-
PDF
- Split View
-
Views
-
Cite
Cite
Arun Saini, Ralph E. Delius, Shivaprakash Seshadri, Henry Walters, Christopher W. Mastropietro, Passive peritoneal drainage improves fluid balance after surgery for congenital heart disease, European Journal of Cardio-Thoracic Surgery, Volume 41, Issue 2, February 2012, Pages 256–260, https://doi.org/10.1016/j.ejcts.2011.05.037
Close - Share Icon Share
Abstract
In some centers, passive peritoneal drainage (PD) is implemented following surgery for congenital heart disease. The utility of this practice has yet to be studied. We hypothesized that passive PD can promote negative fluid balance without compromising intravascular volume.
A retrospective review of infants who underwent repair of complete atrioventricular septal defect (AVSD) between 6/2006 and 8/2010 was completed. Data are represented as mean ± standard deviation.
Thirty-six infants underwent AVSD repair, 18 of whom had PD catheters placed without complication. Infants with passive PD had longer duration of cardiopulmonary bypass (211 ± 59 vs 137 ± 41 min, P < 0.001) and aortic cross-clamp (148 ± 29 vs 102 ± 21 min, P < 0.001); had higher Aristotle complexity score (12.6 ± 3 vs 10.7 ± 2, P = 0.03) and ventilatory support immediately after surgery (ventilation index score 19.5 ± 6.5 vs 14.3 ± 2.5, P = 0.004); and received greater fluid administration (225 ± 6 3 vs 168 ± 45 ml kg−1, P = 0.002) in the first 48 postoperative hours. Despite these differences, infants with passive PD achieved negative fluid balance more rapidly (12 ± 10 vs 27.3 ± 13 h, P < 0.0001) and to a greater extent (-73 + 55 vs +2.6 + 39 mL kg-1 at 48 h, P = 0.002). Moreover, postoperative hemodynamics, urine output, creatinine clearance, blood urea nitrogen, peak lactate, and duration of mechanical ventilation were similar between groups.
Passive PD is safe and promotes negative fluid balance after repair of complete AVSD without adversely affecting intravascular volume.
INTRODUCTION
Infants who undergo surgery for congenital heart disease are prone to developing extravascular fluid overload [1]. Preoperative congestive heart failure, cardiopulmonary bypass-related hemodilution, and intraoperative administration of fluids and blood products contribute to a state of markedly positive fluid balance upon arrival to the cardiac intensive care unit (ICU). This clinical condition is further exacerbated by postoperative renal dysfunction common to this patient population [2] as well as by postoperative capillary leakage [3,4] and low cardiac output states that often necessitate aggressive fluid resuscitation. Excessive pulmonary, peritoneal, and subcutaneous extravascular fluid can decrease respiratory system compliance, impair gas exchange, alter hemodynamics, and delay metabolic recovery [5,6]. Accordingly, removal of extravascular fluid in this patient population has been shown to improve lung compliance and gas exchange [3]. Therefore, achieving negative fluid balance quickly and safely postoperatively in these patients is desirable. Traditional methods employed to this end include intraoperative modified ultrafiltration, postoperative fluid restriction, and aggressive diuretic therapy [7]. The vigor with which one is able to implement these modalities to mobilize extravascular fluid, however, is often limited by the need to maintain adequate intravascular volume and end organ perfusion.
In some centers, peritoneal drainage (PD) catheters are placed intraoperatively and peritoneal dialysis is employed in the immediate postoperative period to rapidly achieve negative fluid balance and provide ultrafiltration for patients with renal dysfunction [8–13]. Other authors have alluded to the use of PD catheters to drain fluid passively as a less aggressive means of promoting negative fluid balance [8,9,14–17]. We use the latter technique at our institution, though there is a paucity of published literature regarding its effectiveness and limitations. We hypothesized that passive PD has been a safe and helpful means of promoting negative fluid balance without detrimentally depleting the intravascular volume of our patients recovering from surgery for congenital heart disease.
