.69 |
| RIMA (%) | 50.0 | 81.7 | <0.001 | 58.8 | 83.2 | <0.001 |
| Radial artery (%) | 44.3 | 50.0 | 0.19 | 66.9 | 50.3 | <0.001 |
| Completeness of revascularization (%) | 82.1 | 73.1 | 0.01 | 75.0 | 67.7 | 0.11 |
| ICOR | 1.04 ± 0.37 | 1.09 ± 0.37 | 0.02 | 1.00 ± 0.38 | 1.03 ± 0.33 | 0.19 |
| Parameter . | OPCAB, (n = 526) . | ONCABG, (n = 186) . | P-value . | TVD . | P-value . | |
|---|---|---|---|---|---|---|
| OPCAB (n = 272) . | ONCABG (n = 155) . | |||||
| CPB conversion (%) | 6.5 | — | — | 6.3 | — | |
| CPB time (min) | — | 95 ± 37 | — | — | 96 ± 37 | — |
| Aortic X-clamp time (min) | — | 42 ± 29 | — | — | 43 ± 29 | — |
| IABP intraoperative (%) | 2.9 | 6.5 | 0.04 | 3.3 | 7.1 | 0.09 |
| Number of diseased vessels | 2.36 ± 0.73 | 2.87 ± 0.39 | <0.001 | 3.00 | 3.00 | 1.00 |
| Total number of anastomoses per patient | 2.42 ± 1.15 | 3.06 ± 0.98 | <0.001 | 2.99 ± 1.14 | 3.10 ± 0.98 | 0.19 |
| Complete in situ grafting (%) | 73.4 | 76.9 | 0.38 | 37.1 | 23.9 | 0.005 |
| LIMA (%) | 93.7 | 94.1 | 1.00 | 93.0 | 94.2 | 0.69 |
| RIMA (%) | 50.0 | 81.7 | <0.001 | 58.8 | 83.2 | <0.001 |
| Radial artery (%) | 44.3 | 50.0 | 0.19 | 66.9 | 50.3 | <0.001 |
| Completeness of revascularization (%) | 82.1 | 73.1 | 0.01 | 75.0 | 67.7 | 0.11 |
| ICOR | 1.04 ± 0.37 | 1.09 ± 0.37 | 0.02 | 1.00 ± 0.38 | 1.03 ± 0.33 | 0.19 |
CPB: cardiopulmonary bypass; LIMA: left internal mammary artery; RIMA: right internal mammary artery; ICOR: index of complete revascularization; IABP: intra aortic balloon pump.
In the subgroup analysis for patients suffering from TVD, the number of distal anastomoses (2.99 ± 1.14 vs. 3.10 ± 0.98; P = 0.19) and the ICOR (1.00 ± 0.38 vs. 1.03 ± 0.33; P = 0.19) was comparable between groups. The frequency of CR (75 vs. 67.7%; P = 0.11) was higher after the OPCAB approach, but failed to achieve statistical significance. With regards to the type of used grafts, ONCABG received significantly more RIMA grafts (58.8 vs. 83.3%; P < 0.001), whereas patients who underwent OPCAB received more radial artery grafts (66.9 vs. 50.3%; P < 0.001). The proportion of complete in situ grafting was significantly higher among OPCAB patients (37.1 vs. 23.9%; P = 0.005).
The need for intra-operative placement of an IABP was lower among OPCAB patients for all-comers (2.9 vs. 6.5%; P = 0.04) as well as in the subgroup analysis (3.3 vs. 7.1%; P = 0.09).
DISCUSSION
This study demonstrates the feasibility and safety of aortic no touch, total arterial off-pump surgery in patients with TVD. The overall outcome data presented here confirm the beneficial effect of OPCAB-TAR with regards to MACCE and, in particular, neurological complications. Furthermore, the study highlights that a standardized OPCAB-TAR approach does not come at price of incomplete revascularization in patients with TVD and complex coronary lesions.
Randomized, prospective trials comprising low-risk patients did not show significant differences between OPCAB and ONCABG [9–11]. In contrast, numerous observational studies demonstrate significant beneficial effects of OPCAB with regards to risk-adjusted morbidity such as MACCE and neurological complications [2–4, 6, 8, 13, 15]. The combination with aortic no touch, total arterial grafting strategies has been demonstrated to further decrease the risk for stroke. This may apply in particular for ‘high-risk’ patients presenting with numerous comorbidities, multi-vessel disease and a general high calcific load due to the presence of concomitant LMD or PAD [16]. A recent meta-analysis of Edelman et al. has highlighted the beneficial effect of aortic no-touch techniques when compared with both, the conventional on-pump technique and partial-clamping approaches during off-pump or on-pump beating heart surgery [6]. In this study, a standardized no-touch OPCAB-TAR technique was associated with a very stroke rate for all-comers (1.0%) and for the subgroup with TVD (0.4%). Our findings are in line with several studies reporting a low stroke rate ranging from 0 to 1% associated with this technique [1, 3, 5, 6, 8].
The no touch, all arterial grafting approach may be carried out in different ways by either using double internal mammary grafts, radial arteries or both as well as different composite grafting strategies such as Y- or T-grafting depending on the degree of coronary artery disease (one-/two-vessel disease versus TVD) and the individual anatomy of the patient, [5, 8, 17]. The use of in situ grafts/composites is preferable whenever possible; but in patients with TVD, the use of free arterial grafts requiring a proximal anastomosis may be preferable in certain circumstances [3, 13, 18, 19]. In this case, the proximal anastomosis can be safely performed in a clamp-less fashion to avoid any aortic manipulation yielding similar results for stroke as achieved with no touch, all arterial grafting approach [3, 13, 18].
