-
PDF
- Split View
-
Views
-
Cite
Cite
Gabor Erdoes, Reto Basciani, Christoph Huber, Stefan Stortecky, Peter Wenaweser, Stephan Windecker, Thierry Carrel, Balthasar Eberle, Transcranial Doppler-detected cerebral embolic load during transcatheter aortic valve implantation, European Journal of Cardio-Thoracic Surgery, Volume 41, Issue 4, April 2012, Pages 778–784, https://doi.org/10.1093/ejcts/ezr068
Close - Share Icon Share
Abstract
Transcatheter aortic valve implantation (TAVI) is an alternative to surgery for high-risk patients with severe aortic valve stenosis. Periprocedural stroke is reported at an incidence up to 10%. Magnetic resonance imaging studies have identified new onset of clinically silent ischaemic cerebral lesions more frequently (68–84%). So far, few data are available about cerebral embolism during TAVI. The aim of this study was to determine the frequency of high-intensity transient signals (HITS) and to explore differences in the HITS pattern between transfemoral and transapical access and between self-expanding (SE) and balloon-expandable (BE) deployment technique.
Transcranial Doppler (TCD) ultrasound recordings of 44 patients undergoing TAVI (age 78 ± 6 years; logistic EuroSCORE 28 ± 15%; transfemoral access, n = 32; transapical access, n = 12; SE, n = 27; BE, n = 17) were analysed for HITS during the following intervals: (i) instrumentation prior to valvuloplasty, (ii) balloon valvuloplasty, (iii) prosthesis deployment (DP) and (iv) post-implantation (PI) including any re-dilatation episodes. The total procedural load of HITS and HITS frequency in procedural intervals were compared between different access routes and DP techniques. Periprocedural neurocognitive impairment was assessed clinically and by the confusion assessment method (CAM) prior to TAVI and on post-procedural days 1 and 4–6.
TCD recordings demonstrated the occurrence of HITS in all patients. DP was associated with the highest load of HITS. Access route did not significantly influence the total burden of periprocedural HITS. During procedures using the SE type, a slightly larger total load of HITS was observed than with the BE type (P = 0.024). This was mainly due to more HITS during the DP (P = 0.027) and the PI interval (P = 0.002). No incidence of delirium was detected by CAM ratings. Two patients suffered a new onset of stroke within the 2 weeks following the procedure. In-hospital death and 30-day mortality were 0/44.
HITS are observed during all procedural intervals in TAVI. The embolic events appear to peak during DP. In our series, the overall cerebral embolic load did not differ between the transfemoral and the transapical access route. TCD monitoring in TAVI is useful to identify periods and manipulations associated with an increased cerebral embolic load and may help to further enhance the safety of this procedure.
INTRODUCTION
Since the first case report by Cribier, transcatheter aortic valve implantation (TAVI) has emerged as a treatment option for elderly patients with severe valvular aortic stenosis deemed at high risk for conventional surgery [1, 2]. Although TAVI has been shown to decrease mortality and improve the quality of life in selected high-risk patients who are not suitable candidates for surgical aortic valve replacement (SAVR), the transcatheter method involves interventional techniques with a higher degree of invasiveness than simple cardiac catheterization [3, 4]. During cardiac catheterization and accompanying manoeuvres, however, studies have reported both clinically apparent and silent brain embolism by the disruption of vascular plaques and/or calcific debris of the aortic valve [5, 6].
TAVI requires even more extensive intravascular use of guide wires and catheters [4, 7]. In selected cases, concomitant coronary, intracardiac or carotid interventions become necessary due to patients' co-morbidities or intraprocedural complications. Also, the population undergoing TAVI consists of patients of advanced age with a high prevalence of aortic atheromatosis. Therefore, cerebral embolization during TAVI might be even more frequent than during cardiac catheterization alone.
The incidence of periprocedural stroke during TAVI is similar to that reported in conventionally operated patients with aortic stenosis and rates vary widely (3.8–10%; P. Wenaweser et al., submitted for publication) [8–10]. Clinically silent new ischaemic brain lesions have been reported to occur even more frequently (68–84%), as observed in recent diffusion-weighted cerebral magnetic resonance imaging (DW-MRI) studies [11–14].
In order to develop strategies for reducing the risk of cerebral embolism during TAVI, the recognition of cerebral embolic events and their relationship with procedural intervals would be desirable. For this purpose, transcranial Doppler (TCD) ultrasound is commended monitoring modality to detect and quantify, non-invasively and in real-time, high-intensity transient signals (HITS), which represent solid or gaseous cerebral microemboli passing the middle cerebral artery (MCA) [15, 16].
The aim of this study was to measure the quantity of HITS during TAVI and to relate it to different procedural intervals, deployment techniques of the aortic bioprosthesis, access routes and early post-procedural neurological outcome.
MATERIALS AND METHODS
Patients
During the study period, a total of 67 consecutive patients underwent TAVI at our institution and were followed prospectively within an institutional registry. Patients were considered for TAVI if they had severe symptomatic aortic valve stenosis with a calculated aortic valve area <1 cm2 or an aortic valve mean pressure gradient of >40 mmHg on echocardiography, age ≥ 80 years and/or a logistic EuroSCORE ≥ 15%, or age > 70 years with a predicted high or prohibitive risk of morbidity or/and mortality for SAVR. Patient selection, indication for TAVI and contraindications for SAVR were reviewed and had to be agreed upon by a consensus team of interventional cardiologists and cardiac surgeons.
Registry data and TCD recordings were collected in a prospective observational fashion, without any study intervention, and were analysed with written informed consent of the patients and the approval of the Institutional Review Board.