MATERIALS AND METHODS
We performed a chart review of infants less than 1 year of age who underwent repair of complete atrioventricular septal defects (AVSDs) at Children’s Hospital of Michigan between July 2006 and August 2010. This review was approved by the Institutional Review Boards at Wayne State University and the Detroit Medical Center. At Children’s Hospital of Michigan, PD catheters are placed in the majority of neonates undergoing surgery for congenital heart disease as well as those infants undergoing repair of Tetralogy of Fallot. By contrast, for patients with AVSD, the decision of PD catheter placement is at the subjective discretion of the cardiac surgeon, such that only a portion of the infants undergoing repair of AVSD has a PD catheter placed intraoperatively. This practice incidentally provided an intervention and a nonintervention group, allowing us to retrospectively study the impact of passive PD on postoperative fluid balance. Restricting the study to patients with AVSD also maintained study homogeneity.
Operative protocol
All patients received methylprednisolone 30 mg kg−1 prior to surgical incision. Cardiopulmonary bypass (CPB) was performed using a Terumo System One heart lung machine with a roller arterial pump (Terumo Cardiovascular Systems, Ann Arbor, MI, USA) and a Jostra HCU-30 heater cooler unit (Maquet Cardiopulmonary, Solna, Sweden). CPB was instituted with target flow rate of 2.5–3.0 l min−1 m−2. After cross-clamp removal, zero-balanced ultra-filtration was started. Twenty minutes of modified ultrafiltration was initiated after terminating CPB.
Prior to chest closure, a stab incision was made in the right subxyphoid area through which the peritoneal catheter (Kendall Quinton, 37 cm, Tyco Healthcare Group, Mansfield, MA, USA) was passed, leaving the cuff outside the skin to facilitate removal. A purse-string suture was then put in the peritoneum just inferior to the insertion of the diaphragm fibers. An opening was made in the peritoneum within that purse-string suture and the catheter was introduced into the left upper quadrant of the peritoneal cavity. The catheter therefore entered the peritoneal cavity above the level of the umbilicus, generally resting in the left upper part of the peritoneal cavity where it drained freely by gravity without interference by the omentum. All patients also underwent intraoperative placement of mediastinal tubes and some patients, depending on the clinical situation and anatomy, underwent intraoperative placement of pleural tubes.
Postoperatively, peritoneal catheters continued to drain to gravity only; no suction was applied. Laboratory analysis of PD was not performed in any patient. Peritoneal catheters were left in place until drainage was <1 mL kg−1 per 8 h shift. All patients met this criterion and had their catheters removed prior to transfer from the ICU.
Postoperative fluid management
During the first 24 h postoperative period, all patients received 750–1000 ml m−2 day−1 of 5% dextrose solution with 0.2% sodium chloride to maintain fluid and electrolyte requirements; packed red blood cells to maintain hematocrit >30%; fresh frozen plasma and platelets as needed to reverse postsurgical coagulopathy; and 5% albumin administration for additional fluid resuscitation to maintain hemodynamic stability as deemed necessary by the ICU team. In addition, on the first postoperative night, 5% albumin or fresh frozen plasma is used to replace passive PD each hour at a ratio of 0.5 ml:1 ml. The decision of which fluid to use is based on the patient’s coagulation studies. Continuous furosemide infusions (0.1–0.4 mg kg−1 h−1) were initiated in all patients on the morning of postoperative day 1, and some patients received additional intermittent boluses of intravenous furosemide (1 mg kg−1) and chlorothiazide (2 mg kg−1) during the study period.
Postoperative mechanical ventilation management
All patients underwent mechanical ventilation to maintain adequate ventilation and oxygenation as predetermined by the ICU team. Inhaled nitric oxide was used in some with clinical evidence of elevated pulmonary vascular reactivity. Patients were extubated from ventilatory support when inhaled nitric oxide was no longer needed and they were breathing comfortably with good gas exchange on the following settings: respiratory rate ≤5%, pressure support ≤10%, positive end-expiratory pressure ≤5%, and fraction of inspired oxygen concentration ≤40%.
