-
PDF
- Split View
-
Views
-
Cite
Cite
Elisa Meacci, Alfredo Cesario, Giacomo Cusumano, Filippo Lococo, Rolando D’Angelillo, Valentina Dall’Armi, Stefano Margaritora, Pierluigi Granone, Surgery for patients with persistent pathological N2 IIIA stage in non-small-cell lung cancer after induction radio-chemotherapy: the microscopic seed of doubt, European Journal of Cardio-Thoracic Surgery, Volume 40, Issue 3, September 2011, Pages 656–663, https://doi.org/10.1016/j.ejcts.2010.12.062
Close - Share Icon Share
Abstract
Objective: The surgical treatment of residual N2 disease following induction radio-chemotherapy (IT) for locally advanced cIIIA-N2 non-small-cell lung cancer (NSCLC) is still debated. The long-term results after resection in a pN2 series are at the focus of this study. Methods: From January 1992 to December 2008, 161 consecutive pathologically proven Stage IIIA-N2 NSCLC patients underwent IT. Among these, 40 pN2s were included in this retrospective analysis. The associations between the mortality and the disease-free status with potential risk factors were explored by means of the Kaplan–Meier and Cox regression analysis. Results: Mean age and male/female ratio were 58.7 ± 9.7 years and 36/4, respectively. Twelve patients (30%) showed a clinical partial response and 28 (70%) showed stable disease. There was one (3%) perioperative death and four (10%) major complications. In the total group, the 3- and 5-year survival rates were 24.2% and 19.3%, respectively. The Cox regression analysis suggested that the macroscopic pN2 status proved to be a negative prognostic factor (hazard ratio (HR) = 2.8, confidence interval (CI) 95%: 1.1–7.3; p = 0.04). The recurrence rate flattened at 30.8% at the 3rd year. Furthermore, the bilobectomy–pneumonectomy group had a risk of relapse 6.9 times higher than the lobectomy group (CI 95%: 2.5–18.8; p ≪ 0.001). Conclusions: The persistence of disease at the N2 level after IT and surgery for cIIIa-N2 NSCLC does not exclude favorable outcome after resection, in particular in those patients with minor residual disease.
1 Introduction
Concurrent radio-chemotherapy is the standard of treatment in stage IIIa non-small-cell lung cancers (NSCLCs). In some cases, a multidisciplinary approach, including induction therapy (IT) and surgery, enhanced the chances of completeness of the surgical operation and allowed to obtain a significant improvement of prognosis [1]. In particular, the surgical approach is normally reserved in patients with stage IIIA-N2 NSCLC that showed a mediastinal lymph-node downstaging after IT. Although this last point is widely accepted, the role of surgery in those patients with persistent N2 disease after IT is still a forum of open discussion. Focusing on the details of the results of this particular group, surgical resection after IT led to a 5-year survival of 30–40% in patients with pN0 disease and of 20% in patients with persistent pN2 [2,3]. Poor prognosis within this setting is a fact. However, some aspects stay questionable and sow the seed of doubt in this topic.
Data from randomized trials available so far would advocate for a substantial absence of benefit from surgery in these patients. Still, some evidence indicates that these conclusions should be taken into consideration with some degree of flexibility. In fact, the clinical restaging is often inaccurate if compared with the pathological restaging [4]; and the N2 status is heterogeneous, where different levels of involvement (involvement of station ‘X’ instead of station ‘Y’, single level vs multilevel, intracapsular vs extracapsular) along with micro- or macroscopic presence of disease may indeed represent significantly different prognostic classes [5–7].
On these premises, we have run the analysis presented herein to deepen the knowledge of the natural history and outcome of this very peculiar class of patients.
2 Materials and methods
We retrospectively reviewed the clinical records of 40 Stage IIIa-N2 NSCLC patients, who had pathological persistence of N2 involvement, and who had undergone IT protocols followed by surgery indicated with curative intent, from 1992 to 2008.
Institutional Review Board approval had been preliminarily obtained for the use of data stemming out from standard clinical practice for research purposes. No additional interventions were planned (retrospective observational study).
Based on the information available from the clinical records, demographic and clinical features were collected and taken into consideration in the statistical analysis. Follow-up data were obtained from our database or, in some cases, by direct telephonic interview (privacy-related issues covered by comprehensive informed consent) with patients or, in the case of a death, with representatives from the family (next of kin). All patients had at least 1 year of follow-up.