Transcatheter aortic valve implantation procedure
Depending on anatomical conditions, feasible access sites and indications for aortic bioprosthesis type and size, either the transfemoral, the transapical or the trans-subclavian access was chosen. All procedures were performed as reported previously in detail by our group and others [4, 7]. General endotracheal anaesthesia using a total intravenous technique (propofol combined with remifentanil) was administered in all transapical and trans-subclavian access TAVI. In transfemoral TAVI (TF-AVI), local anaesthesia with monitored anaesthesia care was chosen for the majority of cases, using a sedative and analgesic combination of propofol and racemic ketamine. Continuous anaesthesia monitoring consisted of ECG, pulse oximetry, capnography, invasive arterial and central venous pressure as well as TCD. Either the balloon-expandable (BE) Edwards SAPIEN valve (Edwards Lifesciences Inc., Irvine, CA, USA) or the self-expanding (SE) Core Valve Revalving system (Medtronic Inc., Minneapolis, MN, USA) was implanted.
The periprocedural antithrombotic regimen consisted of intravenous administration of a weight adjusted bolus of heparin (70–100 IU/kg) in order to achieve an activating clotting time of >250 s for the duration of the procedure. The antiplatelet regimen consisted of acetylsalicylic acid of 100 mg and a loading dose of 300 mg clopidogrel in TF-AVI patients on the day prior to the procedure. For patients undergoing transapical TAVI (TA-AVI), the clopidogrel loading dose was administered 6 h after apical access closure. Patients were discharged with the prescription of acetylsalicylic acid 100 mg/day indefinitely and clopidogrel 75 mg/day for 3–6 months. In the case of an indication for oral anticoagulation, warfarin was combined with either acetylsalicylic acid or clopidogrel alone.
Transcranial Doppler ultrasonography and embolus detection
As part of the institutional practice in cerebral monitoring prior to and during TAVI, a TCD probe (2 MHz pulsed-wave transducer; Spencer Technologies, Seattle, WA, USA) was placed bilaterally at the temporal bone acoustic window. A commercially available probe-holding head frame system for continuous HITS acquisition (Marc 600 series; Spencer Technologies) was used. Power output and gain settings were adjusted on the TCD machine (ST3, Model # PMD150; Spencer Technologies) to provide an optimal signal-to-noise ratio. The sample volume was 3–6 mm3. Using the 33 gate Power M-mode™ function for rapid vessel localization, characteristic blood flow velocity spectra were easily detected with high signal quality by insonation of the MCA at a depth between 48 and 56 mm.
HITS detection was performed automatically using the multidepth embolic detection with artefact rejection. Because certain technical criteria must be met to qualify HITS as microemboli, all TCD recordings were reviewed (G.E.) in a blinded fashion after the procedures to confirm the quantity and the quality of microembolic signals [17]. Thereafter, TCD recordings were analysed according to the defined protocol. The cumulative counts of HITS recordable during the period between establishment and closure of vascular access were analysed, as well as the load of HITS accumulated during the following procedural intervals: (i) instrumentation prior to balloon valvuloplasty of the native aortic valve (IN), defined as the period from first femoral vessel puncture until the transcatheter introduction of the valvuloplasty balloon; (ii) balloon valvuloplasty of the native aortic valve (BV), defined as the period from the transcatheter introduction of the valvuloplasty balloon until the transcatheter introduction of the balloon-mounted valve; (iii) prosthesis deployment (DP), defined as the period from the transcatheter introduction of the balloon-mounted valve until the removal of the deployment system and (iv) the post-implantation (PI) phase, defined as the period from the removal of the valve deployment system until the closure of the access, also including any re-dilatation episodes. The duration of each interval was recorded in the interventional protocols and was used to calculate the average rate of HITS per minute for each interval.
Neurologic and neurocognitive assessment
A gross clinical neurologic status was documented 1 day prior to TAVI and after TAVI at days 1–6 by the attending anaesthesiologist. Patients were screened for stroke and post-procedural delirium by the confusion assessment method (CAM), which was applied prior to TAVI and on post-procedural days 1 and 4–6 [18]. As per institutional policy, any pathological finding at clinical examination or CAM had to be verified by a neurology consultant, and if appropriate, neuroimaging studies were performed.
Statistical analysis
Categorical data are given as numbers and percentages and were compared using the chi-squared or Fisher's exact test where appropriate. Continuous data are described using medians with interquartile ranges; for comparisons between groups (deployment technique and access route) or repeated measures (procedural intervals), a non-normal distribution was assumed, and non-parametric testing was applied (Mann–Whitney rank-sum test; Friedman repeated-measures ANOVA on ranks). A P-value of <0.05 was considered as statistically significant. Statistical analyses were performed using Sigma Plot for Windows, Version 10.0, and Sigma Stat for Windows, Version 3.0 (Systat Software, Inc., Germany).
RESULTS
Demographics of the study population and procedure characteristics
Of 67 consecutive TAVI procedures in the observation period, 44 patients (66%) exhibited MCA spectra of good quality throughout the whole TAVI procedure and were included in the study. In the remaining 23 patients (34%), either no TCD could be performed for logistic reasons or MCA signals were inconsistently or not obtained due to the absence of an acoustic window, prohibiting continuous bilateral TCD recording and HITS detection throughout the procedure. Thus, these patients were excluded.
The TAVI access route was transfemoral in 32 patients, and transapical in 12 patients. In the majority of the cases, an SE prosthesis type was implanted (n = 27). In contrast to SE prostheses, BE prostheses were implanted both transfemorally (n = 5) and transapically (n = 12).