Data collection
For each of these patients we recorded the following data: age, weight, and gender; comprehensive Aristotle complexity score [18]; duration of CPB and duration of aortic cross-clamp time; intraoperative transesophageal echocardiogram (TEE); postoperative mechanical ventilator support; total fluid input, urine output, chest tube output and PD in the first 48 h postoperatively; mean central venous pressure and peak lactate in the first 48 h postoperatively; blood urea nitrogen and estimated creatinine clearance at postoperative day 2 (Schwartz formula) [19]; serum electrolytes at day 0, 1, and 2 postoperatively; vasoactive medication and diuretic use; and duration of passive PD. The following outcome measures were also recorded: time to the first 8-h shift with negative fluid balance, duration of vasopressor use, duration of mechanical ventilation, and ICU length of stay. Vasoactive-inotropic score (VIS) was calculated according to the following formula [20]: VIS = dopamine (μg kg−1 min−1) + dobutamine (μg kg−1 min−1) + 100 × epinephrine (μg kg−1 min−1) + 10 × milrinone (μg kg−1 min−1) + 10,000 × vasopressin (U kg−1 min−1) + 100 × norepinephrine (μg kg−1 min−1). Ventilation index (VI) was calculated according to the following formula [6]: VI = RR × (PIP-PEEP) × PaCO2/1000, where RR is respiratory rate, PIP is peak inspiratory pressure, and PEEP is positive end-expiratory pressure.
Statistical analyses
Anthropometric, perioperative data, and data from the first 48 postoperative hours are represented as mean with standard deviation. Due to skewness of the data, duration of mechanical ventilation is represented as median with interquartile range. Data from patients with passive PD were compared to those without, using student t or Mann–Whitney U-tests as appropriate for individual variables.
RESULTS
Anthropometric and perioperative data
Thirty-six infants underwent repair of complete AVSD, 18 of which had PD catheters placed intraoperatively. No complications occurred as a result of PD catheter placement. Anthropomorphic and perioperative data are provided in Table 1. Patients with passive PD had evidence of greater surgical complexity, with greater Aristotle complexity score, duration of CPB, and aortic cross-clamping. Intraoperative TEE performed following repair showed normal ventricular function in all patients.
| Variable . | PD (n = 18) . | No PD (n = 18) . | P-value . |
|---|---|---|---|
| Age (days) | 155 ± 87 | 146 ± 41 | 0.70 |
| Weight (kg) | 5.40 ± 1.6 | 5.38 ± 0.8 | 0.93 |
| Female (n) | 10 | 7 | 0.50 |
| Aristotle complexity score | 12.6 ± 2.9 | 10.7 ± 2.0 | 0.03* |
| Cardiopulmonary bypass (min) | 211 ± 59 | 137 ± 41 | 0.001* |
| Aortic cross clamp (min) | 148 ± 29 | 102 ± 21 | <0.001* |
| Trisomy 21 (n) | 16 | 11 | 0.26 |
| Inhaled nitric Oxide (n)a | 7 | 2 | 0.12 |
| Residual valvular insufficiency (n) | 4 | 3 | 0.50 |
| Variable . | PD (n = 18) . | No PD (n = 18) . | P-value . |
|---|---|---|---|
| Age (days) | 155 ± 87 | 146 ± 41 | 0.70 |
| Weight (kg) | 5.40 ± 1.6 | 5.38 ± 0.8 | 0.93 |
| Female (n) | 10 | 7 | 0.50 |
| Aristotle complexity score | 12.6 ± 2.9 | 10.7 ± 2.0 | 0.03* |
| Cardiopulmonary bypass (min) | 211 ± 59 | 137 ± 41 | 0.001* |
| Aortic cross clamp (min) | 148 ± 29 | 102 ± 21 | <0.001* |
| Trisomy 21 (n) | 16 | 11 | 0.26 |
| Inhaled nitric Oxide (n)a | 7 | 2 | 0.12 |
| Residual valvular insufficiency (n) | 4 | 3 | 0.50 |
a Initiated intra-operatively. *Statistical significance set at P-value <0.05.