Pre-treatment evaluation included patient history, physical examination, lung function tests, complete blood chemistry, computed tomography (CT) scan of the chest, brain and upper abdomen, brain magnetic resonance imaging (MRI), bone radionuclide scan, and fiberotic bronchoscopy. The diagnosis of NSCLC was obtained by pathological examination of the material obtained via endo-bronchial biopsy or CT-guided fine needle aspiration (CT-FNAC). The mediastinal involvement was always pathologically proven by endoscopy (trans-bronchial/trans-oesophageal) or via collar mediastinoscopy or anterior procedures (Chamberlain’s mediastinotomy). During the study period, the positron emission tomography (PET) scan was not always available in our institution; however, since 2005, it has been used for staging purposes in selected patients.
2.1 Induction therapy
In 2002, there was a change from two- to three-dimensional conformal radiotherapy methodology. Radiotherapy was administered with an angled-field technique modulated on the volume and location of the disease, so as to include in the isodose of 100% (±5%) area all the target volume (TV), with a maximum dose of 36 Gy to the spine. The TV was considered to be the primary tumor and the metastatic lymph-node area plus the surrounding 1.5-cm margin. The gross tumor volume (GTV) was the clinical target volume (CTV), and the planning target volume (PTV) was the CTV plus the 1.5-cm surrounding margin. The total referred dose was 50.4 Gy with classical or hyperfractionation. In every case, the treatment was CT supported. The treatment was CT, planned with lung parenchyma correctional factors, and a linear photon accelerator (nominal energy 6–10 MV) was used in all cases.
The chemotherapy protocol was not uniform during the study period. Different chemotherapy regimens were used: carboplatin 70 mg m−2 day−1 in continuous venous infusion, during days 1–4 of the first and last week of treatment (1991–2002); cisplatin 20 mg m–2 day–1/bolus plus 5-fluoruracil (5-FU) 1 g mq–1 day–1 in continuous venous infusion, during days 1–4 of the first and last week of treatment (1998–2002); cisplatin 50 mg m–2 on days 1 and 8, gemcitabine 1000 mg m–2 on days 1 and 8, and paclitaxel 125 mg m–2 on days 1 and 8, every 21 days’ cycle (2002 onwards).
A complete clinical and radiological re-evaluation was performed 4 weeks after the end of treatment. Clinical restaging was realized with CT scan, and the status assigned using the modified World Health Organization criteria [8].
2.2 Surgery
In the IT group, surgery was performed, on average, 2 weeks (from 10 to 17 days) after the clinical restaging (thus, as an average, 6 weeks after the last pulse of the IT). A parenchymal resection to an extent lesser than a lobectomy was considered as oncologically inappropriate and never performed. Pneumonectomy was indicated only if less extensive resections were not adequate in planning a radical approach for the removal of the diseases. Systematic mediastinal lymph-node dissection of levels 2, 4, 7, and 10 was performed in all cases [9]. Regardless of the gross nodal involvement, Stations 5 and 6 were removed in the case of left-upper-lobe lesions, whereas a modal dissection of Station 9 was performed after lower lobectomy. All the mediastinal tissue containing the lymph nodes was dissected and removed systematically within typical anatomical landmarks. Surgical–pathological stage was assigned according to the International Staging System for Lung Cancer [10], and the resection was considered complete if the proximal resection margins and the highest mediastinal resected node were free of tumor.
In the definition of the histological response at final pathology, the review of all the specimens to evaluate the macro/microscopic mediastinal lymph-node involvement was performed and defined as follows: (1) the persistent minor mediastinal involvement (microscopic residual disease), as any percentage of viable tumor cells in the primary tumor and 1–10% viable tumor cells in mediastinal lymph nodes; (2) persistent major mediastinal involvement (macroscopic residual disease), as any percentage of viable tumor cells in the primary tumor and >10% viable tumor cells in the mediastinal lymph nodes [5,11].
With respect to the surgical and pathological features, we collected details on the extent of resection, the pathological staging, the completeness of resection, the type of mediastinal involvement and the perioperative morbidity and mortality rates.