Baseline characteristics of the study population are shown in Table 1. Procedural characteristics are given in Table 2 and demonstrate, in TA-AVI procedures, a significantly longer duration of IN than in TF-AVI procedures (P = 0.01). There were, however, no other significant differences between time requirements (overall procedure duration: TF-AVI, 67 min vs. TA-AVI, 83 min; P = 0.7). In both groups, the longest procedural interval was IN.
| . | All TAVI . | TF-AVI . | TA-AVI . |
|---|---|---|---|
| n | 44 | 32 | 12 |
| Age (years)a | 78 ± 6 | 79 ± 5 | 74 ± 8 |
| Male sexb | 24 (55) | 17 (53) | 8 (67) |
| NYHA classc | III (III; III) | III (III; III) | III (III; III) |
| Log EuroSCORE (%)a | 28 ± 15 | 29 ± 17 | 25 ± 10 |
| LV-EF (%)a | 48 ± 17 | 46 ± 16 | 55 ± 18 |
| Valve area (cm2)a | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 |
| History of atrial fibrillationb | 3 (7) | 3 (9) | 0 (0) |
| Coronary artery diseaseb | 27 (61) | 19 (59) | 8 (67) |
| History of strokeb | 1 (2) | 1 (3) | 0 (0) |
| ICA stenosis >50%b | 7 (16) | 6 (19) | 1 (8) |
| . | All TAVI . | TF-AVI . | TA-AVI . |
|---|---|---|---|
| n | 44 | 32 | 12 |
| Age (years)a | 78 ± 6 | 79 ± 5 | 74 ± 8 |
| Male sexb | 24 (55) | 17 (53) | 8 (67) |
| NYHA classc | III (III; III) | III (III; III) | III (III; III) |
| Log EuroSCORE (%)a | 28 ± 15 | 29 ± 17 | 25 ± 10 |
| LV-EF (%)a | 48 ± 17 | 46 ± 16 | 55 ± 18 |
| Valve area (cm2)a | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 |
| History of atrial fibrillationb | 3 (7) | 3 (9) | 0 (0) |
| Coronary artery diseaseb | 27 (61) | 19 (59) | 8 (67) |
| History of strokeb | 1 (2) | 1 (3) | 0 (0) |
| ICA stenosis >50%b | 7 (16) | 6 (19) | 1 (8) |
NYHA: New York Health Association class; Log EuroSCORE: European System for Cardiac Operative Risk Evaluation Score; LV-EF: left ventricular ejection fraction; ICA: internal carotid artery; TAVI: transcatheter aortic valve implantation; TF-AVI: transfemoral aortic valve implantation; TA-AVI: transapical aortic valve implantation. aValues are the mean ± SD.
bValues are n (%).
cValues are the median (25th; 75th percentiles).
| . | All TAVI . | TF-AVI . | TA-AVI . |
|---|---|---|---|
| n | 44 | 32 | 12 |
| Age (years)a | 78 ± 6 | 79 ± 5 | 74 ± 8 |
| Male sexb | 24 (55) | 17 (53) | 8 (67) |
| NYHA classc | III (III; III) | III (III; III) | III (III; III) |
| Log EuroSCORE (%)a | 28 ± 15 | 29 ± 17 | 25 ± 10 |
| LV-EF (%)a | 48 ± 17 | 46 ± 16 | 55 ± 18 |
| Valve area (cm2)a | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 |
| History of atrial fibrillationb | 3 (7) | 3 (9) | 0 (0) |
| Coronary artery diseaseb | 27 (61) | 19 (59) | 8 (67) |
| History of strokeb | 1 (2) | 1 (3) | 0 (0) |
| ICA stenosis >50%b | 7 (16) | 6 (19) | 1 (8) |
| . | All TAVI . | TF-AVI . | TA-AVI . |
|---|---|---|---|
| n | 44 | 32 | 12 |
| Age (years)a | 78 ± 6 | 79 ± 5 | 74 ± 8 |
| Male sexb | 24 (55) | 17 (53) | 8 (67) |
| NYHA classc | III (III; III) | III (III; III) | III (III; III) |
| Log EuroSCORE (%)a | 28 ± 15 | 29 ± 17 | 25 ± 10 |
| LV-EF (%)a | 48 ± 17 | 46 ± 16 | 55 ± 18 |
| Valve area (cm2)a | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 |
| History of atrial fibrillationb | 3 (7) | 3 (9) | 0 (0) |
| Coronary artery diseaseb | 27 (61) | 19 (59) | 8 (67) |
| History of strokeb | 1 (2) | 1 (3) | 0 (0) |
| ICA stenosis >50%b | 7 (16) | 6 (19) | 1 (8) |
NYHA: New York Health Association class; Log EuroSCORE: European System for Cardiac Operative Risk Evaluation Score; LV-EF: left ventricular ejection fraction; ICA: internal carotid artery; TAVI: transcatheter aortic valve implantation; TF-AVI: transfemoral aortic valve implantation; TA-AVI: transapical aortic valve implantation. aValues are the mean ± SD.
bValues are n (%).
cValues are the median (25th; 75th percentiles).