| Variable . | PD (n = 18) . | No PD (n = 18) . | P-value . |
|---|---|---|---|
| Age (days) | 155 ± 87 | 146 ± 41 | 0.70 |
| Weight (kg) | 5.40 ± 1.6 | 5.38 ± 0.8 | 0.93 |
| Female (n) | 10 | 7 | 0.50 |
| Aristotle complexity score | 12.6 ± 2.9 | 10.7 ± 2.0 | 0.03* |
| Cardiopulmonary bypass (min) | 211 ± 59 | 137 ± 41 | 0.001* |
| Aortic cross clamp (min) | 148 ± 29 | 102 ± 21 | <0.001* |
| Trisomy 21 (n) | 16 | 11 | 0.26 |
| Inhaled nitric Oxide (n)a | 7 | 2 | 0.12 |
| Residual valvular insufficiency (n) | 4 | 3 | 0.50 |
| Variable . | PD (n = 18) . | No PD (n = 18) . | P-value . |
|---|---|---|---|
| Age (days) | 155 ± 87 | 146 ± 41 | 0.70 |
| Weight (kg) | 5.40 ± 1.6 | 5.38 ± 0.8 | 0.93 |
| Female (n) | 10 | 7 | 0.50 |
| Aristotle complexity score | 12.6 ± 2.9 | 10.7 ± 2.0 | 0.03* |
| Cardiopulmonary bypass (min) | 211 ± 59 | 137 ± 41 | 0.001* |
| Aortic cross clamp (min) | 148 ± 29 | 102 ± 21 | <0.001* |
| Trisomy 21 (n) | 16 | 11 | 0.26 |
| Inhaled nitric Oxide (n)a | 7 | 2 | 0.12 |
| Residual valvular insufficiency (n) | 4 | 3 | 0.50 |
a Initiated intra-operatively. *Statistical significance set at P-value <0.05.
Postoperative fluid balance
Patients with passive PD received significantly more postoperative intravenous fluid administration than those without (Fig. 1. Despite this finding, those with passive PD achieved negative fluid balance more quickly and to a greater extent at 48 postoperative hours as compared to those without PD (Figs. 1 and 2). Patients with PD achieved negative fluid balance by 12 ± 10 h as compared to 27 ± 13 h in those without PD (P < 0.001). Contributions to total output from urine, chest (mediastinal and/or pleural), and PD in both groups are provided in Fig. 3. Of note, 29% of total output in those with passive PD was contributed by PD (85 ± 65.2 ml kg−1). Median duration of PD catheterization was 4 days (IQR: 3–5.25).)
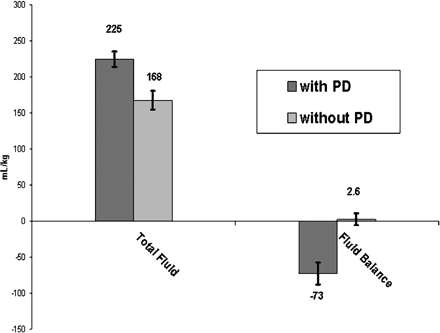
Total fluid input and fluid balance for the first 48 post-operative hours. Despite significantly greater fluid input (P = 0.002), patients with passive PD had significantly more negative fluid balance (P < 0.0001).
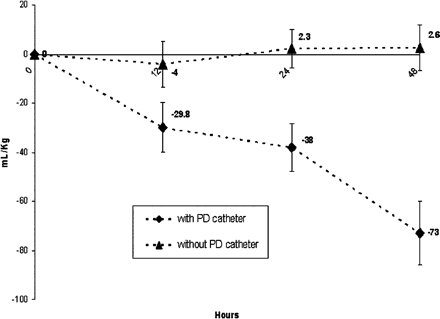
Fluid balance over time. Patients with passive PD became increasingly more negative over time following surgery, while those without remained approximately in even fluid balance during the same time period.
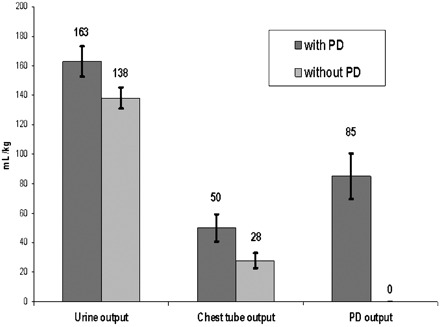
Total fluid output at 48 post-operative hours. Urine output was similar between groups (P = 0.08) and chest tube output was greater in patients with passive PD (P = 0.02). PD was most contributory to the difference in fluid balance between groups.
