-
PDF
- Split View
-
Views
-
Cite
Cite
Chun Soo Park, Jae Gun Kwak, Cheul Lee, Chang-Ha Lee, Sang Yoon Lee, Eun Young Choi, Jin Young Song, Soo-Jin Kim, Partial anomalous pulmonary venous connection to the superior vena cava: the outcome after the Warden procedure, European Journal of Cardio-Thoracic Surgery, Volume 41, Issue 2, February 2012, Pages 261–265, https://doi.org/10.1016/j.ejcts.2011.05.043
Close - Share Icon Share
Abstract
Partial anomalous pulmonary venous connection (PAPVC) draining into the superior vena cava (SVC) has been repaired with various techniques. We investigated the outcome of the Warden procedure for repair of this anomaly.
From December 1994 to January 2011, 30 patients underwent a Warden procedure for repair of PAPVC to the SVC in our center. Their median age at the time of the operation was 4.9 years (range, 1 month to 55 years). Follow-up data were obtained through a review of medical records, correspondence with the patients' cardiologists, and direct telephone contact. The mean follow-up duration was 5.3 ± 5.1 years (range, 1 month to 16 years).
One patient died of an underlying cardiac condition and cerebral complication unrelated to the Warden procedure. One patient had transient postoperative sinus node dysfunction. During follow-up, pulmonary venous pathway obstruction occurred in one patient, and systemic venous pathway obstruction occurred in three patients. Re-operation or re-intervention for systemic venous pathway obstruction was required in younger (<2 years) and smaller (<7 kg) patients within 1 year after the Warden procedure. All patients were in regular sinus rhythm in the latest electrocardiogram.
The Warden procedure is a safe and effective surgical option for repair of PAPVC to the SVC in terms of preserving the sinus node function and non-obstructive pulmonary venous pathway. However, more attention must be paid to the reconstruction of non-obstructive systemic venous pathway, especially in younger and smaller children. Patch augmentation could be considered and effectively performed, if there is any doubt regarding tension-free anastomosis.
INTRODUCTION
Partial anomalous pulmonary venous connection (PAPVC) is an anomaly in which some, but not all, of the pulmonary veins connect to the right atrium or to one or more of its venous tributaries [1]. This anomaly is frequently present with sinus- venosus-type atrial septal defect but can occur as an isolated form. The right pulmonary veins are commonly affected, and drainage into the superior vena cava (SVC) is not uncommon.
When repairing the PAPVC to the SVC, the goal is the creation of unobstructed pulmonary and systemic venous pathways without compromising sinus node function. Although various techniques have been developed to correct the PAPVC to the SVC [2–5], partitioning of the SVC into two venous pathways and/or manipulation of the junction between the right atrium and the SVC still raise concerns regarding obstruction of venous pathways and sinus node dysfunction. In addition, some reports showed different outcomes with the same techniques [6,7]. Warden and colleagues [8] first described the technique, in which the SVC is transected above the uppermost insertion of the anomalous pulmonary vein, the cephalic portion of the transected SVC is implanted to the right atrial appendage, and the caudal portion of the transected SVC serves as a conduit for pulmonary venous drainage to the left atrium. The outcomes of this technique (Warden procedure) seem to be promising [9–15], but most series had only a small number of patients and limited duration of follow-up.
In this study, we investigate the outcomes after the Warden procedure for repair of PAPVC to the SVC at our center.
