-
PDF
- Split View
-
Views
-
Cite
Cite
Severin Semsroth, Robert G. Stigler, Oliver Y. Bernecker, Elfriede Ruttmann-Ulmer, Jakob Troppmair, Karin Macfelda, Johannes O. Bonatti, Guenther Laufer, Everolimus attenuates neointimal hyperplasia in cultured human saphenous vein grafts, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 3, March 2009, Pages 515–520, https://doi.org/10.1016/j.ejcts.2008.11.035
Close - Share Icon Share
Abstract
Objective: Neointimal hyperplasia is the first step in a cascade leading to a reduced patency rate of saphenous vein grafts in comparison to arterial grafts in coronary artery bypass grafting. Using cultured human saphenous vein grafts as a model for coronary artery bypass grafting, we investigated if the mammalian target of rapamycin inhibitor everolimus attenuates neointimal hyperplasia. Methods: Saphenous vein grafts from 10 patients undergoing coronary artery bypass grafting were processed as follows: from each patient, one segment served as baseline control at day 0. Two segments were cultured in a neointimal hyperplasia model separately. One received no treatment and the other everolimus (1 μM). All vein grafts underwent histomorphometric analysis, assessment of proliferation by Ki-67 immunostaining and quantification of phospho-S6 ribosomal protein using western blot analysis. Results: Everolimus treatment resulted in reduced neointimal hyperplasia (thickness 3.7 ± 1.2 μm) compared to untreated controls (10.1 ± 2.5 μm, p = 0.008). The intima/intima + media-ratio was reduced in the everolimus group (0.10 ± 0.02) compared to untreated controls (0.24 ± 0.07, p = 0.008). The number of Ki-67 positive proliferating cells in everolimus treated vein grafts (15 ± 7 cells/high power field) showed a tendency of reduction compared to untreated controls (36 ± 20 cells/high power field, p = 0.036). Finally, everolimus treatment resulted in downregulation of S6 ribosomal protein phosphorylation in comparison to untreated controls. Conclusion: Everolimus is able to reduce neointimal proliferation in cultured human saphenous vein grafts by inhibition of the mammalian target of rapamycin, even though different transfection methods are to be evaluated for a clinical application in coronary artery bypass grafting.
1 Introduction
Coronary artery bypass grafting (CABG) has been the gold standard in the treatment of multivessel and left main coronary artery disease (CAD). Saphenous vein grafts are the most widely used type of bypass graft and have historically early graft failure due to thrombosis with a rate of 10–15% within a year after surgery. Ten years after CABG, around 65% of all grafts are patent and half of those have angiographic signs of atherosclerosis. Twenty years after CABG, the patency rate of saphenous vein grafts appears to be around 25% [1]. It is now clear that treatment with platelet inhibitors and statins has improved patency of saphenous vein grafts, but long-term data regarding these pharmacological strategies are lacking so far [2]. The so called ‘vein graft disease’ is a pathological process, caused by distinctive triggers (endothelial injury, ischemia/reperfusion) leading to activation of cell cycle progression [3]. Activated vascular smooth muscle cells migrate from the media into the intima of venous vascular walls transforming from a contractile into a proliferative phenotype by forming a neointima, resulting eventually in graft atherosclerosis influenced by common coronary risk factors. The consequence is vein graft failure [4]. The mammalian target of rapamycin (mTOR) is a serine/threonine-specific kinase and a key regulator of cell growth and proliferation (Fig. 1 ) [5]. This pathway is activated via insulin, growth factors and the nutritional status. Growth factors are directly involved in the pathogenesis of neointimal hyperplasia. Platelet derived growth factor (PDGF) is released by activated platelets and binds to the PDGF-receptors on vascular smooth muscle cells (VSMC) [6]. Insulin like growth factor-1 (IGF-1) plays an important role in mammalian growth and development. Vascular endothelial growth factor (VEGF) binds to the VEGF-receptors on smooth muscle cells [7]. Upstream components of the mTOR pathway are controlled through the PI3K/Akt pathway [8,9]. Currently, the mTOR inhibitors rapamycin and everolimus are licensed as an immunosuppressive drug in organ transplantation. Rapamycin inhibits fibronectin-induced SMC migration through a pathway that involves phosphoinositide 3-kinase (PI3K), mTOR and S6K [10]. On the other hand, everolimus is a proliferation signal inhibitor that blocks vascular SMC proliferation [11].