Postoperative data
Postoperative data are listed in Table 2. Ventilation index upon arrival to the ICU was significantly higher in those with passive PD. Median duration of mechanical ventilation was however similar between groups, 3 days (IQR: 3–4.75) in those with passive PD and 2.5 days (1.25–4) in those without (P = 0.21). VISs were also similar at 6, 12, 24, and 48 postoperative hours between groups, as were mean CVP, peak serum lactate, and markers of renal function during the same time period. Two patients with passive PD developed junctional tachyarrhythmia as compared to four without PD (P = 0.66) postoperatively; all other patients remained in normal sinus rhythm.
| Variables . | PD (n = 18) . | No PD (n = 18) . | P-value . |
|---|---|---|---|
| Ventilation indexa | 19.5 ± 6.5 | 14.3 ± 2.5 | 0.004* |
| Vasoactive inotrope score (VIS)b | |||
| VIS - 6 h | 14.5 ± 5.6 | 13.9 ± 7.7 | 0.78 |
| VIS - 12 h | 13.6 ± 4.8 | 12.9 ± 7.1 | 0.74 |
| VIS - 24 h | 14.1 ± 6.0 | 12.8 ± 9.1 | 0.61 |
| VIS - 48 h | 10.7 ± 4.4 | 7.7 ± 7.2 | 0.13 |
| Mean CVPc (mmHg) | 11.2 ± 2.4 | 10.4 ±1.7 | 0.22 |
| Peak lactate (mg/dl) | 3.0 ± 1 | 2.7 ± 1.2 | 0.23 |
| Marker of renal functiond | |||
| CrCl (ml/min) | 69.7 ± 19 | 76.5 ± 14.9 | 0.24 |
| Blood urea nitrogen (mg/dl) | 13.8 ± 3.7 | 15.9 ± 4.2 | 0.12 |
| Creatinine (mg/dl) | 0.39 ± 0.09 | 0.37 ± 0.09 | 0.46 |
| Cumulative diuretic dosinge | |||
| Furosemide | 5 ± 2.1 | 6.3 ± 2.8 | 0.18 |
| Chlorothiazide | 1.5 ± 2.8 | 2.6 ± 3.8 | 0.32 |
| Variables . | PD (n = 18) . | No PD (n = 18) . | P-value . |
|---|---|---|---|
| Ventilation indexa | 19.5 ± 6.5 | 14.3 ± 2.5 | 0.004* |
| Vasoactive inotrope score (VIS)b | |||
| VIS - 6 h | 14.5 ± 5.6 | 13.9 ± 7.7 | 0.78 |
| VIS - 12 h | 13.6 ± 4.8 | 12.9 ± 7.1 | 0.74 |
| VIS - 24 h | 14.1 ± 6.0 | 12.8 ± 9.1 | 0.61 |
| VIS - 48 h | 10.7 ± 4.4 | 7.7 ± 7.2 | 0.13 |
| Mean CVPc (mmHg) | 11.2 ± 2.4 | 10.4 ±1.7 | 0.22 |
| Peak lactate (mg/dl) | 3.0 ± 1 | 2.7 ± 1.2 | 0.23 |
| Marker of renal functiond | |||
| CrCl (ml/min) | 69.7 ± 19 | 76.5 ± 14.9 | 0.24 |
| Blood urea nitrogen (mg/dl) | 13.8 ± 3.7 | 15.9 ± 4.2 | 0.12 |
| Creatinine (mg/dl) | 0.39 ± 0.09 | 0.37 ± 0.09 | 0.46 |
| Cumulative diuretic dosinge | |||
| Furosemide | 5 ± 2.1 | 6.3 ± 2.8 | 0.18 |
| Chlorothiazide | 1.5 ± 2.8 | 2.6 ± 3.8 | 0.32 |
a Ventilation index upon arrival to the ICU.
b VIS at that point post-operatively, represented as median with interquartile range.
c Mean central venous pressure during first 48 post-operative hours.