MATERIALS AND METHODS
Patients
The Institutional Review Board of Sejong General Hospital approved this retrospective study and waived the requirement for informed consent. From December 1994 to January 2011, 30 patients who underwent the Warden procedure for repair of PAPVC to the SVC were identified from our database. Their median age at the time of the operation was 4.9 years (range, 1 month to 55 years) and 13 patients were male (43.3%). Diagnosis was made from echocardiographic exam, and chest computed tomography was done as necessary. Anomalous pulmonary veins connected to the SVC were right sided in all patients and involved more than one lobe in 11 patients (36.7%) (Table 1). One patient had the left-upper-pulmonary vein connected to the left innominate vein as well as the right-upper-pulmonary vein connected to the SVC. Atrial septal defect was sinus-venosus type in 14 patients (46.7%), secundum type in seven patients (23.3%), and patent foramen ovale in four patients (13.3%) (Table 1). In five patients (16.7%), the atrial septum was intact (Table 1). Associated cardiovascular anomalies included ventricular septal defect (n = 4), persistent left SVC (n = 3), tetralogy of Fallot (n = 3), absent pulmonary valve syndrome (n = 1), multiple pulmonary vein stenosis (n = 1), patent ductus arteriosus (n = 1), and vascular ring (n = 1) (Table 1). Cardiac catheterization was performed in six patients for evaluation of associated anomalies or pulmonary hypertension. Their mean pulmonary arterial pressure averaged 30.8 ± 5.7 mmHg (range, 20—36 mmHg). Preoperatively, all patients had a normal electrocardiogram. Functional class assessed using the New York Heart Association (NYHA) classification was 1 or 2 in 21 patients and 3 or 4 in nine patients.
| . | . | N (%) . |
|---|---|---|
| Anomalous pulmonary vein | RUPV only | 19 (63.3%) |
| More than RUPV | 11 (36.7%) | |
| Atrial septal defect | Sinus venosus | 14 (46.7%) |
| Secundum | 7 (23.3%) | |
| Patent foramen ovale | 4 (13.3%) | |
| Intact atrial septum | 5 (16.7%) | |
| Associated anomaly | Ventricular septal defect | 4 |
| Persistent left SVC | 3 | |
| Tetralogy of Fallot | 3 | |
| Absent pulmonary valve syndrome | 1 | |
| Multiple pulmonary vein stenosis | 1 | |
| Patent ductus arteriosus | 1 | |
| Vascular ring | 1 |
| . | . | N (%) . |
|---|---|---|
| Anomalous pulmonary vein | RUPV only | 19 (63.3%) |
| More than RUPV | 11 (36.7%) | |
| Atrial septal defect | Sinus venosus | 14 (46.7%) |
| Secundum | 7 (23.3%) | |
| Patent foramen ovale | 4 (13.3%) | |
| Intact atrial septum | 5 (16.7%) | |
| Associated anomaly | Ventricular septal defect | 4 |
| Persistent left SVC | 3 | |
| Tetralogy of Fallot | 3 | |
| Absent pulmonary valve syndrome | 1 | |
| Multiple pulmonary vein stenosis | 1 | |
| Patent ductus arteriosus | 1 | |
| Vascular ring | 1 |
RUPV: right upper pulmonary vein; SVC: superior vena cava.
| . | . | N (%) . |
|---|---|---|
| Anomalous pulmonary vein | RUPV only | 19 (63.3%) |
| More than RUPV | 11 (36.7%) | |
| Atrial septal defect | Sinus venosus | 14 (46.7%) |
| Secundum | 7 (23.3%) | |
| Patent foramen ovale | 4 (13.3%) | |
| Intact atrial septum | 5 (16.7%) | |
| Associated anomaly | Ventricular septal defect | 4 |
| Persistent left SVC | 3 | |
| Tetralogy of Fallot | 3 | |
| Absent pulmonary valve syndrome | 1 | |
| Multiple pulmonary vein stenosis | 1 | |
| Patent ductus arteriosus | 1 | |
| Vascular ring | 1 |
| . | . | N (%) . |
|---|---|---|
| Anomalous pulmonary vein | RUPV only | 19 (63.3%) |
| More than RUPV | 11 (36.7%) | |
| Atrial septal defect | Sinus venosus | 14 (46.7%) |
| Secundum | 7 (23.3%) | |
| Patent foramen ovale | 4 (13.3%) | |
| Intact atrial septum | 5 (16.7%) | |
| Associated anomaly | Ventricular septal defect | 4 |
| Persistent left SVC | 3 | |
| Tetralogy of Fallot | 3 | |
| Absent pulmonary valve syndrome | 1 | |
| Multiple pulmonary vein stenosis | 1 | |
| Patent ductus arteriosus | 1 | |
| Vascular ring | 1 |
RUPV: right upper pulmonary vein; SVC: superior vena cava.