| . | All TAVI . | TF-AVI . | TA-AVI . | P-value . |
|---|---|---|---|---|
| n | 44 | 32 | 12 | |
| Overall duration (min)a | 75 ± 18 | 67 ± 16 | 83 ± 22 | 0.7 |
| Duration (min)b | ||||
| Instrumentation (IN) | 36 (17–58) | 30 (23–40) | 43 (39–52) | 0.01 |
| Valvuloplasty (BV) | 3 (2–6) | 4 (2–5) | 3 (3–3.5) | 0.4 |
| Deployment (DP) | 2 (1–5) | 2 (2–3) | 2 (2–2.5) | 0.6 |
| Post-implantation | 26 (15–60) | 26 (20–36) | 26 (16–50) | 0.9 |
| . | All TAVI . | TF-AVI . | TA-AVI . | P-value . |
|---|---|---|---|---|
| n | 44 | 32 | 12 | |
| Overall duration (min)a | 75 ± 18 | 67 ± 16 | 83 ± 22 | 0.7 |
| Duration (min)b | ||||
| Instrumentation (IN) | 36 (17–58) | 30 (23–40) | 43 (39–52) | 0.01 |
| Valvuloplasty (BV) | 3 (2–6) | 4 (2–5) | 3 (3–3.5) | 0.4 |
| Deployment (DP) | 2 (1–5) | 2 (2–3) | 2 (2–2.5) | 0.6 |
| Post-implantation | 26 (15–60) | 26 (20–36) | 26 (16–50) | 0.9 |
TAVI: transcatheter aortic valve implantation; TF-AVI: transfemoral aortic valve implantation; TA-AVI: transapical aortic valve implantation. aValues are the mean ± SD.
bValues are the median (25th; 75th percentile).
| . | All TAVI . | TF-AVI . | TA-AVI . | P-value . |
|---|---|---|---|---|
| n | 44 | 32 | 12 | |
| Overall duration (min)a | 75 ± 18 | 67 ± 16 | 83 ± 22 | 0.7 |
| Duration (min)b | ||||
| Instrumentation (IN) | 36 (17–58) | 30 (23–40) | 43 (39–52) | 0.01 |
| Valvuloplasty (BV) | 3 (2–6) | 4 (2–5) | 3 (3–3.5) | 0.4 |
| Deployment (DP) | 2 (1–5) | 2 (2–3) | 2 (2–2.5) | 0.6 |
| Post-implantation | 26 (15–60) | 26 (20–36) | 26 (16–50) | 0.9 |
| . | All TAVI . | TF-AVI . | TA-AVI . | P-value . |
|---|---|---|---|---|
| n | 44 | 32 | 12 | |
| Overall duration (min)a | 75 ± 18 | 67 ± 16 | 83 ± 22 | 0.7 |
| Duration (min)b | ||||
| Instrumentation (IN) | 36 (17–58) | 30 (23–40) | 43 (39–52) | 0.01 |
| Valvuloplasty (BV) | 3 (2–6) | 4 (2–5) | 3 (3–3.5) | 0.4 |
| Deployment (DP) | 2 (1–5) | 2 (2–3) | 2 (2–2.5) | 0.6 |
| Post-implantation | 26 (15–60) | 26 (20–36) | 26 (16–50) | 0.9 |
TAVI: transcatheter aortic valve implantation; TF-AVI: transfemoral aortic valve implantation; TA-AVI: transapical aortic valve implantation. aValues are the mean ± SD.
bValues are the median (25th; 75th percentile).
High-intensity transient signal-derived cerebral embolic load and neurologic performance
Counts of HITS were symmetrically distributed between left and right MCA territories. Symmetry of hemispheric distribution was independent from the procedural period, access route and deployment technique (Table 3).
Total, left and right hemispheric HITS counts in relation to procedural intervals
| Period and access . | Total HITS . | HITS left MCA . | HITS right MCA . | aP-value . |
|---|---|---|---|---|
| All TAVI (n = 44) | ||||
| Overall | 548 (386–692) | 255 (190–354) | 274 (185–356) | 0.93 |
| Instrumentation (IN) | 137 (102–190)* | 73 (47–94) | 73 (50–103) | 0.90 |
| Valvuloplasty (BV) | 63 (32–109) | 32 (17–52) | 29 (12–54) | 0.38 |
| Deployment (DP) | 183 (112–316)* | 93 (48–157) | 98 (53–142) | 0.63 |
| Post-implantation | 50 (22–97) | 28 (12–47) | 23 (10–45) | 0.07 |
| Period and access . | Total HITS . | HITS left MCA . | HITS right MCA . | aP-value . |
|---|---|---|---|---|
| All TAVI (n = 44) | ||||
| Overall | 548 (386–692) | 255 (190–354) | 274 (185–356) | 0.93 |
| Instrumentation (IN) | 137 (102–190)* | 73 (47–94) | 73 (50–103) | 0.90 |
| Valvuloplasty (BV) | 63 (32–109) | 32 (17–52) | 29 (12–54) | 0.38 |
| Deployment (DP) | 183 (112–316)* | 93 (48–157) | 98 (53–142) | 0.63 |
| Post-implantation | 50 (22–97) | 28 (12–47) | 23 (10–45) | 0.07 |
Values are the median (25th; 75th percentile). TAVI: transcatheter aortic valve implantation; HITS: high-intensity transient signal; MCA: middle cerebral artery.
aComparison between left and right MCA.
*P < 0.05 vs. BV, PI.