2.3 Statistical analysis
Survival and disease-free survival times were analyzed by means of the Kaplan–Meier function. The survival curves for the various potential prognostic factors were compared with the log-rank test. The Cox multiple regression approach was applied to identify the risk factors for mortality and relapse of the tumor and to estimate the respective hazard risks. The small sample size, and, thus, the limited number of deaths and relapses of tumor, constitute a limit to the number of covariates that it was possible to implement into a proportional hazards regression model. Therefore, based on the clinical relevance and on the actual representativeness in the study sample, the following factors were selected as potential risk factors: (2) positive response to the induction therapy (N2 downstaging vs stable disease); (2) N2 multilevel (vs single level) and macroscopic (vs microscopic) involvement; (3) need for bilobectomy or pneumonectomy (vs lobectomy); and (4) adenocarcinoma (vs squamous or large cells’ tumor). Recurrence was also considered when assessing mortality. These factors were fitted into the model one at a time. A forward stepwise-model-selection approach was applied to identify the most significant factors associated with the event of death or relapse of tumor.
A p ≪ 0.05 was set as the statistical threshold for statistical significance. STATA/SE release 10.0 was used to perform all statistical analyses.
3 Results
From January 1992 to December 2008, 161 consecutive patients with locally advanced Stage IIIa-N2 NSCLC entered in a multimodal treatment with induction-concurrent chemotherapy and radiotherapy (IT group). As showed in the Consort Type Diagram (Fig. 1 ), all patients (in good clinical and functional conditions), with partial/complete response or stable disease after IT, were explored surgically. At pathological staging, 40 patients showed persistent N2 disease, and were considered for this retrospective analysis. The mean age within this group was 58.7 ± 9.7 years. The male/female ratio was 36/4. Table 1 shows data regarding demographic, clinical, and surgical characteristics and staging of patients before IT (tumor, node, metastasis classification, TNM), after IT (yTNM), and after surgery (pathological TNM, pTNM).
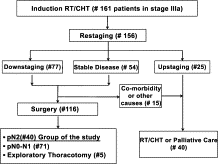
Consort type diagram of the population in cStage IIIa undergone multimodality approach.
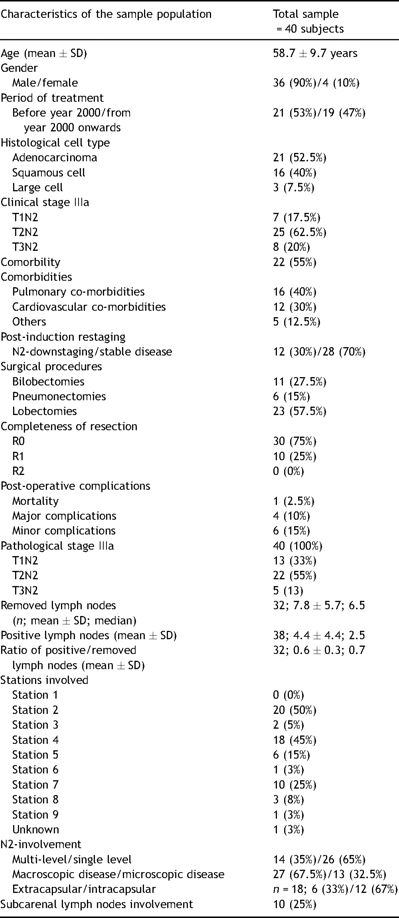
Preoperatively, 12 out of 40 patients (30%) showed a ‘radiological’ mediastinal downstaging, whereas 28 patients (70%) were restaged T1-3N2 (no change). Postoperatively, the whole population exhibited pathologically confirmed persistent N2 disease. In 27 (68%) cases, there was a macroscopic residual N2 disease, while a microscopic residual N2 disease was observed in 13 (33%) cases. Intracapsular involvement was found in 12 (66%) patients, while an extracapsular involvement was detected in six (33%) of them (data available in 18 subjects). The median number of lymph nodes removed was 6.5 (range: 1–21), while the median node ratio (involved/removed) was 0.7 (range: 0.1–1). A standard lobectomy was performed in 23 (58%) cases, bilobectomy in 11 (28%), and pneumonectomy was needed in six (15%) cases. The overall 30-day mortality rate was 3% (one case of pulmonary embolism after right pneumonectomy), and the major complications’ rate was 10% (n = 4). One patient experienced a pulmonary embolism and pneumonia after a lobectomy; one had lobar pneumonia, which required 48 h of mechanical ventilation and prolonged antibiotic therapy (over 7 days); one had broncho-pleural fistula conservatively treated, and, lastly, one myocardial infarction occurred. Among the six minor complications (15%) observed, four were supra-ventricular arrhythmias (medically treated), one was a persistent air-leak, and one was a case of disorientation. In 10 cases (25%), some residual microscopic remnants of the tumor were found on the bronchial resection margins and/or in the highest mediastinal resected nodes (R1); no grossly incomplete (R2) resections occurred.
