-
PDF
- Split View
-
Views
-
Cite
Cite
Jessica A. Yu, Marvin Pomerantz, Amy Bishop, Michael J. Weyant, John D. Mitchell, Lady Windermere revisited: treatment with thoracoscopic lobectomy/segmentectomy for right middle lobe and lingular bronchiectasis associated with non-tuberculous mycobacterial disease, European Journal of Cardio-Thoracic Surgery, Volume 40, Issue 3, September 2011, Pages 671–675, https://doi.org/10.1016/j.ejcts.2010.12.028
Close - Share Icon Share
Abstract
Objective: Lady Windermere syndrome is a well-known but poorly understood female predominant phenotype of isolated right middle lobe and lingular bronchiectasis associated with non-tuberculous mycobacterial (NTM) infection. Despite lengthy multidrug antibiotic treatment, the presence of damaged parenchymal tissue leads to symptomatic disease recurrence, often with resistant organisms. The use of surgical resection as an adjunct to medical therapy may alter this cycle, although little is known about the use of thoracoscopic lung resection in this patient population. Methods: This is a retrospective review of a prospectively collected database of patients with pulmonary NTM disease from July 2004 to December 2009. All patients had focal bronchiectasis of the right middle lobe and lingula, treated with targeted antimicrobial therapy for several months prior to resection. Results: A total of 134 patients underwent 172 operations, with 38 patients having staged bilateral resections. The cohort was predominately female (96%) and Caucasian (95%), with a mean age of 59 years (range 34–81 years). Using a thoracoscopic approach in all patients, 102 middle lobectomies and 70 lingulectomies were performed. Conversion to open thoracotomy occurred in five cases (3%). Secondary procedures were performed in 20 cases (12%). There was no operative mortality. Postoperative morbidity was noted following 12 operations (7%), primarily consisting of prolonged air leak. The mean length of stay was 3.3 days (range 1–15 days). Conclusions: Although medical therapy remains the primary treatment modality for patients with pulmonary NTM disease, the selective use of pulmonary resection may reduce the incidence of symptomatic disease recurrence. The addition of thoracoscopic resection to treatment regimens for patients with Lady Windermere syndrome can be accomplished with minimal morbidity and mortality.
1 Introduction
Within the spectrum of pulmonary non-tuberculous mycobacterial (NTM) disease, there is a well-known but poorly understood female predominant phenotype of isolated right middle lobe and lingular bronchiectasis (Fig. 1(A) and (B) ). In 1992, Reich and Johnson [1] proposed that voluntary cough suppression may lead to post-obstructive bronchiectasis in these patients. They offered the term ‘Lady Windermere syndrome’ referencing an Oscar Wilde play, Lady Windermere’s Fan, to convey the fastidious behavior of women who do not cough. Subsequent publications have characterized distinct morphologic features associated with this subset of patients including thin body habitus and mitral valve prolapse [2–4]. In addition, though studies [2] have investigated several immune phenotypes in these patients, no consistent finding has been established; thus, these patients remain in the category of immune-competent pulmonary NTM patients.
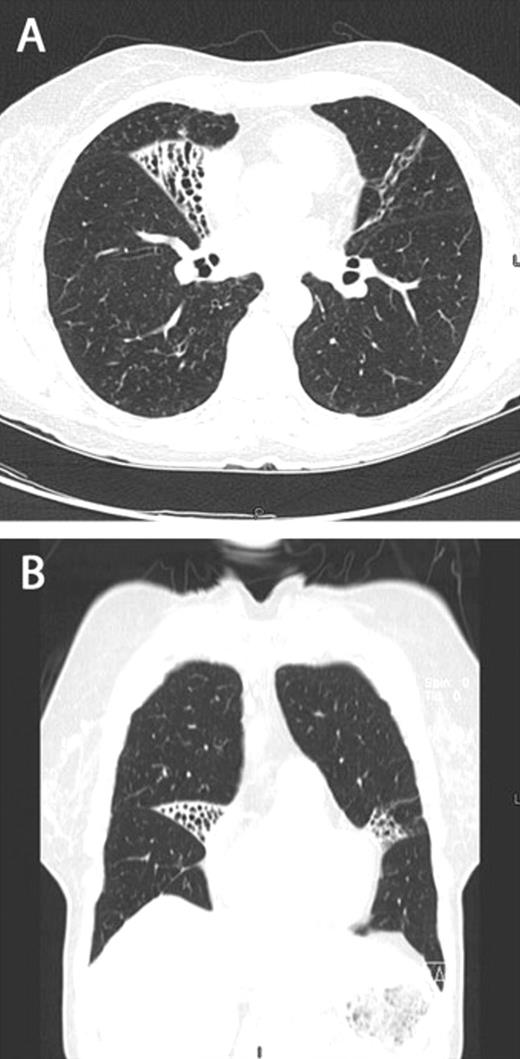
Axial (A) and coronal (B) computed tomography images demonstrating focal bronchiectasis in both the right middle lobe and lingula, characteristic of Lady Windermere syndrome.