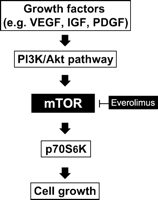
The involvement of the mTOR pathway in the development of neointimal hyperplasia.
The aim of the study was to evaluate whether everolimus has the potential to attenuate neointimal hyperplasia in cultured human saphenous vein grafts via inhibition of the mTOR pathway.
2 Methods
2.1 Organ culture
The study was approved by the local institutional review board. Saphenous veins from 10 patients undergoing coronary artery bypass grafting were harvested in a common open no touch technique and flushed with cold physiologic saline solution without additives. The surgeon performed a leakage test of the graft with gentle, but unmonitored intraluminal pressure, before vein grafts were anastomosed to the coronary arteries. Remnants of these saphenous veins were transported in serum free Roswell Park Memorial Institute (RPMI)-1640 tissue culture medium at 4 °C, containing 4 units/ml heparin, 25 g/ml gentamicin, 2.5 g/ml amphotericin B, 100 units/ml penicillin, 100 g/ml streptomycin and 2 mM l-glutamine (Sigma–Aldrich, St. Louis, USA) from the operating room to the laboratory within minutes. Immediately after laboratory arrival, the vein grafts were carefully dissected from connective adventitial tissue, cut longitudinally and sliced into three pieces of about 7 mm under sterile conditions. One segment served as baseline control (day 0), two segments were pinned up on a mesh with a silicon pad (the endothelial side facing upside) and cultured with 10 ml of culture medium containing RPMI 1640, 30% fetal bovine serum (FBS), 4 units/ml heparin, 25 (g/ml gentamicin, 2.5 g/ml amphothericin, 100 units/ml penicillin, 100 g/ml streptomycin and l-glutamine (Sigma–Aldrich, St. Louis, USA). From graft harvesting to incubation of the vein graft, the estimated total time of ischemia was around 30 min. The specimens were cultured in an incubator (Hera Cell, Hanau, Germany) at 37 °C, 5% carbon dioxide for 14 days, as described previously by Porter et al. [12]. Everolimus was added to the culture medium of one segment, the remaining segment received no treatment (untreated control). The concentration of 1 μM everolimus was chosen as suggested by Schuler et al. [11]. The growth factor-driven in vitro proliferation of cells from non-hematopoietic origin is inhibited by 1 μM everolimus to achieve IC 50 values (IC 50: concentration needed to reach 50% inhibition). Medium and drugs were renewed every 48 h. All saphenous vein grafts, either cultured (treatment with everolimus or no treatment) for 14 days or immediately fixed after graft harvesting (baseline) were divided as follows: one section was fixed in 4% paraformaldehyde for histomorphometric evaluation and assessment of cell proliferation by Ki-67 immunostaining. The other sections were frozen in liquid nitrogen for western blot analysis of the phosphorylation of serine 235/236 on the ribosomal S6 protein, a downstream effector of mTOR used as an indicator of mTOR pathway activity.
2.2 Histomorphometric analysis for quantification of neointimal growth
Sections of vein grafts were fixed in 4% paraformaldehyde for 18 h, dehydrated, paraffin-embedded, sectioned (4 μm) and stained with elastica van Gieson (Acustain Elastic Stain Kit, HT25A, Sigma–Aldrich, St. Louis, MO, USA). Quantitative histomorphometric analysis was performed (ImageJ 1.32 for Macintosh, National Institutes of Health, USA) as follows: neointimal hyperplasia and media (both divided by the lamina elastica interna) were measured at 30 sections across the high power field and mean values were determined. The intima/intima + media (I/I + R) ratio was subsequently calculated.
