-
PDF
- Split View
-
Views
-
Cite
Cite
Aydin Yilmaz, Sezgi Sahin Duyar, Ebru Cakir, Ertan Aydin, Funda Demirag, Jale Karakaya, Ulku Yazici, Yurdanur Erdogan, Clinical impact of visceral pleural, lymphovascular and perineural invasion in completely resected non-small cell lung cancer, European Journal of Cardio-Thoracic Surgery, Volume 40, Issue 3, September 2011, Pages 664–670, https://doi.org/10.1016/j.ejcts.2010.12.059
Close - Share Icon Share
Abstract
Objectives: This study is conducted to show the relationship between visceral pleural, lymphovascular, and perineural invasion, and other clinicopathologic characteristics and their significance as prognostic factors. Methods: The clinicopathologic characteristics of 289 patients who underwent a potentially curative surgical resection between 2000 and 2009 in our clinic were reviewed retrospectively. The prognostic factors were then evaluated by univariate and multivariate analysis. The patients who were given neoadjuvant–adjuvant chemotherapy and/or radiotherapy and who died due to postoperative mortality were excluded. Data from 188 patients were analyzed. Results: Out of the 188 patients (108 diagnosed as adenocarcinoma and 80 squamous cell carcinoma), 66 patients had lymphovascular invasion, 53 patients had perineural invasion, and 92 patients had visceral pleural invasion. Visceral pleural invasion was related with T factor, tumor histology, dimension, stage, and differentiation. Lymphovascular invasion was related with N status and stage. Perineural invasion was observed more frequently in tumors with moderate/poor differentiation. Visceral pleural and lymphovascular invasion were found to be poor prognostic factors but we could not show statistically meaningful effect of perineural invasion on survival. Conclusion: The presence of visceral pleural or lymphovascular invasion can show higher risk of mortality whereas perineural invasion has no effect on prognosis.
1 Introduction
Definition of the stage is important in determining prognosis, choosing therapy modality, and follow-up of the patients with lung cancer. In 2009, a new version of TNM (tumor-node-metastasis) staging system was published by the International Association for the Study of Lung Cancer (IASLC). In this new system of restaging, there was no consideration of pathologic features such as lymphovascular, visceral pleural, or perineural invasion in the suggestions. This was because there were not sufficient numbers of patients for whom reliable data were available to investigate the impact of visceral pleural invasion. Also, lymphovascular or perineural invasion was not mentioned in the goals of the IASLC study [1]. In addition to anatomic factors, pathologic characteristics of resected non-small-cell lung cancer (NSCLC) may help us to understand the heterogeneity of survival patterns and different biological behaviors of the tumors.
In the present study, we aimed to show relationship between visceral pleural (VPI), lymphovascular (LVI), perineural invasion (PNI), and other clinicopathologic characteristics and their significance as prognostic factors.
2 Patients and methods
The clinicopathologic characteristics of 289 patients who underwent a potentially curative surgical resection between June 2000 and June 2009 in our clinic were reviewed retrospectively. In the preoperative evaluation, the results of biochemistry panel including renal and liver function tests, alkaline phosphatase and serum calcium level and complete blood count, postero-anterior and lateral chest radiographs, and computed tomographic (CT) scans of the thorax including the upper abdomen and bronchoscopy were obtained for all of the patients. The patients with a sign or symptom of cranial and/or bone metastasis underwent cranial CT and/or bone scintigraphy. The prognostic factors evaluated by univariate and multivariate analysis were age, gender, histological type of tumor, pathologic T–N status, pathologic stage (according to the 7th version of TNM staging system), greatest tumor dimension, type of the operation, grade of differentiation, and visceral pleural, lymphovascular, and perineural invasion.
The patients who were given neoadjuvant chemotherapy (two patients), adjuvant chemotherapy or curative radiotherapy (87 patients), who died due to postoperative mortality (mortality within the first month of the surgery) (six patients), and the patients with an evidence of residual tumor at resection margin (15 patients) were all excluded. The remaining patients with advanced stage of tumor did not receive adjuvant chemotherapy/radiotherapy due to the poor performance status or the refusal of chemotherapy/radiotherapy by themselves. The pathologic stage of each patient was re-evaluated according to the IASLC staging system, revised in 2009. Lymph nodes were classified according to the American Thoracic Society guidelines.