Beyond the difficulties in treating bronchiectasis, the additional burden of pulmonary NTM infection poses a significant therapeutic challenge to clinicians. Current recommendations for multidrug antibiotic regimens yield sputum conversion rates of 55–67% [3,5,6]. Patients suffer high rates of antibiotic-related side effects or intolerance, and relapse or reinfection rates remain around 20–44%, even with the addition of macrolide therapy [5,6]. The limitations of medical treatment can perpetuate a cycle of symptomatic disease recurrence and progressive parenchymal tissue damage. The spectrum of damage to the lung ranges from focal bronchiectasis or cavitary disease, to a completely destroyed lung [2,4,7–11].
A number of studies have suggested that the use of surgical resection as an adjunct to medical therapy in pulmonary NTM disease may alter this cycle [3,4,7–11], although little is known about the use of thoracoscopic lung resection in this patient population.
2 Materials and methods
We retrospectively reviewed hospital records of 134 patients, who underwent 172 consecutive thoracoscopic anatomic lung resections for NTM disease at our institution between July 2004 and December 2009. The study was approved by the University of Colorado Hospital’s Institutional Review Board.
2.1 Patient selection
All patients had a diagnosis of pulmonary NTM disease according to American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) guidelines [3], and underwent pulmonary function testing and high-resolution computed tomography demonstrating focal parenchymal lung disease in the right middle lobe and lingula. Cardiopulmonary function was assessed and deemed adequate for surgery. Careful attention was paid to nutritional status, and nutritional specialist consultation was obtained as needed. The patients were treated with targeted antimycobacterial therapy for at least 2–3 months prior to evaluation for surgical intervention. In many cases, satisfactory completion of appropriate antibiotic treatment dictated the timing of surgical resection. Indications for surgery included localized fibronodular bronchiectasis and/or cavitation, symptoms such as chronic productive cough, hemoptysis, or recurrent pulmonary infection, and failure of medical management.
2.2 Surgical technique
Video-assisted thoracoscopic lobectomy and segmentectomy were performed using two 10-mm ports and a 4-cm ‘utility’ incision in the anterior axillary line over the anterior hilar structures. No rib spreading was used. Adhesions were commonly encountered upon entering the thorax and were divided with blunt or sharp dissection, or electrocautery. Proceeding with the anatomic resection, the bronchial circulation was often hypertrophied and accompanied by surrounding lymphadenopathy. The pulmonary vessels and bronchus were divided using Endo GIA staplers (US Surgical Corp., Norwalk, CT, USA), identical to those used in cases of pulmonary malignancy. Not infrequently, there was incomplete development of the major fissure secondary to underlying anatomy or significant inflammatory changes, in which case resection favored the non-involved side of the fissure. An Endobag (US Surgical Corp., Norwalk, CT, USA) was used to remove the specimen from the hemithorax, taking care to avoid contamination of the port sites. Cultures were sent to two separate microbiology labs familiar with culture techniques for mycobacterial organisms.
2.3 Postoperative care
Preoperative antimycobacterial antibiotic regimens were continued throughout the perioperative period. Routine postoperative care emphasized pain control, pulmonary toilet, early mobilization, and maintenance of nutrition. Prophylactic low-dose beta blockade was used to reduce the incidence of perioperative atrial fibrillation. Patients who presented with bilateral disease underwent staged procedures, with the second procedure usually planned for 4–6 weeks later. Culture results from tissue obtained at operation were used to later adjust antimicrobial regimens as needed.
2.4 Statistical analysis
All percentages given are percent of patients, unless otherwise stated.