Operative technique
After median sternotomy, the SVC and the innominate vein were dissected and the azygos vein was divided. Arterial cannula and dual venous cannulae were instituted and cardiopulmonary bypass was initiated. The operation was performed under moderate hypothermic cardiopulmonary bypass with ischemic arrest using a cold crystalloid cardioplegic solution. Surgical correction for associated anomalies was performed before or after the Warden procedure as appropriate. Concomitant procedures included closure of ventricular septal defect in four patients, total correction of tetralogy of Fallot in three patients, tricuspid valve repair in three patients, pulmonary valve replacement in one patient, sutureless repair of stenotic pulmonary vein in one patient, and division of patent ductus arteriosus in one patient. The details of the Warden procedure are as follows: the SVC was divided above the uppermost insertion of the anomalous pulmonary vein and the caudal end of the divided SVC was closed directly or using a patch. For reconstruction of pulmonary venous pathway, the atrial septal defect was further enlarged or newly created in 17 patients (56.7%), and baffling from the SVC orifice to the atrial septal defect was performed using various materials (Table 2). In cases with intact atrial septum, we resected the floor of the oval fossa and a part of the superior limbus as necessary. If the superior limbus was resected, we repaired that area for restoring endocardial continuity and preventing the unexpected extracardiac communication. The right-atrial appendage was amputated or prepared for a pedicled flap and the pectinate muscle was carefully resected. The systemic venous pathway was reconstructed using various techniques (Table 2). In patients with isolated PAPVC (n = 18), the mean cardiopulmonary bypass (CPB) time and ischemic time were 126.2 ± 51.8 min and 59.9 ± 26.9 min, respectively. In patients with concomitant cardiac anomaly to be repaired, the mean CPB time and ischemic time were 193.1 ± 57.1 min and 120.8 ± 40.7 min, respectively.
| . | . | N (%) . |
|---|---|---|
| Atrial septal defect | Extension | 11 (36.7%) |
| Creation | 6 (20%) | |
| Left untouched | 13 (43.3%) | |
| Pulmonary venous | Treated autologous pericardium | 19 (63.3%) |
| pathway | Bovine pericardium | 6 (20%) |
| Right atrial freewall | 2 (6.7%) | |
| 0.6 mm thickness PTFE patcha | 2 (6.7%) | |
| 0.1 mm thickness PTFE membranea | 1 (3.3%) | |
| Systemic venous | Direct implantation | 20 (66.7%) |
| pathway | Patch augmentation of anterior walla,b | 6 (20%) |
| Right atrial wall pedicled flap | 3 (10%) | |
| PTFE tube grafta,c | 1 (3.3%) |
| . | . | N (%) . |
|---|---|---|
| Atrial septal defect | Extension | 11 (36.7%) |
| Creation | 6 (20%) | |
| Left untouched | 13 (43.3%) | |
| Pulmonary venous | Treated autologous pericardium | 19 (63.3%) |
| pathway | Bovine pericardium | 6 (20%) |
| Right atrial freewall | 2 (6.7%) | |
| 0.6 mm thickness PTFE patcha | 2 (6.7%) | |
| 0.1 mm thickness PTFE membranea | 1 (3.3%) | |
| Systemic venous | Direct implantation | 20 (66.7%) |
| pathway | Patch augmentation of anterior walla,b | 6 (20%) |
| Right atrial wall pedicled flap | 3 (10%) | |
| PTFE tube grafta,c | 1 (3.3%) |
N: number of patients; PTFE: polytetrafluoroethylene.