Data from the remaining 188 patients were analyzed. Univariate and multivariate analyses were performed to identify prognostic factors. Informed consent was not required because the study was observational and retrospective.
2.1 Surgical procedure
The surgical procedures consisted of 143 lobectomies/bilobectomies (76.0%), 32 pneumonectomies (17.0%), 11 lobectomy+ chest wall resections (5.9%), and two pneumonectomy+ chest wall resections (1.1%). Systematic nodal dissection was performed in all cases and all nodal stations were labeled according to the American Thoracic Society (ATS) guidelines.
2.2 Pathologic evaluation
The excised tumors had been fixed in 10% buffered formalin. From each tumor, one block per cm was sampled, embedded in paraffin, sectioned at a thickness of 5 μm and stained with hematoxylin and eosin (H&E). All of the histological slides were evaluated according to the World Health Organization criteria [2] for both histology and grade by two pathologists. Discrepancies were resolved by simultaneous re-examination of the slides by both of the pathologists. Visceral pleural invasion was classified by using the Japan Lung Cancer Society criteria: p0, tumor with no pleural involvement beyond its elastic layer; p1, tumor that extends beyond the elastic layer of the visceral pleura but is not exposed on the pleural surface; p2, tumor that is exposed on the pleural surface but does not involve adjacent anatomic structures; and p3, tumor that involves adjacent anatomic structures [3]. When it was difficult to evaluate the degree of the visceral pleural invasion by H&E staining, an elastic stain (Verhoeff’s elastic van Gieson) was used. p2 and p3 can be evaluated by H&E staining. The slides, in which the distinction between p0 and p1 is unclear on H&E section, were stained with Verhoeff’s elastic van Gieson. Lymphovascular invasion was defined as tumor cells identifiable in the lymphatic or blood vessel lumen. Tumoral involvement of epineurium was defined as perineural invasion.
2.3 Statistical analyses
Categorical variables were expressed as count and percent. Chi square test (Pearson, Yates’, or Fisher exact chi square tests) was used to compare differences between groups for all categorical variables as univariate analysis. The survival curves were estimated using the Kaplan–Meier method. Patient survival was expressed by using time zero as the date of pathologic diagnosis and death as the end point. The log-rank test or Breslow test was used for comparison of the survival curves in univariate analysis. Cox’s Proportional Hazard Model (Cox PH Model) was used for multivariate analysis. A multivariate Cox PH Model was constructed to examine the effect of lung cancer on the risk of mortality. Tumor histology, age, gender, type of invasions (LVI, PNI, and VPI), stage, and differentiation were accepted as independent variables in Cox PH Model. Binary logistic regression with backward method was used to evaluate which independent variables (tumor histology, gender, age, tumor dimension, pathologic T–N status, p stage, and grade of differentiation) were statistically significant predictors of the binary dependent variable (LVI and PNI). Multinomial logit model was used to determine the risk factors affecting the visceral pleural invasion in three categories. In all the statistical analyses, p ≪ 0.05 was considered statistically significant. All statistical analyses were performed by SPSS for Windows, version 15.0 (Statistical Package for Social Sciences, SPSS Inc., Chicago, IL, USA, 2006).
3 Results
The clinical and pathological characteristics of the patients are summarized in Table 1 . Out of 188 patients (108 diagnosed as adenocarcinoma, 80 squamous cell carcinoma), 66 had lymphovascular invasion (LVI), 53 had perineural invasion (PNI), and 92 had visceral pleural invasion (VPI). All types of invasions were analyzed independently by dividing the patients in two groups for each kind of invasion (invasion+ and invasion−).