3.1 Long-term outcome
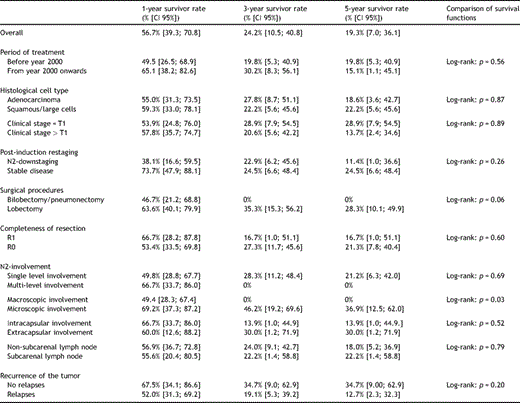
The mean follow-up duration was 24.4 ± 32.6 months. One, 3- and 5-year estimated overall survival rates were 56.7% (confidence interval (CI) 95%: 39.3–70.8%), 24.2% (CI 95%: 10.5–40.8%), and 19.3% (CI 95%: 7–36.1%), respectively (Fig. 2(A) ). From Fig. 3(A) and (B) , it emerges that the different degree of lymph-node involvement (micro/macroscopic) and the different types of surgical resection (bilobectomy or pneumonectomy vs lobectomy) correlated with a different survival response. This finding was also supported by the log-rank test (Table 2 ). With respect to both variables, the survivor curves were similar for the first year of follow-up, but started to differentiate after then. From the Cox multivariable regression analysis, it emerged that the macro/microscopic residual N2 disease factor was the most significantly associated with overall mortality. In particular, it was estimated that the group of patients with macroscopic disease had a risk of death 2.8 times higher than the group with microscopic residual N2 (CI 95%: 1.1–7.3%). None of the other selected factors were identified as additional risk factors for decease with large enough statistical evidence.
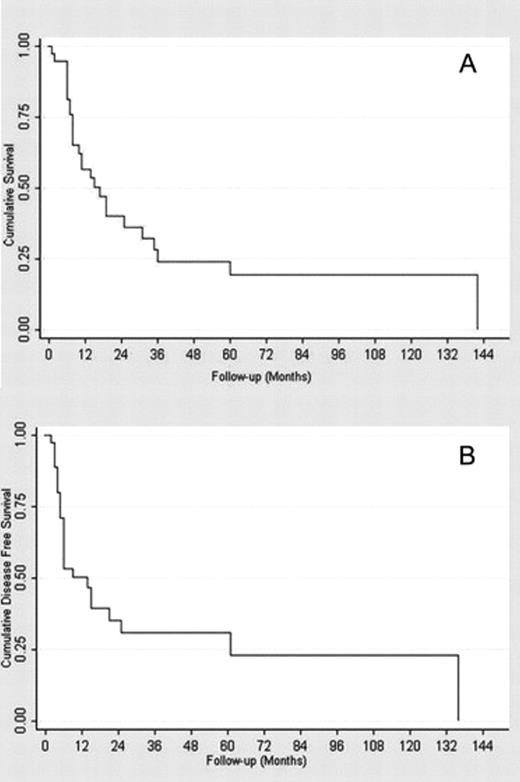
(A) Overall survival function. (B) Overall disease free survival function.