a PTFE (GoreTex; W.L. Gore & Associates, Inc., Newark, USA).
b Treated autologous pericardium in 2, bovine pericardium in 2 and 0.1 mm thickness PTFE membrane in 2.
c Early mortality.
| . | . | N (%) . |
|---|---|---|
| Atrial septal defect | Extension | 11 (36.7%) |
| Creation | 6 (20%) | |
| Left untouched | 13 (43.3%) | |
| Pulmonary venous | Treated autologous pericardium | 19 (63.3%) |
| pathway | Bovine pericardium | 6 (20%) |
| Right atrial freewall | 2 (6.7%) | |
| 0.6 mm thickness PTFE patcha | 2 (6.7%) | |
| 0.1 mm thickness PTFE membranea | 1 (3.3%) | |
| Systemic venous | Direct implantation | 20 (66.7%) |
| pathway | Patch augmentation of anterior walla,b | 6 (20%) |
| Right atrial wall pedicled flap | 3 (10%) | |
| PTFE tube grafta,c | 1 (3.3%) |
| . | . | N (%) . |
|---|---|---|
| Atrial septal defect | Extension | 11 (36.7%) |
| Creation | 6 (20%) | |
| Left untouched | 13 (43.3%) | |
| Pulmonary venous | Treated autologous pericardium | 19 (63.3%) |
| pathway | Bovine pericardium | 6 (20%) |
| Right atrial freewall | 2 (6.7%) | |
| 0.6 mm thickness PTFE patcha | 2 (6.7%) | |
| 0.1 mm thickness PTFE membranea | 1 (3.3%) | |
| Systemic venous | Direct implantation | 20 (66.7%) |
| pathway | Patch augmentation of anterior walla,b | 6 (20%) |
| Right atrial wall pedicled flap | 3 (10%) | |
| PTFE tube grafta,c | 1 (3.3%) |
N: number of patients; PTFE: polytetrafluoroethylene.
a PTFE (GoreTex; W.L. Gore & Associates, Inc., Newark, USA).
b Treated autologous pericardium in 2, bovine pericardium in 2 and 0.1 mm thickness PTFE membrane in 2.
c Early mortality.
Follow-up data
Follow-up data were obtained through a review of medical records, correspondence with the patients' cardiologists, and direct telephone contact between 1 and 15 March 2011. The mean follow-up duration was 5.3 ± 5.1 years (range, 1 month to 16.3 years). In early survivors, the most recent echocardiography and electrocardiography were performed during follow-up at a mean interval of 3.3 ± 3.9 years and 3.3 ± 4.0 years, respectively.
Statistical analysis
Categorical variables are presented as frequency with percentage and continuous variables are expressed as mean ± standard deviation or median with range as appropriate. Time-to-event analyses were performed using the Kaplan—Meier method. We analyzed the data using Statistical Package for the Social Sciences (SPSS) version 13.0 (SPSS, Chicago, IL, USA).
RESULTS
Mortality and morbidity
One early death involved a 12-year-old boy who underwent the Warden procedure concomitant with pulmonary valve replacement and tricuspid valve repair. Before the operation, he had undergone a total repair of absent pulmonary valve syndrome at 1 year of age and had since had two more re-operations for pulmonary valve implantation. In this patient, PAPVC was not diagnosed at the time of initial operation. According to the medical records and physicians' communication reports, PAPVC was detected at the time of the second reoperation on cardiac catheterization. However, since the patient was 6 years old and the ratio of pulmonary to systemic blood flow was 1.5 at cardiac catheterization, pediatric cardiology and cardiac surgery staff decided to defer corrective surgery for PAPVC till the next surgery for pulmonary valve. Preoperative NYHA functional class was 3 and preoperative work-up showed decreased both ventricular function and enlarged right-heart chambers and markedly elevated both ventricular end-diastolic pressures. Postoperatively, the patient was required to be mechanically supported with left-ventricular-assisted device owing to elevated left-atrial pressure (30 mmHg) and fulminant pulmonary edema. Although the patient could be weaned from the device on postoperative day 2, he died of diffuse cerebral injury on postoperative day 8. There was no late death.