2.3 Ki-67 immunostaining for evaluation of cellular proliferation
From each vein paraffin-embedded sections were cut at a distance of 4 μm. The slides were rehydrated for 5 min in 50 mM Tris buffered saline (TBS) at pH 7.4, with 1% bovine serum albumin (BSA) and 0.1% Triton X (Sigma–Aldrich, St. Louis, MO, USA). The endogenous peroxidase activity was quenched by incubating the specimens for 5 min with the peroxidase block (Dako, Glostrup, Denmark). After a washing-step using 50 mM TBS, 1% BSA and 0.1% Tween 20, the specimen were incubated with the primary antibody or negative control reagent (washing solution) for 30 min. Antibodies recognizing Ki-67 antigen, (anti-Ki-67, Dako, Glostrup, Denmark) were diluted 1:40 in 50 mM TBS and 1% BSA. Following the primary antibody, the specimen were coated with labeled polymer (EnVision Systems, Dako, Glostrup, Denmark) for 30 min and washed. Staining was completed by a 10 min incubation with 3,3′-diaminobenzidine (DAB) + substrate-chromogen, that resulted in a brown-colored precipitate at the antigen site (EnVision Systems, Dako, Glostrup, Denmark). Counterstaining was performed with Mayer’s Hemalaun (Merck, Darmstadt, Germany).
2.4 Quantification of phosphorylated-S6 ribosomal protein using western blot analysis
We investigated the phosphorylation of the S6 ribosomal protein (s6rp), a downstream protein of mTOR leading to translation and cell cycle progression. Three samples of each group (baseline control, no treatment and treatment with everolimus) were homogenized, quantified (Bradford Assay, Biorad) and each well was loaded with 20 (g of protein. Electrophoresis was performed for the first 5 min at 50 V, 3.00 A, 300 W, the following 50 min with 150 V, 3.00 A, 300 W in a mini protean three Cell (Biorad, Hercules CA, USA). Protein transfer to a nitrocellulose membrane (Schaerer, Rothrist, Switzerland) was done with the transfer unit (Biorad, Hercules CA, USA) according to the protocol. Subsequent washing with 50 mM TBS and 0.1% Tween 20 (TBS-T), blocking for 60 min in skim milk (Sigma–Aldrich, St. Louis, USA) and incubation with a phospho-S6-ribosomal protein (Ser235/236) antibody (Cell Signaling Technology, Inc. Danvers, MA, USA) overnight at 4 °C was performed. After a second washing step (TBS-T), the secondary antibody was added as recommended by the manufacturer (Amersham, Buckinghamshire, United Kingdom) and incubated for 60 min at 4 °C. For final detection the blot was covered with 2 ml of ECL detection solution (Amersham, Buckinghamshire, United Kingdom), and exposed to an X-ray film (Hyperfilm, Amersham, Buckinghamshire, United Kingdom). Equal protein loading was confirmed by reprobing the blot with a monoclonal antibody against GAPDH (AM4300, Ambion, Austin, USA)
2.5 Data analysis
For statistical analysis (SPSS 12.0, Chicago, IL, USA), a Wilcoxon’s signed rank test was performed for pairwise comparison between baseline and cultured vein grafts on day 14 receiving everolimus or no treatment. Since multiple comparisons were performed, a Bonferroni correction (<0.05/3 = 0.017) was applied. Therefore, p values <0.017 were considered to indicate statistical significance. Data are presented in mean values ± standard deviation, except S6-ribosomal protein that has been analyzed in total protein concentration.
3 Results
3.1 Inhibition of m-TOR reduces neointimal hyperplasia in saphenous vein grafts
After two weeks of organ culture, saphenous vein grafts treated with the mTOR-inhibitor everolimus showed a significant reduction of neointimal hyperplasia (3.7 ± 1.2 μm) compared to untreated vein grafts (10.1 ± 2.5 μm) and no significant reduction compared to baseline control (2.8 ± 1.7 μm). A significant increase of neointimal thickness after two weeks of organ culture was observed in the untreated vein grafts in comparison to baseline control (Figs. 2A and 3 ). There was no statistically significant difference between the thicknesses of the media between all three groups (Fig. 4 ). Everolimus treated vein grafts showed significantly reduced intima/intima + media-ratio (0.10 ± 0.02) compared to untreated vein grafts (0.24 ± 0.07) and no difference compared to baseline control (0.08 ± 0.05) (Fig. 5 ).