3 Results
Over a 5.5-year period from July 2004 to December 2009, 134 patients with NTM disease underwent 172 operations, with 38 patients having staged bilateral resections (38/134, 28%). The majority of patients (118/134, 88%) had preoperative cultures positive for Mycobacterium avium complex, and 14 of these patients also had a history of infection with Mycobacterium abscessus or Mycobacterium chelonae. Fourteen (10%) of patients grew primarily M. abscessus or M. chelonae preoperatively. The remaining two patients (1.5%) had a history of Mycobacterium simiae, or Mycobacterium triplex.
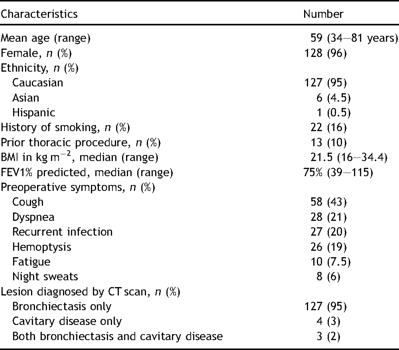
A total of 128 of the patients were female (96%); the patients were predominantly Caucasian (95%) and the median body mass index (BMI) was 21.5 kg m−2 (range 16–34.4 kg m−2). The mean age of the cohort was 59 years (range 34–81 years) and the median forced expiratory volume in 1 s (FEV1) was 75% of predicted (range 39–115%). Thirteen patients had a history of prior thoracic procedures (10%). The most common preoperative symptoms reported were cough (43%), followed by dyspnea (21%), recurrent pulmonary infection (20%), and hemoptysis (19%). Patients frequently reported intolerance of an antibiotic requiring its discontinuation at some point during their therapy. High-resolution computed tomography scans demonstrated fibronodular bronchiectasis in 95% of patients (Fig. 1). Occasionally, dependent drainage from the severely bronchiectatic areas would secondarily involve other lung segments (Fig. 2 ) The remaining 5% of patients had focal cavitary lesions, or a combination of bronchiectasis and cavitation (Table 1 ).
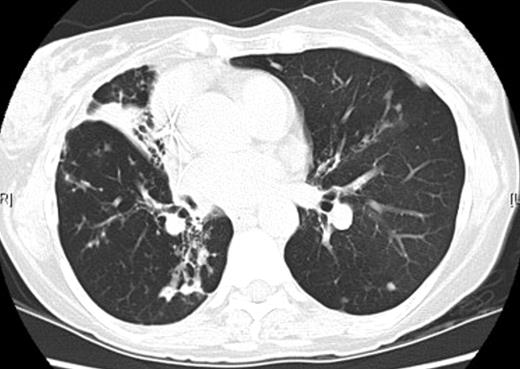
Computed tomography image demonstrating isolated bronchiectasis in the right middle lobe with associated inflammatory changes in the superior segment of the right lower lobe.
A video-assisted thoracoscopic approach was used in all patients. A total of 102 middle lobectomies and 70 lingulectomies were performed. One patient with situs inversus required a left middle lobectomy and right lingulectomy. Secondary procedures were performed in 20 cases (12%), including 10 wedge resections, seven segmentectomies, two bronchoplasties, and one Nissen fundoplication. The wedge resections were primarily for isolated small cavitary lesions, and the segmentectomies addressed additional areas of localized fibronodular bronchiectasis. In two cases, there was an injury to the bronchus requiring bronchoplasty. Conversion to open thoracotomy occurred in five cases (3%). In four patients, thoracotomy was required secondary to extensive adhesions. In one patient, completion of the bronchoplasty required an extension of the utility incision. No attempt was made to buttress the bronchial stump with autologous tissue.
There was no operative mortality. Postoperative morbidity was noted following 12 procedures (7%), consisting of prolonged air leak (3.5%), two pneumothoraces requiring catheter drainage (1.2%), one patient with atrial fibrillation, one wound infection, one patient with atelectasis requiring bronchoscopy, and one re-admission for pleural effusion (Table 2 ). The mean and median length of stay was 3.3 days (range 1–15 days).

Long-term follow-up data were available for 110 patients (82%) for a mean duration of 23 months (range 0–70 months). Ninety-two of these patients (92/110, 84%) had postoperative cultures and smears negative for mycobacterial organisms. Of these patients, 27 (27/92, 29%) converted after resection of culture-positive parenchymal disease. Eight patients who converted subsequently developed recurrent positive sputum cultures, representing either relapse or reinfection. Eighteen patients (18/110, 16%) who underwent surgical resection had not converted their sputum culture at the time of this report, suggestive of failure of the surgical therapy.