One patient, who underwent the Warden procedure concomitant with implantation of the left-upper-pulmonary vein to the left-atrial appendage, had transient sinus node dysfunction early postoperatively. This patient recovered regular sinus rhythm spontaneously before discharge.
Re-operation or re-intervention
Among 29 early survivors, re-operation was required in five patients during follow-up. One patient who underwent the Warden procedure concomitant with a total correction of tetralogy of Fallot at 9 months of age required re-operation for significant residual leak of ventricular septal defect at 1 month after the first operation. In the other four patients, re-operation or re-intervention was required for systemic or pulmonary venous pathway stenosis directly related to the Warden procedure. The rate of freedom from the events related to the Warden procedure was 88.1% at 5 years (Fig. 1). One patient, who was treated with the Warden procedure and left-pulmonary-vein angioplasty for the PAPVC to the SVC and multiple pulmonary-vein stenosis, underwent re-baffling from the SVC orifice to atrial septal defect using bovine pericardium for pulmonary-venous-pathway stenosis at about 7 years after the operation. This patient required catheter-based re-interventions for right-upper-pulmonary- vein-orifice stenosis at 42 months, 48 months, and 66 months after the re-operation, and a recent echocardiography showed mild right-upper-pulmonary-vein stenosis (mean pressure gradient 6 mmHg).
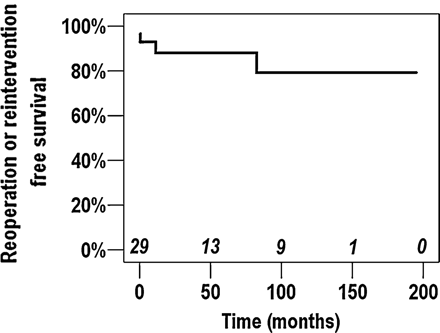
Kaplan—Meier freedom from re-operation or re-intervention curve for the events directly related to the Warden procedure.
There were three patients who required re-operation (n = 2) or re-intervention (n = 1) for systemic-venous-path-way stenosis. Two of these patients underwent more than one re-operation or re-intervention. The age and weight of these three patients at the time of the Warden procedure were <2 years and <7 kg, respectively (Table 3). In all three patients, direct implantation of the SVC to the right-atrial appendage without any modification was performed at the initial operation (Table 3). All re-operations or re-interventions for systemic-venous-pathway stenosis were performed within 1 year after the operation (Table 3).
Patients who required re-operation or re-intervention for systemic venous pathway stenosis (n = 3)
| No. . | Age (month) . | Weight (kg) . | Associated anomaly . | SVC to RA anastomosis . | Interval . | Re-operation or re-intervention . |
|---|---|---|---|---|---|---|
| 1 | 2 | 3.7 | VSD, PDA | Direct implantation | 1 day | SVC angioplasty with AP |
| 2 | 11 | 6.9 | PDA | Direct implantation | 11 months | Balloon angioplasty |
| 3 | 24 | 6.9 | VSD | Direct implantation | 12 days | SVC angioplasty with GoreTex membrane |
| No. . | Age (month) . | Weight (kg) . | Associated anomaly . | SVC to RA anastomosis . | Interval . | Re-operation or re-intervention . |
|---|---|---|---|---|---|---|
| 1 | 2 | 3.7 | VSD, PDA | Direct implantation | 1 day | SVC angioplasty with AP |
| 2 | 11 | 6.9 | PDA | Direct implantation | 11 months | Balloon angioplasty |
| 3 | 24 | 6.9 | VSD | Direct implantation | 12 days | SVC angioplasty with GoreTex membrane |
AP: autologous pericardium; No.: patient number; PDA: patent ductus arteriosus; PV: pulmonary venous; RA: right atrium; SVC: superior vena cava; TOF: tetralogy of Fallot; VSD: ventricular septal defect.