d On post-operative day 2; CrCL: creatinine clearance.
e During first 48 post-operative hours. * Statistical significance set at P-value <0.05.
| Variables . | PD (n = 18) . | No PD (n = 18) . | P-value . |
|---|---|---|---|
| Ventilation indexa | 19.5 ± 6.5 | 14.3 ± 2.5 | 0.004* |
| Vasoactive inotrope score (VIS)b | |||
| VIS - 6 h | 14.5 ± 5.6 | 13.9 ± 7.7 | 0.78 |
| VIS - 12 h | 13.6 ± 4.8 | 12.9 ± 7.1 | 0.74 |
| VIS - 24 h | 14.1 ± 6.0 | 12.8 ± 9.1 | 0.61 |
| VIS - 48 h | 10.7 ± 4.4 | 7.7 ± 7.2 | 0.13 |
| Mean CVPc (mmHg) | 11.2 ± 2.4 | 10.4 ±1.7 | 0.22 |
| Peak lactate (mg/dl) | 3.0 ± 1 | 2.7 ± 1.2 | 0.23 |
| Marker of renal functiond | |||
| CrCl (ml/min) | 69.7 ± 19 | 76.5 ± 14.9 | 0.24 |
| Blood urea nitrogen (mg/dl) | 13.8 ± 3.7 | 15.9 ± 4.2 | 0.12 |
| Creatinine (mg/dl) | 0.39 ± 0.09 | 0.37 ± 0.09 | 0.46 |
| Cumulative diuretic dosinge | |||
| Furosemide | 5 ± 2.1 | 6.3 ± 2.8 | 0.18 |
| Chlorothiazide | 1.5 ± 2.8 | 2.6 ± 3.8 | 0.32 |
| Variables . | PD (n = 18) . | No PD (n = 18) . | P-value . |
|---|---|---|---|
| Ventilation indexa | 19.5 ± 6.5 | 14.3 ± 2.5 | 0.004* |
| Vasoactive inotrope score (VIS)b | |||
| VIS - 6 h | 14.5 ± 5.6 | 13.9 ± 7.7 | 0.78 |
| VIS - 12 h | 13.6 ± 4.8 | 12.9 ± 7.1 | 0.74 |
| VIS - 24 h | 14.1 ± 6.0 | 12.8 ± 9.1 | 0.61 |
| VIS - 48 h | 10.7 ± 4.4 | 7.7 ± 7.2 | 0.13 |
| Mean CVPc (mmHg) | 11.2 ± 2.4 | 10.4 ±1.7 | 0.22 |
| Peak lactate (mg/dl) | 3.0 ± 1 | 2.7 ± 1.2 | 0.23 |
| Marker of renal functiond | |||
| CrCl (ml/min) | 69.7 ± 19 | 76.5 ± 14.9 | 0.24 |
| Blood urea nitrogen (mg/dl) | 13.8 ± 3.7 | 15.9 ± 4.2 | 0.12 |
| Creatinine (mg/dl) | 0.39 ± 0.09 | 0.37 ± 0.09 | 0.46 |
| Cumulative diuretic dosinge | |||
| Furosemide | 5 ± 2.1 | 6.3 ± 2.8 | 0.18 |
| Chlorothiazide | 1.5 ± 2.8 | 2.6 ± 3.8 | 0.32 |
a Ventilation index upon arrival to the ICU.
b VIS at that point post-operatively, represented as median with interquartile range.
c Mean central venous pressure during first 48 post-operative hours.
d On post-operative day 2; CrCL: creatinine clearance.
e During first 48 post-operative hours. * Statistical significance set at P-value <0.05.