4 Discussion
Lady Windermere syndrome refers to a subset of patients with NTM lung disease and isolated right middle lobe and lingular bronchiectasis. This report describes our experience with the thoracoscopic approach to lung resection in a cohort of 134 consecutive patients treated with targeted antimicrobial therapy for several months prior to resection. As a part of multimodality treatment, it has been suggested that selective use of pulmonary resection may improve sputum conversion rates and reduce the incidence of symptomatic disease recurrence in pulmonary NTM patients [3,4,7–11]. However, the majority of reported resections have been with open thoracotomy [4,7–10]. Some authors have actually recommended avoidance of a thoracoscopic approach, claiming palpation of the tissue via an open thoracotomy is necessary for complete resection of diseased lung [7]. This study demonstrates that a thoracoscopic approach to pulmonary resection in patients with pulmonary NTM disease and bronchiectasis is both feasible and safe with minimal mortality and morbidity.
Anatomic lung resection was completed successfully using a thoracoscopic approach in 97% of operations. To date, this is the largest reported cohort of patients undergoing thoracoscopic anatomic lung resection for pulmonary NTM disease or bronchiectasis. Despite concerns for prohibitive adhesions in these chronically infected patients, only four patients required an open thoracotomy for adhesions. Of these four, two had had previous operations in the ipsilateral hemithorax. In addition, secondary procedures may be accomplished thoracoscopically, as evidenced by the 17 patients in our study who underwent minimally invasive secondary resections.
There was no operative mortality in this series, and complications were reported following 12 cases (7%). This is notably lower than complication rates of 18–32% [8–10] in reports of primarily open surgical resection of pulmonary NTM disease that also included patients with more advanced pathology.
The success rate of treating pulmonary NTM disease with multi-agent antimicrobial therapy and medical treatment alone is, at best 55–67% [3,5,6]. Previous studies have reported improved sputum conversion rates when surgical resection is employed in the management of pulmonary NTM [4,7–11]. The experience of our cohort is consistent with the literature with 84% of patients having either cultures and or sputum smears negative for mycobacterial disease after surgical resection, and, specifically, 27 patients (29%) who had operative tissue positive for mycobacteria, and subsequently converted to a sputum-culture-negative status.
There is considerable overlap between our patient cohort and those patients with non-NTM related bronchiectasis. The experience reported here is especially relevant in light of a number of recent studies reinforcing a role for the surgical management of bronchiectasis, particularly for localized lesions [12–15]. The role of surgery for patients with non-NTM associated bronchiectasis parallels that for pulmonary NTM patients: to eliminate damaged segments or lobes contributing to overwhelming secretions, to eliminate sources of hemoptysis, and to remove damaged lung suspected of harboring problematic organisms [16]. Recent studies, including a 790-patient cohort by Zhang et al. [15] used primarily an open approach to resection and reported symptom improvement for 70–98% or more of patients treated [12–15]. Morbidity ranged from 11% to 23% and mortality ranged from 0% to 1.7%. In contrast to these studies, all of our patients underwent a thoracoscopic approach to resection with comparable morbidity, no mortality, and a mean length of stay of 3.3 days.
This study has several limitations. Our review was retrospective in nature, and thus susceptible to flaws typical of such an approach. Further, the median length of follow-up in our study was 17 months, somewhat short for a patient population in which disease recurrence can occur years after cessation of treatment.
In summary, selective pulmonary resection may reduce the incidence of symptomatic disease recurrence for patients with pulmonary NTM disease and bronchiectasis. Although the relative merits of thoracoscopic surgery compared with thoracotomy in lung cancer patients have been well studied [17–19], there is a paucity of data addressing the morbidity and mortality of the thoracoscopic approach in the surgical treatment of inflammatory lung disease. A thoracoscopic approach for patients with Lady Windermere syndrome can be accomplished with minimal morbidity and mortality. In addition, our experience may support the concept of a thoracoscopic approach for the surgical management of bronchiectasis in non-NTM patients.
References
Author notes
Presented at the 24th Annual Meeting of the European Association for Cardio-thoracic Surgery, Geneva, Switzerland, September 11–15, 2010.