Patients who required re-operation or re-intervention for systemic venous pathway stenosis (n = 3)
| No. . | Age (month) . | Weight (kg) . | Associated anomaly . | SVC to RA anastomosis . | Interval . | Re-operation or re-intervention . |
|---|---|---|---|---|---|---|
| 1 | 2 | 3.7 | VSD, PDA | Direct implantation | 1 day | SVC angioplasty with AP |
| 2 | 11 | 6.9 | PDA | Direct implantation | 11 months | Balloon angioplasty |
| 3 | 24 | 6.9 | VSD | Direct implantation | 12 days | SVC angioplasty with GoreTex membrane |
| No. . | Age (month) . | Weight (kg) . | Associated anomaly . | SVC to RA anastomosis . | Interval . | Re-operation or re-intervention . |
|---|---|---|---|---|---|---|
| 1 | 2 | 3.7 | VSD, PDA | Direct implantation | 1 day | SVC angioplasty with AP |
| 2 | 11 | 6.9 | PDA | Direct implantation | 11 months | Balloon angioplasty |
| 3 | 24 | 6.9 | VSD | Direct implantation | 12 days | SVC angioplasty with GoreTex membrane |
AP: autologous pericardium; No.: patient number; PDA: patent ductus arteriosus; PV: pulmonary venous; RA: right atrium; SVC: superior vena cava; TOF: tetralogy of Fallot; VSD: ventricular septal defect.
Follow-up
All early survivors were in NYHA functional class 1 or 2 and did not have any symptoms or signs related to the venous-pathway-stenosis at the latest follow-up. At the latest echocardiography, mild pulmonary-venous-pathway stenosis (mean pressure gradient 6 mmHg) was observed in one patient who underwent re-baffling of pulmonary-venous pathway for pulmonary-venous-pathway stenosis at about 7 years after the operation. No patient displayed a significant stenosis in the systemic-venous pathway. All patients were in regular sinus rhythm in the latest electrocardiogram.
DISCUSSION
Although various surgical techniques have been introduced and performed for repair of the PAPVC to the SVC, it remains unclear which surgical option is better than the other. Traditionally performed methods for repairing of the PAPVC to the SVC are one-patch or two-patch repair, in which the incision crossing the junction between the right atrium and the SVC is required frequently. This incision could be placed away from the sinus node. However, this could injure the blood supply to the sinus node and could cause sinus-node dysfunction [16]. Although some authors noted that the long-term sinus-node dysfunction was 0% in patients who underwent one-patch or two-patch repair [3,5,15,17], other authors reported on patients with long-term sinus-node dysfunction [2,6,7,18,19]. By contrast, in accordance with our results, the most recent studies regarding the outcome of Warden procedure demonstrated that this procedure might be free of sinus-node dysfunction in the long term [7,9,11–13,20]. Thus, it can be inferred that the Warden procedure may be better than the other procedures in terms of preservation of sinus-node function. Nevertheless, transient sinus-node dysfunction has been noted in some reports [10,12,13,20] including our study, and a patient with a late sick sinus syndrome was also observed [10]. We believe that care must be taken not to injure the sinus node or sinus nodal artery by deep stitches when reconstructing the pulmonary-venous pathway in the Warden procedure.