Total, left and right hemispheric HITS counts in relation to procedural intervals
| Period and access . | Total HITS . | HITS left MCA . | HITS right MCA . | aP-value . |
|---|---|---|---|---|
| All TAVI (n = 44) | ||||
| Overall | 548 (386–692) | 255 (190–354) | 274 (185–356) | 0.93 |
| Instrumentation (IN) | 137 (102–190)* | 73 (47–94) | 73 (50–103) | 0.90 |
| Valvuloplasty (BV) | 63 (32–109) | 32 (17–52) | 29 (12–54) | 0.38 |
| Deployment (DP) | 183 (112–316)* | 93 (48–157) | 98 (53–142) | 0.63 |
| Post-implantation | 50 (22–97) | 28 (12–47) | 23 (10–45) | 0.07 |
| Period and access . | Total HITS . | HITS left MCA . | HITS right MCA . | aP-value . |
|---|---|---|---|---|
| All TAVI (n = 44) | ||||
| Overall | 548 (386–692) | 255 (190–354) | 274 (185–356) | 0.93 |
| Instrumentation (IN) | 137 (102–190)* | 73 (47–94) | 73 (50–103) | 0.90 |
| Valvuloplasty (BV) | 63 (32–109) | 32 (17–52) | 29 (12–54) | 0.38 |
| Deployment (DP) | 183 (112–316)* | 93 (48–157) | 98 (53–142) | 0.63 |
| Post-implantation | 50 (22–97) | 28 (12–47) | 23 (10–45) | 0.07 |
Values are the median (25th; 75th percentile). TAVI: transcatheter aortic valve implantation; HITS: high-intensity transient signal; MCA: middle cerebral artery.
aComparison between left and right MCA.
*P < 0.05 vs. BV, PI.
The total procedural load of HITS did not differ significantly between access routes, but was smaller in BE-type than in SE-type prostheses [BE, 412 (354–585); SE, 580 (456–777 ); P = 0.024; Table 4].
The HITS load per procedural interval depending on the deployment technique and the access route
| Deployment technique . | SE (n = 27) . | BE (n = 17) . | P-value . |
|---|---|---|---|
| HITS total procedure | 580 (456–777) | 412 (354–585) | 0.024 |
| Instrumentation | 155 (102–194) | 136 (93–197) | 0.66 |
| Valvuloplasty | 64 (41–97) | 46 (12–161) | 0.46 |
| Deployment | 256 (132–352) | 158 (103–201) | 0.027 |
| Post-implantation | 71 (44–125) | 32 (18–49) | 0.002 |
| Access route | TF (n = 32) | TA (n = 12) | P-value |
| HITS total procedure | 563 (393–731) | 428 (370–664) | 0.22 |
| Instrumentation | 156 (103–203) | 121 (56–152) | 0.16 |
| Valvuloplasty | 62 (32–95) | 72 (30–188) | 0.47 |
| Deployment | 198 (111–431) | 178 (137–207) | 0.40 |
| Post-implantation | 63 (44–124) | 30 (13–49) | 0.004 |
| Deployment technique . | SE (n = 27) . | BE (n = 17) . | P-value . |
|---|---|---|---|
| HITS total procedure | 580 (456–777) | 412 (354–585) | 0.024 |
| Instrumentation | 155 (102–194) | 136 (93–197) | 0.66 |
| Valvuloplasty | 64 (41–97) | 46 (12–161) | 0.46 |
| Deployment | 256 (132–352) | 158 (103–201) | 0.027 |
| Post-implantation | 71 (44–125) | 32 (18–49) | 0.002 |
| Access route | TF (n = 32) | TA (n = 12) | P-value |
| HITS total procedure | 563 (393–731) | 428 (370–664) | 0.22 |
| Instrumentation | 156 (103–203) | 121 (56–152) | 0.16 |
| Valvuloplasty | 62 (32–95) | 72 (30–188) | 0.47 |
| Deployment | 198 (111–431) | 178 (137–207) | 0.40 |
| Post-implantation | 63 (44–124) | 30 (13–49) | 0.004 |
Values are the median (25th; 75th percentile). HITS: high-intensity transient signal; SE: self-expanding deployment technique; BE: balloon-expandable deployment technique; TF: transfemoral access route; TA: transapical access route.
The HITS load per procedural interval depending on the deployment technique and the access route
| Deployment technique . | SE (n = 27) . | BE (n = 17) . | P-value . |
|---|---|---|---|
| HITS total procedure | 580 (456–777) | 412 (354–585) | 0.024 |
| Instrumentation | 155 (102–194) | 136 (93–197) | 0.66 |
| Valvuloplasty | 64 (41–97) | 46 (12–161) | 0.46 |
| Deployment | 256 (132–352) | 158 (103–201) | 0.027 |
| Post-implantation | 71 (44–125) | 32 (18–49) | 0.002 |
| Access route | TF (n = 32) | TA (n = 12) | P-value |
| HITS total procedure | 563 (393–731) | 428 (370–664) | 0.22 |
| Instrumentation | 156 (103–203) | 121 (56–152) | 0.16 |
| Valvuloplasty | 62 (32–95) | 72 (30–188) | 0.47 |
| Deployment | 198 (111–431) | 178 (137–207) | 0.40 |
| Post-implantation | 63 (44–124) | 30 (13–49) | 0.004 |
| Deployment technique . | SE (n = 27) . | BE (n = 17) . | P-value . |
|---|---|---|---|
| HITS total procedure | 580 (456–777) | 412 (354–585) | 0.024 |
| Instrumentation | 155 (102–194) | 136 (93–197) | 0.66 |
| Valvuloplasty | 64 (41–97) | 46 (12–161) | 0.46 |
| Deployment | 256 (132–352) | 158 (103–201) | 0.027 |
| Post-implantation | 71 (44–125) | 32 (18–49) | 0.002 |
| Access route | TF (n = 32) | TA (n = 12) | P-value |
| HITS total procedure | 563 (393–731) | 428 (370–664) | 0.22 |
| Instrumentation | 156 (103–203) | 121 (56–152) | 0.16 |
| Valvuloplasty | 62 (32–95) | 72 (30–188) | 0.47 |
| Deployment | 198 (111–431) | 178 (137–207) | 0.40 |
| Post-implantation | 63 (44–124) | 30 (13–49) | 0.004 |
Values are the median (25th; 75th percentile). HITS: high-intensity transient signal; SE: self-expanding deployment technique; BE: balloon-expandable deployment technique; TF: transfemoral access route; TA: transapical access route.