DISCUSSION
Prompt achievement of negative fluid balance following surgery for congenital heart disease is recognized as an important postoperative goal and has been used in several studies as a positive outcome measure [20–22]. The use of active peritoneal dialysis to this end has been well described [8–13]. This practice has not become widespread, perhaps due to potential complications (e.g., peritonitis, hyperglycemia, and fluid accumulation in pleural and pericardial space during dwell cycles, blood loss during drainage, leakage from the catheter site, bowel perforation, and hemodynamic instability with rapid fluid shift) and/or resource utilization (i.e., required staff and technical expertise for implementation) [9–13]. With passive PD, these risks and disadvantages are considerably less or absent. Previous authors have alluded to the use of this technique [8,9,14–17] but, to our knowledge, this is the first report to study the impact of passive PD on postoperative management following surgery for congenital heart disease. In our cohort, passive PD acted as a simple, less invasive (as compared to active peritoneal dialysis) adjunct to conventional means of facilitating negative fluid balance.
Patients with passive PD received more fluid during the first 48 postoperative hours and yet achieved greater negative fluid balance during this time period. The greater amount of fluid administration is most likely due to our practice of replacing half of the passive PD during the first postoperative night with intravenous fluid. This fluid is given to maintain adequate intravascular volume and prevent the undesirable hemodynamic consequences of intercompartmental fluid shifts that could potentially occur in patients with excessive passive PD. Urine output, creatinine clearance, blood urea nitrogen, peak lactate, central venous pressure, and postoperative inotrope use were similar between groups, suggesting similar intravascular volume. Hence, the net effect of our protocol is likely extravascular fluid removal.
Unlike passive PD, intraoperative chest tube thoracostomy (e.g., mediastinal and/or pleural) as a means of extravascular fluid drainage is routine at most centers. Chest tubes were present in all patients in both groups in this study, yet those with both chest tubes and passive PD achieved negative fluid balance more quickly and to a greater extent. In other words, chest tube drainage alone could not effectively match the efficiency of the combination of chest-tube drainage and passive PD. Interestingly, patients with passive PD had considerably more PD than chest-tube output, demonstrating a predilection for movement of extravascular fluid to the peritoneum as compared to the mediastinum and pleural space, and suggesting a physiologic rationale for PD catheter placement. In addition, Bokesch and colleagues [14] showed a higher concentration of proinflammatory cytokines in peritoneal fluid as compared to serum in neonates recovering from cardiac surgery. The authors postulated that cytokine removal by passive PD may be helpful in minimizing capillary leakage and recovering organ function after cardiopulmonary bypass, representing a possible additional benefit of passive PD.
This study focused solely on patients undergoing repair of AVSD, though the observed beneficial effects of passive PD on fluid balance would likely occur in infants and children with other lesions. This modality may be especially helpful in patients prone to right heart dysfunction such as those with Tetralogy of Fallot, many of whom manifest restrictive right ventricular physiology postoperatively with consequent ascites [23]. While some centers place PD catheters postoperatively at the bedside when such undesirable situations occur in their patients [24,25], we prefer to place these catheters in the environment of the operating suite, under direct visualization. We believe this approach to be safer and more proactive. More research is therefore needed to determine ideal candidates for passive PD.
This study is limited by its retrospective design. As such, the study could not be randomized nor could it be controlled for surgeon, surgical complexity, and disease severity. Though the decision to place a PD catheter was not based on objective criteria, the differences in those with passive PD as compared to those without noted on Table 1 suggest that PD catheters were placed in patients with more complicated intraoperative courses. Longer durations of cardiopulmonary bypass in this group likely led to more severe postoperative capillary leak. This assertion is supported by the greater amount of ventilator support on arrival to the PICU. Surprisingly, despite these differences, patients with passive PD achieved negative fluid balance more expeditiously and had similar duration of mechanical ventilation as compared to those without. We speculate that extravascular fluid removal by passive PD tempered some of the detrimental effect of the prolonged intraoperative courses both by minimizing excess lung water and keeping the abdominal cavity decompressed. We, therefore, hope that our data will provide support for a larger, prospectively controlled multicenter study to better measure the effect of passive PD on postoperative outcomes.
In conclusion, this is the first report to compare the effects of passive PD on patients recovering from surgery for congenital heart disease to a control population. PD catheters can be placed safely and act as an outlet for passive PD, an effective means of facilitating negative fluid balance and preventing extravascular fluid accumulation in the postoperative period.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented in abstract form in January at Cardiology 2011: The 15th Annual International Symposium on Congenital Heart Disease, Scottsdale, AZ, USA.