In the Warden procedure, possible mechanisms of pulmonary-venous-pathway obstruction included crushing the SVC by repairing the caudal end of the transected SVC, baffle stenosis, and restrictive atrial septal defect. Although Baron and colleagues [11] preferred the patch repair of the caudal end of the transected SVC to avoid stenosis at this site, most authors performed direct closure without any related pulmonary-venous-pathway stenosis [10,12,20]. In our study, direct closure of the caudal end of the transected SVC was performed in most patients (n = 28) and patch closure with autologous pericardium in two patients. Re-operation for pulmonary-venous-pathway stenosis was required in one patient with direct closure of caudal end of the transected SVC, but the stenosis was attributed to the restrictive atrial septal defect and baffle stenosis. Various methods and materials [7,9–11,13,14] have been used to redirect the anomalous pulmonary-venous flow into the left atrium through the atrial septal defect. We also used various materials for redirection of the pulmonary venous pathway; one re-operation was required for pulmonary-venous-pathway stenosis in a patient who had undergone redirection of pulmonary venous pathway with treated autologous peri-cardial patch. According to the previous studies and ours, pulmonary-venous-pathway stenosis rarely occurred, regardless of methods or materials used for redirection of pulmonary venous pathway. It seems that there is no method or material better than the others.
To achieve an unobstructed systemic-venous pathway in the Warden procedure, extensive dissection of the SVC and the brachiocephalic vein as well as complete resection of trabeculations in the right atrium is essential. Despite an effort to achieve tension-free cavoatrial anastomosis, systemic-venous-pathway stenosis could occur at the junction of the SVC and the right-atrial appendage. Gustafson and colleagues [10] reported that a patient with evident stenosis of the systemic-venous pathway at 4 months after the operation required the re-operation. Stewart and colleagues [7] also reported that one of five patients who underwent the Warden procedure had severe obstruction of the systemic-venous pathway 2 years after the operation. Alsoufi and colleagues [15] noted the development of systemic-venous-pathway stenosis in one patient among 14 patients who had undergone Warden procedure. In our study, we observed systemic-venous-pathway stenosis in three patients. Two of them required re-operation with anterior wall augmentation using a patch and the other required catheter-based re-intervention. Notably, all three patients were smaller (<7 kg) and younger (<2 years), and re-operations or re-intervention were required within 1 year (Fig. 1). Two of these patients required re-operation for systemic-venous-pathway stenosis 1 day and 12 days after the operation. This finding suggests that the systemic-venous-pathway obstruction was related to the technical problem and the risk might be higher in younger and smaller patients. In addition, systemic-pathway reconstruction was performed with direct implantation of the SVC to the right-atrial appendage without any modification in these patients. When the anomalous pulmonary vein enters the more cephalic portion of the SVC, tension-free anastomosis between the cephalic end of the SVC and the right-atrial appendage cannot be guaranteed, despite sufficient mobilization of the cephalic portion of the SVC. To overcome this concern, some modifications [13,21] including a patch augmentation or right-atrial-wall pedicled flap have been introduced. Among our early survivors, a patch augmentation (with or without right-atrial-wall pedicled flap) was added to the reconstruction of the systemic-venous pathway in nine patients, and all of them had widely patent systemic-venous pathway at the latest echocardiographic follow-up. According to our experience, a patch augmentation could be effectively incorporated into the reconstruction of the systemic-venous pathway, if tension-free anastomosis between the SVC and the right atrium could not be guaranteed.
In conclusion, the Warden procedure is a safe and effective surgical option for repair of PAPVC to the SVC in terms of preserving the sinus-node function and non-obstructive pulmonary venous pathway. However, more attention must be paid to the reconstruction of non-obstructive systemic-venous pathway, especially in younger and smaller children. Patch augmentation could be considered and effectively performed, if there is any doubt regarding tension-free anastomosis.
Conflict of interest: none declared.
REFERENCES
- electrocardiogram
- sinoatrial node
- sinus node dysfunction
- cardiologists
- medical records
- partial anomalous pulmonary venous connection
- lung
- superior vena cava
- anastomosis, surgical
- child
- follow-up
- objective (goal)
- reconstructive surgical procedures
- repeat surgery
- surgical procedures, operative
- telephone
- brain
- heart
- sinus rhythm