Visceral pleural invasion was strongly related to the T factor (p ≪ 0.001), tumor dimension (p = 0.005), stage (p = 0.015), and grade of differentiation (p = 0.007). VPI was also seen more frequently in adenocarcinoma (p ≪ 0.001) (Table 2 ). When VPI was classified according to Japan Lung Cancer Society criteria, 34 of all patients (18.1%) have p1 invasion, 37 of them have p2 invasion (19.7%), and 21 patients have p3 invasion (11.2%). When divided into two groups (p0 vs p1 + p2 + p3) a statistically significant difference in survival was seen (p = 0.036) (Fig. 1 ). When we dismissed p3 group and compared p1 + p2 group versus p0 group, we could also show a survival benefit (p = 0.014) (Fig. 2 ). In multivariate analysis, the risk of mortality for p1 + p2 group was found to be 2,369 times greater than that for the p0 group (Table 3 ). However, there was no statistically significant difference in survival when each level of pleural invasion was analyzed separately (p = 0.122). However, 5-year survival rates were decreasing with level of pleural invasion. These findings prove the poor prognostic effect of visceral pleural invasion. Although median survival is somewhat better in p1 disease, we could not show a statistically meaningful difference between p1 and p2 (Fig. 2). The reason for this situation could be the relatively small number of patients in p1 and p2 groups.
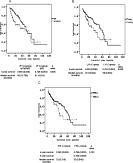
Survival curves, 4-year/5-year survivals and median survival rates of the patients (A) with/without VPI. Five-year survival rates for VPI+ and VPI− groups were 0.584 (0.056) and 0.729 (0.054) respectively (p = 0.036). Median survival rates for VPI+ and VPI− groups were 46 (15.795) and 51 (4.814) months respectively. (B) With/without LVI, 5-year survival rates for LVI+ and LVI− groups were 0.320 (0.090) and 0.761 (0.042) respectively (p = 0.049). Median survival rates for LVI+ and LVI− groups were 33 (10.002) and 56 (8.394) months respectively. (C) With/without PNI, 4-year survival rates for PNI+ and PNI− groups were 0.36 (0.092) and 0.796 (0.036) respectively (p = 0.153). Median survival rates for PNI+ and PNI− groups were 33 (3.316) and 53 (5.648) months respectively. We could not show 5-year survival rate for PNI+ group because none of the patients with PNI lived 5 years long.
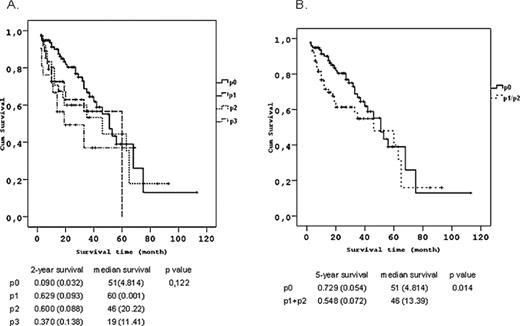
Survival curves and 2-year/5-year survivals according to the degree of VPI. (A) When patients divided into four groups as p0, p1, p2 and p3. Two-year survival rates for p0, p1, p2 and p3 were 0.090 (0.032), 0.629 (0.093), 0.600 (0.088) and 0.370 (0.138) respectively (p = 0.122). We could show only 2-year survival rates for this group because there only three patients who lived longer than 3 years in p3 group. (B) When p3 group is dismissed and patients divided to two groups as p0 and p1 + p2. Two-year survival rates for two groups were 0.729 (0.054) and 0.548 (0.072) respectively (p = 0.014).
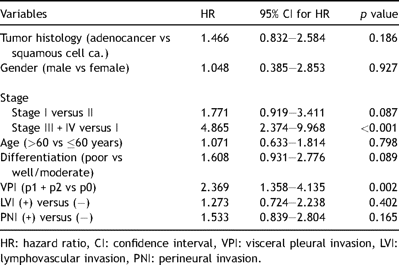
Multivariate analysis of prognostic factors in all NSCLC patients (Cox PH model).
LVI was proven to be related with N status and stage (p = 0.006 and p = 0.024 respectively). We could not demonstrate any relationship with the other factors (Table 4 ). LVI was found as an indicator of poor prognosis in survival analysis by Kaplan–Meier method (p = 0.049) (Fig. 1). However, this result was not supported by multivariate analysis (Table 3). This result may be explained through a relationship between VPI and LVI.