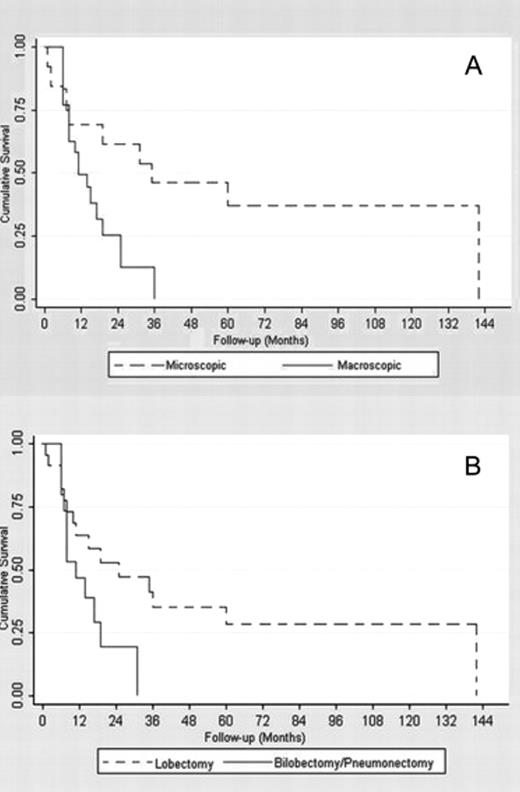
(A) Microscopic and macroscopic lymph node involvement survival functions. (B) Lobectomy and bilobectomy–pneumonectomy survival functions.
For the entire population, the 1- and 3-year estimated disease-free rates were 50.4% (CI 95%: 32.8–65.6%) and 30.8% (CI 95%: 15.2–48%). The 5-year disease-free rate coincided with the 3-year one (Fig. 2(B)).
The visual inspection of the disease-free curves suggested four factors potentially associated with the recurrence rate (Fig. 4 ). In particular, the log-rank test confirmed that (1) the patients who needed bilobectomy or pneumonectomy and those who needed lobectomy had a different response (p ≪ 0.0001) and (2) the micro-/macroscopic involvement also had a different disease-free survival (p = 0.04). However, the single-/multilevel lymph-node involvement curves (p = 0.10), the intra-/extracapsular nodal involvement (p = 0.14), and the completeness of resection curves (p = 0.18) were not proved to be different. The Cox multivariable regression analysis confirmed that the need for bilobectomy or pneumonectomy increased the risk of recurrence compared with the need for lobectomy. As reported in Table 4, a hazard ratio (HR) of 6.9 (CI 95%: 2.5–18.8%) was estimated. No evidence for any other additional risk factor was found from the analysis of our sample data. The main results from the Kaplan–Meier survival analysis are reported in Tables 2 and 3 . Table 4 shows a selected outcome from the Cox proportional hazards model regression analysis: the overall mortality HR for the macroscopic N2 involvement and the disease-recurrence HR for the need for bilobectomy or pneumonectomy are reported, with relative 95% CIs and p values. Estimates provided for all other selected factors were obtained by fitting each single variable, one at a time, additively to the macroscopic N2 involvement – for the model on overall mortality, and to the need for bilobectomy or pneumonectomy – for the model on disease recurrence, according to a forward stepwise model selection procedure.
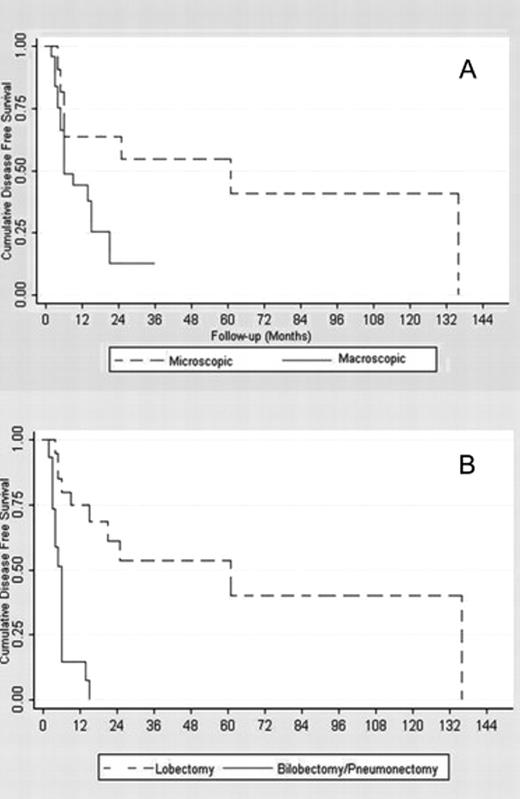
Microscopic and macroscopic disease free survival functions. (B) Lobectomy and bilobectomy–pneumonectomy disease free survival functions.