Another important aspect of this study was to focus on the feasibility of CR in patients with complex coronary lesions. OPCAB has been repeatedly criticized to be associated with incomplete revascularization due to the technical challenge in patients presenting with multi-vessel disease [11, 12, 20]. In a recent study, evaluating 8081 consecutive patients who underwent isolated surgical revascularization, Filardo et al. [12] have suggested that OPCAB is not the appropriate strategy in patients with multi-vessel disease and that ONCABG might be preferable as it may achieve a more complete and durable revascularization.
In contrast to this report, this study highlights that a standardized OPCAB-TAR approach is not associated with a less CR in patients with TVD. This is clearly reflected by a comparable ‘ICOR’ as well as an overall higher frequency of achieved CR in patients who underwent OPCAB-TAR. The overall number of diseased vessels was lower in the all-comers analysis for OPCAB patients, but in the subgroup analysis for patients with TVD (being equal in the number of diseased vessels), the ICOR remained comparable and the frequency of CR was still slightly higher in the OPCAB-TAR group.
One obvious reason for the reported less CR may be associated with the principal ‘learning curve’ of surgeons relatively inexperienced in the OPCAB technique [15, 21]. Currently, only ∼20% of coronary bypass procedures are performed in off-pump fashion in the Western World [12, 21, 22]. Experience in OPCAB surgery may have an important impact on the CR [14, 23]. The feasibility of CR in off-pump surgery has been demonstrated by several groups considered as ‘high volume’ OPCAB centres [2, 4, 15, 21, 22]. A recent article of Patel and Angellini suggested the idea of so-called expert centres to optimize the potential benefits of OPCAB while decreasing associated problems such as high conversion rates and incomplete revascularization due to inexperience and low case loads [15, 21, 22].
In summary, this report highlights the importance of no-touch OPCAB-TAR when considering the outcomes of surgical revascularization versus PCI. The higher incidence of stroke that is brought forward as a major drawback for surgery [24] can be compensated with this technique and has to be considered in consecutive future trials. This may strengthen the argument for surgical revascularization to be the most appropriate therapy in patients with TVD.
Limitations
First, due to the retrospective, non-randomized design all established disadvantages apply. Although, PS adjustment is a valuable tool, it cannot equal the advantages of a prospective, randomized trial representing the highest level of evidence [25]. On the other hand, and as exemplarily seen in the ROOBY trial [11], prospective trials may often be subject to a certain selection bias enrolling preferably low-risk patients what may not represent the ‘real world’ in the daily clinical practice. Secondly, the study period was long and being a surgical centre with a continuously increasing frequency of the OPCAB technique (nowadays >95% of OPCAB procedures), most of the on-pump procedures were done in the earlier part of the study.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr T. Kieser(Alberta, AB, Canada): To my mind, your hospital in Zurich uses one of the best ways to perform total arterial off-pump bypass surgery: as much bilateral internal thoracic artery as possible, as much in situ BITA as possible, use of the HEARTSTRING™ device for proximal anastomoses, use of shunts to perform the distals, and the use of transit-time flow intraoperatively to assess your grafts. I have two questions.
The many endpoints and combination of endpoints are confusing. Ideally, there is one primary objective, two or three secondary, and the rest can be exploratory. This would cut down the number of P values and reduce the chance of a Type 1 error. I would like to know what you would choose to be your main primary endpoint.
Secondly, the comparison of the overall group is questionable because there is such a difference in the percentage of patients with triple vessel disease: 51.8% in the off-pump group and 83.3% in the on-pump. Propensity analysis, although useful to balance groups, may not be statistically valid when the event rates are low and/or the sample size is not large enough. Would you agree that a sample size estimate calculation might help determine if the number of subjects in your study is sufficient to include significance for the events?
Dr Emmert: Your first question actually is a very good one. Of course, we have included a lot of endpoints also in the full paper and I agree that this might be a bit confusing. This was actually due to the fact that we are performing a lot of subgroup analysis at the moment. Professor Taggart nicely pointed out that off-pump should be applied to a margin of patients and especially to high-risk patients. That's why we need to first define what a high-risk patient is. Is a high-risk patient a patient presenting with renal failure or pulmonary failure, or a patient presenting with a calcified aorta? That's why we have developed a statistical analysis tool where we put in all the different subgroups to get a better insight into which patients benefit most of from the off-pump technique. But I agree, and that's why I have actually only presented the cardiac-related complications in this talk. On the other hand, I think it's worthwhile for the interested reader that we tell them the full results for the subgroups.
Regarding your second question, I fully agree with you, I would love to have had 7,000 patients instead of 700 to get a higher significance in terms of propensity adjustment. We took advice from an expert on that and he also mentioned, of course, that we have some limitations there; however, we always go for the best we have and tried to perform the adjustment wherever possible. I think propensity scoring is of great value to adapt your study findings and to further improve the significance. And as I mentioned, for example, with regards to the stroke rate, this was a good example where we were not able to go further and to perform a propensity-adjusted analysis.
Dr Kieser: It's just your event rates are so low, it's hard, which is good.
Author notes
Presented at the 25th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Lisbon, Portugal, 1–5 October 2011.
- aorta
- myocardial infarction
- anterior descending branch of left coronary artery
- circumflex branch of left coronary artery
- right coronary artery
- cerebrovascular accident
- anastomosis, surgical
- coronary vessels
- objective (goal)
- surgical procedures, operative
- therapeutic touch
- heart
- morbidity
- mortality
- surgery specialty
- transplantation
- revascularization
- triple vessel disease
- off-pump coronary artery bypass
- surrogate endpoints
- touch sensation
- complete arterial revascularization
- multi vessel coronary artery disease