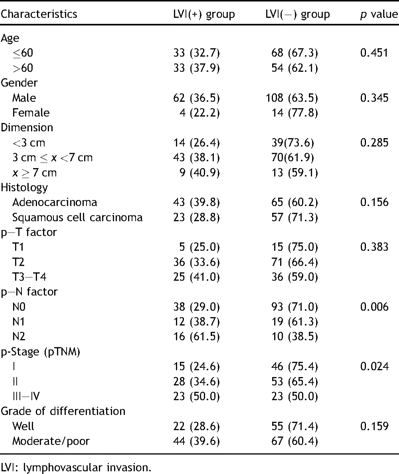
PNI was observed more frequently in tumors with moderate/poor differentiation (p = 0.041). Univariate analysis did not yield statistically meaningful results for the other factors (Table 5 ). There was not a statistically meaningful effect of PNI on survival but median survival of PNI(−) group was quite higher than PNI(+) grouping (20 months) (Fig. 1).

Multivariate analysis (with the same variables used in univariate analysis in Tables 2, 3, and 5) was also performed to determine the independent risk factors for each type of invasion. For VPI (p1 and p2 disease), only histology of adenocarcinoma was proven as an independent risk factor [Odds ratio (OR):4,62 (95% Confidence interval (CI): 2,117–10,088), p = 0,0]. For LVI, histologic type of adenocarcinoma (OR: 2.0 (95% CI: 1.026–3.881), p = 0.042) and N2 lymph node involvement (OR: 4.7 (95% CI: 1.862–11.860), p = 0.001) were found as independent risk factors whereas only the degree of differentiation was seen as an independent risk factor for PNI (OR: 2.4 (95% CI: 1.170–4.805), p = 0.017).
4 Discussion
Despite the fact that a new staging system seems powerful in determining prognosis, attempts to find new molecular biologic markers and histopathologic factors are still ongoing. These nonanatomic measures may help us to reach a more accurate staging system for lung cancer. Molecular techniques may require more expensive laboratory equipment and skilful personnel but detailed histopathological examination may be more beneficial [4]. There are several histopathological factors such as visceral pleural invasion [5–9], vascular invasion [10], perineural invasion [4,11], histologic grade [12], mitotic index, and nuclear atypia [13] which were identified as prognostic factors.
In this study, we found that VPI adversely affected long-term survival. There are many recently published studies on this topic. Shimuzu et al. reported that visceral pleural invasion was significantly associated with poor survival and a higher frequency of lymph node involvement in a review of 1074 patients with surgically resected T1–T2 NSCLC [5]. In another study, they also recommended that tumors of greater than 3 cm with visceral pleural invasion should be upgraded to T3 status [3]. There are other authors who agree with this restagement with the cut-off point for diameter as 4 cm or 3 cm [6,9]. We showed that T status, diameter, stage, grade of differentiation, and histology of tumor (adenocarcinoma vs squamous cell cancer) was related with VPI. Among these prognostic factors only histology of adenocarcinoma was proven as an independent risk factor for VPI (OR: 4.62 (95% CI: 2.117–10.088), p = 0.0). When the prognostic significance of VPI was stratified by tumor size, some authors reported that VPI did not affect prognosis adversely in tumors ≤3 cm in size [6]. In this present study, 71% of the patients had tumors >3 cm.
According to the Japan Lung Cancer Society tumors with a size of 3 cm or less and with VPI at a degree of p1 are classified as T1 disease. In a study by Osaki et al., VPI at a degree of p1 was proven as an important component of staging system and they suggested that p1–p2 status should be regarded as T2 disease [7]. We could not demonstrate a clinical advantage of classifying VPI as p1 and p2 except in terms of clinical trials. But the presence of VPI must be highlighted in a pathologic examination and elastic stain would help to get better results in distinguishing p1 from p0 in some cases [5]. By performing elastic stain for histologic review and giving special attention to VPI, we reached a higher positive rate for VPI (48.9%) than from previous studies by Manac’h et al. [14] or Takizawa et al. [15] (19.1% and 23.6% respectively). It is important to remember that inclusion of patients with advanced stage and T3–T4 tumors might also contribute to the higher rate of VPI in this study, depending on the results showing the positive relation between T status, stage and VPI. The pathologic stage of nine cases in this study was upstaged by the help of elastic stain.