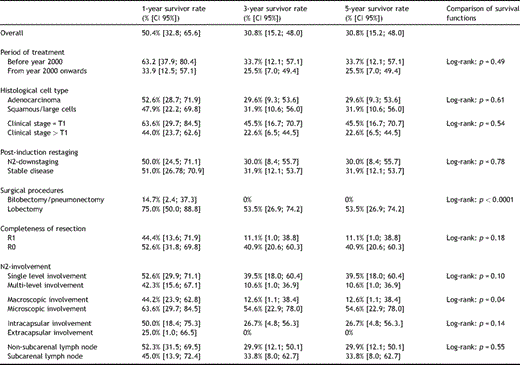
Long-term survival analysis: 1, 3 and 5-year disease free survival rates.
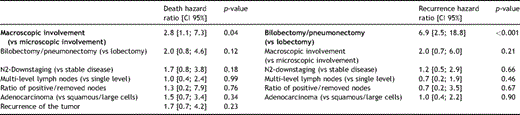
Proportional hazards regression analysis: a selected output from the Cox regression on long-term survival and disease free survival (factors highlighted in bold are significantly (p ≪ 0.05) associated to the recorded event as risk factors. Factors not highlighted in bold were fitted in the Cox proportional hazards regression model one at a time, according to a forward stepwise model selection approach).
4 Discussion
The standard of cure for IIIa-N2 patients is still a controversial issue. Primary surgery allows a very low 5-year survival rate (near 9%) in patients with positive N2 lymph nodes at mediastinoscopy [12]. Conversely, the multimodality therapy followed by surgery has shown an improvement of these outcomes [13]. Even if the role of surgery in the multimodality treatment is criticized and its utility not yet completely demonstrated as shown from recent reports [14,15], surgery is the most accepted treatment after IT in the subset of patients with a complete or partial response to the IT itself. In fact, downstaging after IT followed by surgery has shown to be directly associated with an improvement of the disease-free survival and with a lower distant recurrence rate [16,17]; but, at the same time, clinical restaging after IT is often inaccurate and the clinical response does not always correspond to the pathological one [5,18,19]. Besides, some teams [5,6] consider for surgical exploration not only the responders, but also patients with stable disease, notwithstanding the fact that the presence of residual N2 disease has been proposed as a contraindication to surgery. Consequently, the pathological N2 persistent surgically resected disease comprised two different conditions: (1) patients considered responders before surgery, but with evidence of viable neoplastic lesions in the pathological specimens; and (2) patients with clinically and pathologically persistent N2, intentionally treated by surgical teams on the assumption that surgery can achieve a better result compared with palliative care. The overall accuracy (and thus reliability in clinical terms) of the restaging process is still in debate and the introduction of innovative endoscopic and imaging techniques has somewhat increased the level of correlated complexity; however, this is beyond the scope of the present discussion where all the data available so far indicate that the outcome of surgery in terms of long-term prognosis and the presence of risk factors remains to be further explored in this subset of patients.
In fact, even if many experiences showed poor prognosis in persistent N2 patients [2,5], several authors reported encouraging results in some subsets of these patients and gave evidence of the great variability of the N2 disease [7,8,10,20,21]. Specifically, the experiences of Bueno et al. [2], Dooms et al. [5], and Lorent et al. [17] reported a 5-year survival rate of 9%, 0%, and 14%, respectively. By contrast, Cerfolio et al. [6] reported a 5-year survival rate of 42% for a highly selected group of persistent N2 patients (with unsuspected microscopic persistent N2 disease), who underwent R0 resections after IT. Similarly, Port et al. [3] reported a 5-year survival of 19% in the same group of patients, concluding that ‘Surgical resection may be a viable option for patients with residual N2 disease after induction chemotherapy….’ Our study confirms the positive results in terms of long-term survival and disease-free survival of the entire pN2 persistent group. In fact, the overall 3- and 5-year survival rates were 24.2% and 19.3%, respectively. Besides, the 3- and 5-year disease-free survival rates were both 30.8%. These data are not surprising and are in line with our previous results [1], and those of the IALSC committee survival rate N2 population [22].