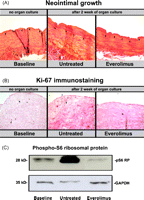
(A) Tissue sections of a saphenous vein grafts used during routine CABG were stained with elastica van Giesson. Black arrows indicate the lamina elastica interna dividing the media from the (neo)intima compartment. Left panel shows a saphenous vein immediately after harvesting (baseline control), representing the status of the vein at this stage. It shows few endothelial layers in the intima compartment and an intact vessel wall structure. The middle panel represents the vein graft after two weeks of untreated organ culture. A massive neointima has developed. In the structure of the intimal compartment, proliferative vascular smooth muscle cells have formed a neointima. The panel at the right shows a representative picture of a vein graft after two weeks of organ culture treated with everolimus. It demonstrates the antiproliferative effect of mTOR inhibition through a reduced intimal proliferation. (B) Sections were immunostained for Ki-67, positive reaction color is dark brown. Treatment of saphenous vein grafts with the mTOR inhibitor everolimus shows significantly less Ki-67 positive cells, indicating effective blockage of cell proliferation by mTOR inhibition. (C) Whole cell lysates from vein grafts were examined by western blot analysis for expression of phospho-S6 ribosomal protein with a specific antibody. Everolimus inhibits neointimal growth by suppressing the downstream protein of mTOR S6-ribosomal protein. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
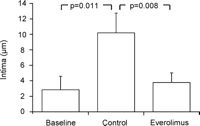
Intima thickness (μm) was significantly reduced in the everolimus treated vein grafts in comparison to untreated vein grafts (control). Further, a significant increased intima thickness was observed in the control group in comparison to baseline.
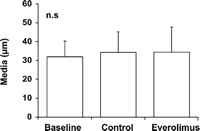
Media thickness (μm) in baseline and cultured vein grafts showed no difference in all three groups.
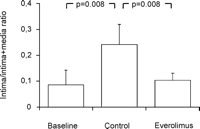
Intima/intima + media-ratio showed a significantly reduced ratio in the everolimus treated vein grafts in comparison to untreated vein grafts. Further, a significantly increased ratio was observed in the control group in comparison to baseline.
3.2 The anti-proliferative effect of the mTOR inhibition is demonstrated by the analysis of Ki-67 labeled proliferating cells
The Ki-67 positive cells showed a clear tendency of reduction in the everolimus treated vein grafts (15 ± 7 cells per high power field) in comparison to untreated (36 ± 20 cells per high power field) and to baseline control vein grafts (35 ± 23 cells per high power field), but were not considered statistically significant. Remarkably, baseline vein grafts showed Ki-67 expression already after short surgical manipulation (Figs. 2B and 6 ).
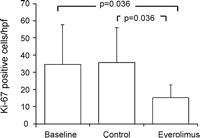
Everolimus reduces cell proliferation. Interestingly, cell proliferation was identical in baseline and in the no treatment group, indicating cell proliferation during graft harvesting.
3.3 Downregulation of the mTOR pathway by everolimus
Potent inhibition of the mTOR pathway could be demonstrated by the downregulation of the mTOR dependent phosphorylation of ribosomal protein (Ser235/236) (Figs. 2C and 7 ). Densometric total protein quantification showed a reduction of S6-ribosomal protein phosphorylation (0.78 relative densitometry units) in the treatment group compared to untreated controls (2.16 relative densitometry units) both in relation to baseline vein grafts (1 relative densitometry unit).
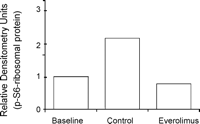
Western blots were scored for relative densitometry, indicating blockage of mTOR pathway through downregulation of the downstream protein phospho-S6 ribosomal protein.
4 Discussion
The present study demonstrated an antiproliferative effect of everolimus in cultivated human saphenous veins frequently used as conduits for coronary artery bypass grafting. This antiproliferative effect was demonstrated by histomorphometric analysis and supported by immunohistochemical staining of Ki-67, a marker for cell proliferation. Furthermore, a downregulation of the phosphorylation of S6-ribosomal protein (Ser235/236) in everolimus treated vein graft was observed. This indicates an effective blockage of the mTOR signaling pathway and demonstrates not only an involvement of mTOR in neointimal hyperplasia development, but also suggests a possible therapeutic approach. Everolimus, a macrolide antibiotic and derivate of sirolimus, blocks cell cycle progression by forming a complex with the immunophilin FK506-binding protein 12 (FKBP12). This complex inhibits mTOR, resulting in an arrest at the G1-phase of the cell cycle [13].
Everolimus is currently licensed as an immunosuppressive drug. Several studies have suggested everolimus as a potent substance for the reduction of cardiac-allograft vasculopathy by inhibiting chronic inflammatory response [16]. Further, everolimus coated stents are being used for prevention of in-stent restenosis after percutaneous coronary intervention in patients with coronary heart disease [14]. Inhibition of mTOR by everolimus reduces macrophages in an atherosclerotic plaque without influencing the viability of SMC favoring plaque stability [15].