For both access routes and prosthesis types, the largest embolic load occurred during the few minutes of DP. In particular, SE-type prostheses released more HITS than BE-type valves during this interval [SE, 256 (132–352); BE, 158 (103–201); P = 0.027; Table 4].
During instrumentation, a relatively large embolic load occurred, too (Table 4). This interval lasted, however, more than half an hour, i.e. much longer than the deployment interval. The access type and the deployment technique did not exhibit differences in HITS during the instrumentation period.
In the PI interval, transfemoral implants generated more HITS compared with transapical implants [TF-AVI, 63 (44–124); TA-AVI, 30 (13–49); P = 0.004; Table 4). Also, the deployment technique had a small influence on PI HITS [SE, 71 (44–125); BE, 32 (18–49); P = 0.02; Table 4). Figure 1 summarizes the relevant results.
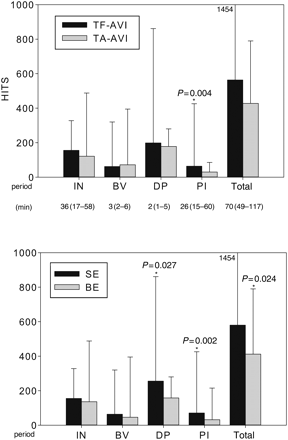
HITS load according to the TAVI access route and the deployment technique.
There was no incidence of delirium (as measured by CAM) during hospital stay; however, one patient of the TF-AVI group and one patient of the TA-AVI group suffered new stroke on the post-interventional days 4 and 13, respectively. No in-hospital death occurred, and the 30-day mortality was 0/44 in the entire cohort.
DISCUSSION
In patients undergoing TAVI, we determined the quantity and the distribution of HITS by TCD ultrasound. We related the occurrence of HITS to procedural intervals, types of vascular access and deployment techniques.
The main finding is that the peak HITS load occurs during the very short interval of valve deployment. Pre-implantation instrumentation releases almost as many HITS, although over a longer time period. The other intervals are characterized by both shorter duration and lower incidence of HITS. Our main results are in good accordance with a recent TCD study performed exclusively in TA-AVI procedures, where microembolic signals were detected in all patients and where peak embolic frequency was also recorded during valve delivery [19].
Additional findings of our study describe the effect of access route and deployment technique on the incidence of HITS: a larger total load of HITS was observed with the use of SE prostheses. This difference was attributable to more HITS during both DP and PI. Several hypotheses may be discussed to explain these observations: counts of HITS during instrumentation, balloon valvuloplasty, deployment and total procedure were found to be quite similar in transfemoral and transapical approaches. Although the transapical access is associated with less catheter traffic through ascending aorta and aortic arch, this appears to have less impact on the embolic load than the interaction of rigid wires and stent/frame structures with aortic valvular and root calcifications. The interaction between hardware and aortic root calcifications may become aggravated by the specific deployment technique of the SE-type prosthesis. In our series, SE-type valves were exclusively released without rapid right ventricular pacing. Thus, antegrade ejection continued during most of the gradual release process of the framed valve. This may lead to prolonged friction between prosthetic frame and calcifications, and the antegrade embolization of debris may be promoted by sustained cardiac ejection. In this regard, stopping ejection briefly by rapid pacing, until the frame is seated, may even be protective for the brain. At the present stage, however, these considerations still remain theoretical and require further study.
Further, there was the finding of a low HITS rate during PI, which was nevertheless slightly higher with transfemoral access and SE deployment technique. More frequent re-dilation manoeuvres of deployed but regurgitant TF/SE prostheses may have played a role [the number of patients with re-dilatation: SE = 5 (18%) vs. BE = 1 (6%)]. Also, the removal of large-bore transfemoral introducers through the aortic arch may have led to the contact with and friction at atheromatous wall areas upstream of cephalic branch vessels.
Given the lack of other TCD studies comparing the transfemoral with the transapical access route in TAVI, discussions of pathomechanism must rely on studies into micro-embolization during cardiac catheterization [20, 21]. These reports also emphasize the potentially harmful consequences of stiff large-bore catheters and of flushing and contrast injection in the atherosclerotic ascending aorta and the higher risk of cerebral embolization in patients with coronary artery disease.
At present, the clinical significance of the observed incidence and periprocedural distribution of HITS remains unclear. Despite reliable and objective TCD recordings, as shown by the symmetrical distribution of HITS between brain hemispheres, we found no clinically apparent differences in the gross periprocedural neurocognitive outcome to associate them with the HITS load, deployment technique or access route. Likewise, the strokes detected in two patients in the post-procedural phase were not associated with increased counts of HITS during TAVI, which reflects multifactority and complexity of cerebral ischaemic events. With low event rates of new-onset stroke (4%), delirium and in-hospital death (0% each), this study was not powered to detect associations between the HITS load and these major adverse clinical outcomes.