Lymphatic vessel invasion was shown as a significant determinant for survival in a number of reports [4,16–18] whereas the prognostic relevance of blood vessel invasion (BVI) remains controversial [4,11,18,19]. In another study only venous invasion (but not arterial) was related with poor outcome [20]. We also found that LVI was correlated with N status and stage. 76% of LVI (−) group was N0. LVI was not seen in 75.4% of the patients with stage I disease. Multivariate analysis revealed that the histologic type of adenocarcinoma [OR: 2.0 (95% CI: 1.026–3.881), p = 0.042] and N2 lymph node involvement [OR: 4.7 (95% CI: 1.862–11.860), p = 0.001] were independent risk factors for LVI. Tsuchiya et al. suggested that upstaging IB group with vessel invasion to IIA group could improve the prediction of prognosis [10]. In pathologic examination, blood vessel and/or lymphatic vessel invasion in tumor tissues was accepted as LVI (+) in this study. So, we could not show the prognostic effect of blood vessel or lymphatic vessel invasion separately. However, LVI was found as a poor prognosis in survival analysis by Kaplan–Meier method (p = 0.049). However, it was not proven in multivariate analysis. It may be due to a relationship between LVI and VPI. In a study of Shimizu et al., it was stated that there were significantly more tumors with VPI patients with positive lymphatic and vascular invasion [5].
Intratumoral perineural invasion has been defined as a poor prognostic factor in many kinds of extrapulmonary cancer. However, there are a few studies for NSCLC. Sayar et al. confirmed that perineural invasion in tumor tissue was an independent factor for survival prediction in their study of 82 patients in which the prevalence of PNI was 29% [4]. However, in a larger study by Poncelet et al. (including 346 patients), they did not find that intratumoral permeation had adverse affects on long-term survival [11]. But the prevalence of PNI in the latter study was about 3%. We had a similar prevalence with Sayar et al. (28.2%) but our results were consistent with those by Poncelet et al. We observed that PNI was more frequent in tumors with moderate/poor differentiation (p = 0.041), which was a well-identified prognostic factor. Grade of differentiation was proven as an independent risk factor for PNI in multivariate analysis too (OR: 2.4 (95% CI: 1.170–4.805) p = 0.017). However, we could not demonstrate perineural invasion as a poor prognostic factor for NSCLC. But there was a high difference in median survival between invasive and non-invasive groups for PNI (20 months), which suggests a clinical impact.
To the best of our knowledge, this is the first study in which relationship between visceral pleural, lymphovascular, and perineural invasion, and other clinicopathologic characteristics and their significance as prognostic factors were explored together. But our study had some limitations like inclusion of T3–T4 tumors respectively lower number of patients, probability of having more patients with tumors that have a certain undetermined/unknown negative prognostic factor or with a higher SUV (standardized uptake value), which may shadow the prognostic impact of these invasions [21].
In conclusion, despite the limitations mentioned above we found that the presence of VPI was an independent risk factor, LVI was correlated with poor outcome and although it is not statistically meaningful, PNI seemed to have a clinical impact. Also our findings highlighted the relationships between these pathologic factors and age, gender, histological type of tumor, pathologic T–N status, pathologic stage (according to the 7th version of TNM staging system), greatest tumor dimension, and grade of differentiation.
We may suggest that in NSCLC if histopathologic evaluation reveals one of these invasions (PNI, VPI, or LVI) or a combination of them, adjuvant therapy may be recommended or at least these patients must be closely followed up after surgery. Maybe a pathologic scoring system including these invasions can be framed to yield a better staging system for NSCLC. We also believe that large-scale studies must be carried out to clarify the validity of these results.