Inhibition of the mTOR/p70 S6K1 pathway is an important factor in transcriptional control of vascular smooth muscle cell gene expression regulating vascular smooth muscle cell differentiation by expression of the cyclin-dependent kinase inhibitors p21(cip) and p27(kip). Cultured vascular smooth muscle cells treated with the mTOR inhibitor rapamycin maintain a contractile morphology. In comparison, injured vascular smooth muscle cells undergo a phenotypic transformation towards a proliferative, dedifferentiated, migratory phenotype with upregulated extracellular matrix protein synthesis. This cascade results in the development of neointimal hyperplasia [16]. Schachner et al. [17] observed a reduction of neointimal proliferation and increased apoptosis in an experimental animal model of neointimal hyperplasia by blocking mTOR with rapamycin. However, different transfection methods for administration of everolimus have to be evaluated before being introduced in a clinical setting of coronary artery bypass grafting. Local drug administration for prevention of neointimal hyperplasia has been investigated using different carriers. Promising carriers for everolimus could be Pluronic-F-127e gel (BASF, Germany), as demonstrated by Schachner et al. [17], a drug-eluting biodegradable poly l-lactic acid and epsilon-caprolactone copolymer (PLA-CL) film as used by Kawatsu et al. [18], a drug-delivery system using fibrin glue as shown by Qi et al. [19]. These carriers have in common the fact that timing and dosage of drug delivery can be modified by adjustment of the chemical composition. The advantages of local perivascular drug delivery systems are less systemic side effects as well as direct drug delivery at the target tissue.
To demonstrate the antiproliferative effect of everolimus, cell proliferation was assessed by Ki-67 immunostaining, which showed a trend in reduction of cell proliferation in vein grafts treated with the mTOR inhibitor everolimus in comparison to untreated vein grafts after two weeks of organ culture. A question that could not be addressed by the study was to find out how much damage had been inflicted upon the vein graft during surgical handling in routine vein graft harvesting. Due to concerns from the ethical committee, we could not obtain unmanipulated saphenous veins before graft harvesting. Remarkably, we could demonstrate baseline vein grafts already showing some degree of intimal growth. Initial cell proliferation was demonstrated by increased Ki-67 expression. These baseline vein grafts used in the study represent the inhomogeneous quality of grafts at the time of coronary artery bypass grafting. Further, we were able to show how much neointimal growth occurred in the two weeks of organ culture with or without the treatment of everolimus. Regardless of the thickness of intima in baseline vein grafts, everolimus blocked neointimal development equally efficiently in all saphenous vein grafts. Standard surgical handling of vein grafts induces inflammation via activation of NFκB in the vessel wall and impairs vascular function [20]. Mechanical injury of vein grafts during CABG should be reduced as much as possible. S6-ribosomal protein phosphorylation increases cell cycle progression and everolimus blocks cell cycle proliferation effectively by suppressing mTOR. These findings indicate an involvement of the mTOR pathway in cell proliferation [21] with formation of neointimal hyperplasia during surgical handling in routine CABG and suggest that everolimus inhibited the cell cycle proliferation effectively by suppressing the activation of mTOR protein. In the PREVENT IV trial the efficacy and safety of pretreating vein grafts with edifoligide for patients undergoing CABG surgery has been assessed. The ex vivo treatment of saphenous vein grafts with edifoligide, an oligonucleotide decoy that binds to and inhibits E2F transcription factor, did not improve patency rate of vein grafts used in CABG patients. The authors of these disappointing findings concluded that more understanding of the mechanisms and clinical consequences of vein graft failure are necessary to improve the durability of CABG surgery [22].
In conclusion, everolimus is an effective way to attenuate neointimal hyperplasia in cultured human saphenous vein grafts. The mTOR pathway is involved in the development of neointimal hyperplasia by increasing cell proliferation. Inhibition of mTOR pathway with everolimus attenuates expression of the downstream protein, phospho-S6-ribosomal protein (Ser235/236). Therefore, everolimus could prevent the development of neointimal proliferation of saphenous vein-grafts in CABG, though different delivery methods for transfection are to be evaluated for a clinical application in CABG.
Everolimus was kindly provided by Novarits International AG.