Our results are in agreement with previously published neuroimaging studies prior to and after TAVI (Table 5). In a series investigating both the transfemoral and the transapical valve implantation technique, 68% of the treated patients had new cerebral ischaemic lesions on DW-MRI post-interventionally, even though a clinical neurological correlate was detected in only 3.3%. Similarly, other reports, which exclusively studied TF-AVI or TA-AVI, confirmed a periprocedural incidence of permanent neurological impairment in the range of 3.6–5%, with a 15–20× higher rate of clinically silent new ischaemic foci on DW-MRI [11–14].
| Author . | Year . | Patients (n) . | TAVI type . | Neuroimaging modality . | Silent new lesion (%) . | New-onset stroke (%) . |
|---|---|---|---|---|---|---|
| Drews [19] | 2011 | 50 | TA-AVI | CT | 8 | 0 |
| Rodes-Cabau [11] | 2011 | 60 | TF/TA-AVI | DW-MRI | 68 | 3.3 |
| Kahlert [12] | 2010 | 32 | TF-AVI | DW-MRI | 84 | 5 |
| Ghanem [13] | 2010 | 22 | TF-AVI | DW-MRI | 72.7 | 3.6 |
| Arnold [14] | 2010 | 25 | TA-AVI | DW-MRI | 68 | 4 |
| Author . | Year . | Patients (n) . | TAVI type . | Neuroimaging modality . | Silent new lesion (%) . | New-onset stroke (%) . |
|---|---|---|---|---|---|---|
| Drews [19] | 2011 | 50 | TA-AVI | CT | 8 | 0 |
| Rodes-Cabau [11] | 2011 | 60 | TF/TA-AVI | DW-MRI | 68 | 3.3 |
| Kahlert [12] | 2010 | 32 | TF-AVI | DW-MRI | 84 | 5 |
| Ghanem [13] | 2010 | 22 | TF-AVI | DW-MRI | 72.7 | 3.6 |
| Arnold [14] | 2010 | 25 | TA-AVI | DW-MRI | 68 | 4 |
TAVI: transcatheter aortic valve implantation; TF-AVI: transfemoral aortic valve implantation; TA-AVI: transapical aortic valve implantation; CT: computed tomography; DW-MRI: diffusion-weighted magnetic resonance imaging.
| Author . | Year . | Patients (n) . | TAVI type . | Neuroimaging modality . | Silent new lesion (%) . | New-onset stroke (%) . |
|---|---|---|---|---|---|---|
| Drews [19] | 2011 | 50 | TA-AVI | CT | 8 | 0 |
| Rodes-Cabau [11] | 2011 | 60 | TF/TA-AVI | DW-MRI | 68 | 3.3 |
| Kahlert [12] | 2010 | 32 | TF-AVI | DW-MRI | 84 | 5 |
| Ghanem [13] | 2010 | 22 | TF-AVI | DW-MRI | 72.7 | 3.6 |
| Arnold [14] | 2010 | 25 | TA-AVI | DW-MRI | 68 | 4 |
| Author . | Year . | Patients (n) . | TAVI type . | Neuroimaging modality . | Silent new lesion (%) . | New-onset stroke (%) . |
|---|---|---|---|---|---|---|
| Drews [19] | 2011 | 50 | TA-AVI | CT | 8 | 0 |
| Rodes-Cabau [11] | 2011 | 60 | TF/TA-AVI | DW-MRI | 68 | 3.3 |
| Kahlert [12] | 2010 | 32 | TF-AVI | DW-MRI | 84 | 5 |
| Ghanem [13] | 2010 | 22 | TF-AVI | DW-MRI | 72.7 | 3.6 |
| Arnold [14] | 2010 | 25 | TA-AVI | DW-MRI | 68 | 4 |
TAVI: transcatheter aortic valve implantation; TF-AVI: transfemoral aortic valve implantation; TA-AVI: transapical aortic valve implantation; CT: computed tomography; DW-MRI: diffusion-weighted magnetic resonance imaging.
To date, no consensus exists on the clinical impact of HITS, although evidence is accumulating that cerebral embolism plays a contributing role in the pathogenesis of post-operative cognitive decline [22–24]. In patients undergoing coronary artery bypass surgery, the overall number of HITS has been found to correlate with changes in cerebral activity at functional MRI [25].
This study has several limitations besides its non-randomized design, small cohort size and lack of power for low-incidence outcomes. Neurocognitive assessment was based on clinical neurological examination and CAM only. The subgroups defined by the access route and the deployment technique were of imbalanced size; they were also not independent categories, since the SE-type prosthesis was implanted exclusively by the transfemoral route. In addition, the less number of TF-AVI cases in the BE group limits any statistical comparison between the two prosthesis types with regard to the implantation technique. It is thus not yet possible to discriminate the causative role of access and the deployment type for HITS release. Also, procedural techniques may vary between interventionists and institutions; transferability of its results to different institutional settings or patient populations may therefore be limited. Nevertheless, our study confirms procedure-related cerebral embolism during TAVI. As a portable non-invasive cerebral monitoring device, TCD provides the real-time detection of HITS in TAVI patients [15, 16]. Insonation of the MCA permits accurate monitoring of 70% of the total cerebral blood flow and therefore the detection of a majority of cerebral embolic events. Nevertheless, the specificity of HITS detection for clinically apparent embolic stroke is necessarily low, and embolic stroke can also occur post-procedurally after the removal of the TCD monitor.
In conclusion, TCD-detectable cerebral embolism occurs regularly during TAVI. We observed statistical associations of the embolic event rate with deployment techniques and defined procedural phases; specifically, the event rate peaks during valve deployment. Our findings are so far derived from a patient cohort of a size yet to small to allow for robust conclusions. Nevertheless, this study has demonstrated that TCD monitoring in TAVI helps to identify periods and manipulations associated with increased cerebral embolic risk and points out areas for further technical improvement.
ACKNOWLEDGEMENTS
The authors thank the clinical research group of the Department of Anaesthesiology and Pain Medicine, University Hospital Bern, and in particular Ms. Monika Stucki, for professional support.