About the prognostic implications of some features, from our study, microscopic residual N2 emerged as a significant favorable factor for survival, as reported in the Tables 2 and 4 (Log-rank: p = 0.03; Cox multivariable regression analysis: p = 0.04; HR = 2.8, CI 95%: 1.1–7.3%). Patients with microscopic residual N2 involvement (13/40) had a 36.9% 5-year survival rate, while this was 0% (p = 0.03) in patients with macroscopic N2 involvement (Fig. 3). The 5-year disease-free survival rate showed a similar outcome with a statistically significant difference between microscopic and macroscopic residual involvement (54.6% vs 12.6%, p = 0.04). This result confirms the better prognosis, in the case of microscopic residual N2, found by Dooms et al. [5], who reported a 5-year overall survival rate of 0% in patients with persistent major mediastinal-lymph-node involvement. On the basis of these results, one may hypothesize that patients affected by microscopic residual pN2 after IT form a separate subgroup that in terms of outcome is probably more similar to the pN0-N1 patients than to the persistent pN2 ones with manifest macroscopic residual-lymph-node involvement (5-year survival rate of 0%).
Among the analyzed variables, both in terms of the overall survival and disease-free survival, patients who underwent pneumonectomy or bilobectomy showed a worse prognosis than those who underwent lobectomy. The 5-year survival rates was 28.3% and 0%, respectively for the lobectomy and the bilobectomy–pneumonectomy group (p = 0.06); as well, the 5-year disease-free survival analysis showed a clearly better outcome for the lobectomy group (53.5% vs 0%, p ≪ 0.001). The association of pneumonectomy with a worse prognosis in the persistent pN2 patients is well known: a more advanced, massive and/or centrally located tumor is indeed a strong determinant of poor(er) outcome. Although pneumonectomy after IT can be a reasonable option to obtain a complete resection, it has to be restricted to very selected patients. Our data about the negative prognostic effect of pneumonectomy are in line with Albain et al. [16], who reported that surgical treatment after IT should be avoided when pneumonectomy is necessary and should be reserved for patients for whom it is possible to perform minor resections. In this context, the importance of the T-factor in the restaging evaluation should be considered. Indeed, the T-status is crucial in the surgical management and particularly in the decision of performing a pneumonectomy instead of a lobectomy. Pneumonectomy being a high-risk procedure after IT (not clearly recommended by most literature, considering the controversial results) [23], the T-factor should more heavily influence the indication to surgery after IT.
Moreover, the increased perioperative morbidity and mortality, along with negative impact on the quality of life (QoL) correlated with pneumonectomy after IT, assume an enhanced value in the subgroup of the persistent pN2 cases. The residual pulmonary function being strongly decreased in these highly critical subjects, but in general in all patients who underwent surgery after IT, a multimodal specific respiratory rehabilitation program could play an important role in improving postoperative functional long-term outcomes [24], and therefore have a considerable impact on QoL.
Finally, in our experience, we could not confirm the role of other factors as predictors of long-term outcome. In particular, the study of Cerfolio et al. [6] was focused on the number of lymph nodes involved as a predictor of survival, demonstrating that patients with single-lymph-node disease had a better prognosis compared with patients with multiple nodal involvement (p = 0.03). Besides, Decaluwé et al. [25] found a significant difference in the 5-year survival between persistent single versus multilevel N2 at time of resection (37% vs 7.1%; p ≪ 0.005). Perhaps due to the small size of our study sample, we could not find a strong statistical evidence supporting the role of multilevel involvement as a prognostic factor of 5-year survival, although the difference observed (39.5% vs 10.6%, p ≪ 0.10, respectively, for single and multilevel involvement) can be regarded as clinically relevant.
5 Conclusions
The process of ‘ideal’ identification, through restaging procedures, of the best candidates for surgery after IT remains to be clarified. Nevertheless, in our data, the 5-year survival rate of 19.3% confirms the potentially curative role of surgery after IT even in selected patients with persistent pN2 disease. Since the subgroup of pN2-‘Micro’ had a good survival, overall, the persistence of N2 disease does not seem, in principle, to exclude favorable outcome after surgery. Conversely, patients with macroscopic persistent pN2 involvement, and patients who have high probability to undergo bilobectomy–pneumonectomy, should be better identified in a preoperative assessment and probably excluded from surgery. A possible approach, as this appears a rather difficult task, especially in the first group of these cases, could be identified in focusing the predictors of pN2-Micro by validation of every correlation with the imaging process at the base of the restaging procedure.
References
Author notes
Meeting presentation: The article has been presented at the 46th annual meeting of The Society of Thoracic Surgeons, Fort Lauderdale, 25–27 January 2010.