Conflicts of interest: R.B. and B.E. receive lecture fees from Medtronic Inc. C.H. is proctor of Edwards Lifesciences Inc. P.W. and S.W. receive lecture and consultant fees from Edwards Lifesciences Inc. and Medtronic Inc. T.C. receives speaker fees from Edwards Lifesciences Inc., Medtronic Inc. and Sorin Inc.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr J. Seeburger(Leipzig, Germany): There is actually only one thing I would like to ask. When it comes to stroke, it seems that the technology of transcranial Doppler is becoming an issue. So far, you have only shown that this is feasible, that you can detect any embolic growth that you see during TAVI, but has it ever changed the management of such a procedure? So the question I would like to ask is, how do you think you can improve and what is the relevance behind the study other than descriptive? How do you think you can change the management of TAVI using the method that you just presented?
Dr Huber(Bern, Switzerland): There are new device types on the horizon, cerebral embolic protection devices. The questions now are, should these be used routinely or not, in what phases of the intervention should they be used, and what do we do with all the late strokes after the intervention? The other question is, is there a relationship between the device and the stroke rate? I think, as a very first step, transcranial Doppler might be used to help answer such questions. Obviously the results should be related to MRI findings as well, and also to improved and in-depth neurologic examination in the long run during the follow-up period.
The deployment process might be of particular interest. We have observed that patients having self-expanding devices all had implantation without rapid pacing. Therefore it might be of importance to have rapid pacing in order to decrease blood flow through the aortic valve during device implantation. That might be one of the results of this paper that we want to have a look at in the future.
Dr L.K. von Segesser(Lausanne, Switzerland): I have a question. Throughout your presentation you have used the term “HITS” for high-intensity signals and emboli, sometimes quite ambivalently, and I think we have to state that these are not the same: high-intensity signals are signals but they are not necessarily emboli. A typical example, for instance, is that when you test for patency of the foramen ovale with some bubbles, there are signals. You can detect them with the Doppler, but they are not considered emboli but are injected on purpose. Please comment.
Dr Huber: Those are types of gaseous emboli, gas bubbles, that will travel with the blood flow somewhere into an end organ in the patient's body. It is not certain whether they cause any harm. But you are right, we should be careful about the distinction between the word “HITS” and the word “emboli”, as emboli might relate more to something that has a significant clinical impact, as opposed to HITS that might just be turbulence of flow. But the entire transcranial Doppler study has been reviewed by the same operator on all patients to try to exactly identify all the artefacts in the signals that we are measuring.
Dr N.M. Van Mieghem(Rotterdam, The Netherlands): Christoph, I have a question regarding transcranial Doppler. We have also been using this technology, and we went nuts because we had HITS all the time during the procedure that we could not interpret. So I am wondering, have you evaluated the interobserver and intraobserver variability? And the person who is performing the measurements, is he doing simultaneous Doppler measurements on the left and right carotid, and using his two hands during the procedure?
Dr Huber: First of all, there is no interoperator variability because the person who did all the measurements was always the same, Erdos Gabor, our anaesthetist. The transcranial Doppler is placed on both sides of the patient, so that there is simultaneous recording. But you are right, it is very difficult because, for example, initial manipulation at the apex starts to generate HIT signals, and I am really not sure why this should happen when still working on the outside of the heart. We certainly saw differences in procedural steps, and intuitively you would have expected those different steps to have a higher HITS burden, and that is exactly what we found. I think the information is quite accurate and interesting, even though the patient numbers are low.
Dr F. Maisano(Milan, Italy): I understood from your data that the self-expanding devices have a slightly higher amount of HITS. Do you think that this is due to the higher number of patients having post-dilatation?
Dr Huber: In regards to the access route, this is rather a discussion point; the post-implantation interval was the only one with a significant difference. The same difference is again shown in the type of deployment. In the post-implantation phase, one reason for increased HITS might be because of more post-deployment dilatation accounting for five in the self-expanding group and only one in the balloon-expanding group. Obviously this could also be withdrawal of the delivery system scratching the aortic arch, for example; we haven't really sub-analysed the whole phase, but we thought this could have been the reason, although there is no way we can prove this for the time being.
Dr Maisano: The way that HITS are generated in the two systems can be quite different. In a self-expanding device you have the unsheathing process, which is opening the door to air embolism when you unsheath the device which has been prepared outside of the body, while with balloon-expandable devices you don't have this mechanism. Can you discuss this a little bit?
Dr Huber: Well, that certainly also might be an impacting factor. I am pretty sure that everybody using self-expanding devices makes certain that they are well de-aired and that there are no air bubbles, but, if there were, it would certainly impact. However, I think, rather, that the implantation time of a self-expanding device first of all is much longer. There is this kind of device friction to the aortic wall, that is being generated all along during implantation with a heart that continues to beat and has ejection, and I think it is during that process that most HITS will be generated, and I think this is what requires detailed analysis.
Dr T. Drews(Berlin, Germany): I think it is very important to show the rate of embolism during this procedure. Therefore at the beginning of this year we published a paper on transapical Doppler results during transapical valve implantation, and we also found that during valvuloplasty and valve delivery we had the highest rates of microembolism and HITS. I have two questions. At first, we discovered that one-third of patients had a history of stroke before the valve device has been implanted. We found this by computer tomography. Do you have some results from preoperative data? And the second question is, you mentioned self-expanding valves. The CoreValve is self-expanding. Do you have any data on HITS or microembolism from patients treated by retrieval of the partially deployed prosthesis through the aorta and the introducer sheath and reimplantation of the same prosthesis?
Dr Huber: Regarding your first question, it actually wasn't the aim of the study to relate to stroke, so we haven't specifically analysed for strokes in these patients. The aim was to see which procedural step would generate most HITS. As to your second question, no, we haven't had any patient where we had to reposition the self-expanding device in the subset of the population we have included.
Author notes
Presented at the 25th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Lisbon, Portugal, 1–5 October 2011.




