-
PDF
- Split View
-
Views
-
Cite
Cite
Ghassan S. Musleh, Subir S. Datta, Nizar N. Yonan, Geir J. Grotte, Brian A. Prendergast, Ragheb I. Hasan, Abdul K. Deyrania, Association of IL6 and IL10 with renal dysfunction and the use of haemofiltration during cardiopulmonary bypass, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 3, March 2009, Pages 511–514, https://doi.org/10.1016/j.ejcts.2008.10.010
Close - Share Icon Share
Abstract
Objective: Assessment of the effects of haemofiltration during cardiopulmonary bypass (CPB) in coronary artery bypass grafting (CABG) on the renal function and correlation with interleukin 6 (IL6) and interleukin 10 (IL10) levels. Methods: Seventy-nine patients scheduled for elective CABG were prospectively randomised into two groups. Group A with a haemofilter attached to arterial line of the CPB circuit and group B without a haemofilter. The two groups were comparable in their symptoms, sex, and previous history of myocardial infarction, left ventricular function, cross-clamp time, bypass time and total grafting per patients. Blood urea and creatinine levels were measured the day before operation, 12 h after operation and on the 3rd postoperative day. IL6 and IL10 were measured in blood samples collected 1 h before surgery, on arrival to ITU and after 12 h. IL6 and IL10 levels were measured using ELISA test. Results: High levels of IL6 (>100 pg/ml) postoperatively were associated with increased incidence of renal dysfunction (p < 0.017). Additionally, high IL10 (>30 pg/ml) levels postoperatively were associated with increased incidence of renal dysfunction (p < 0.014). There were no effects of the haemofilter on postoperative IL6 and IL10 levels. Use of haemofiltration during CPB was found not to be protective against renal dysfunction (p < 0.071). Conclusions: Haemofilter use during cardiopulmonary bypass does not have a protective effect on postoperative kidney function. Haemofilter has no effect on the level of IL6 and IL10.
1 Introduction
Cardiopulmonary bypass is associated with systemic inflammatory response. This inflammatory response varies from simple elevation of inflammatory markers to multiple organ failure. This inflammation may affect every organ in the body including the kidney causing renal dysfunction. Postoperative renal dysfunction is associated with significant increase in morbidity and mortality. It is believed that this systemic inflammation is mediated by cellular and humoral mediators like cytokines, compliments and white blood cells. Interleukin 6 (IL6), a proinflamatory cytokine, plays an important role in the development, pathogenesis and outcome of systemic inflammatory response syndrome (SIRS). Plasma levels of IL6 are elevated in patients with sepsis and high IL6 concentrations are associated with increased mortality [1] and [2]. IL10 is an anti-inflammatory cytokine, it controls the inflammatory processes by suppressing the expression of proinflammatory cytokines, chemokines, adhesion molecules, as well as antigen-presenting and stimulatory molecules in monocytes, macrophages, neutrophils and T cells [3].
Theoretically using a haemofilter for removal of the mediators may minimise this systemic inflammation. As a result, organ dysfunction will be minimised. This study was designed to find out the effects of the haemofilter on the level of the circulating cytokines level and whether this effect materialised into a positive clinical effect in the form of an improvement in the postoperative kidney function.
2 Materials and methods
Patients with coronary artery disease that came for elective first time coronary artery bypass grafting (CABG) were recruited for the study. Four consultant cardiac surgeons from two teaching hospitals participated in the study. Elective cases with stable symptoms were targeted for inclusion. Diabetes, chronic lung disease, renal impairment, urgent/emergency cases and redo cases were excluded from the study. Ethical approval was obtained from the ethical committee at South Manchester University Hospitals. Power calculation suggested that 25 patients in each group are enough to show significant difference in the levels of the interleukins. Seventy-nine patients who consented for the study were randomised by the computer into two groups. Group A, the study group, with a haemofilter attached to the arterial line of the cardiopulmonary bypass (CPB) circuit and group B as control group, without a haemofilter. Both groups underwent conventional CABG with cardiopulmonary bypass. In the control group a haemofilter (Medos Medizintechnik AG Obere Steinfurt 8–10 d-52222 Stolberg, Germany) was used throughout the whole cardiopulmonary bypass period. The inlet of the filter was attached to a branch tubing from the arterial line. The outflow from the haemofilter returned to the venous reservoir. A separate graduated collection container was attached to the effluent side of the haemofilter. On average 15 ml/kg were filtered from each patient during the cardiopulmonary bypass period depending on the flow, surface area of the patient and blood level in the venous reservoir.
Preoperative clinical data were prospectively collected and included age, sex, weight, symptoms and previous history of myocardial infarction. Intra-operative data included number of grafts, cross-clamp time, bypass time, and fluid balance. Postoperative data included ventilation period, ITU stay, and total hospital stay.
Blood urea and creatinine levels were measured the day before operation, 12 h after operation and on the 3rd postoperative day. IL6 and IL10 were measured in blood samples collected 1 h before surgery, on arrival to ITU and after 12 h. Blood was centrifuged and serum was saved and stored at −70 °C.
Serum levels of IL6 and IL10 were measured using ELISA technique (human IL6 Elisa and human IL10 Elisa kits manufactured by Diaclone/France).
IL6 and IL10 levels were then plotted using the mean absorbance for each standard concentration on the ordinate against the IL6 and IL10 standard concentration. This was achieved using computer-based software (Revelations made by Dynal Tech, UK). Concentrations of IL6 and IL10 were measured in pg/ml.
Statistical evaluation was done using chi-square test (spss 11) to assess the effect of haemofilter on the postoperative renal function. Correlations were then analysed between the interleukin levels and renal dysfunction. Mann–Whitney test was used to compare the interleukin levels between the two groups.
3 Results
The two groups are comparable in angina severity, previous history of myocardial infarction, left ventricular function, cross-clamp time, bypass time, sex distribution and total grafting per patients (Table 1 ).
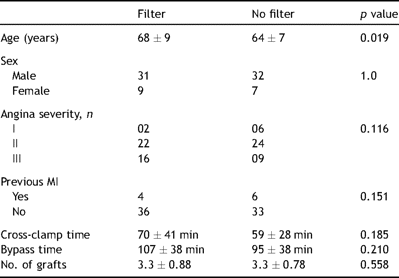
Nine patients developed acute renal dysfunction in the postoperative period (creatinine >176 μmol/l or an increase of 62 μmol/l above the preoperative level). Seven out of the nine were in the filter group and two in the non-filter group. None of the nine patients needed haemofiltration or haemodialysis to treat their renal dysfunction in the postoperative period.
Analysis of the blood for IL6 and IL10 showed no significant difference in the level of IL6 and IL10 between the two groups except IL10 at 12 h after surgery (Table 2 ). However this difference disappears if the IL10 values are adjusted for age.
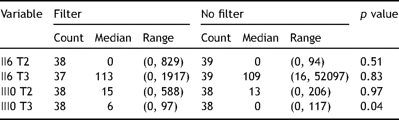
Compared the interleukin levels between the two groups using Mann–Whitney test.
Blood levels of urea and creatinine in the postoperative period showed no significant difference between the two groups; however there was a trend toward reduced levels of both urea and creatinine in the filter group in the third postoperative day (Table 3 ).
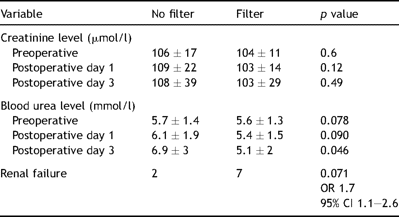
Serum creatinine and urea level of the two groups done on first and third postoperative days.
IL6 level >100 pg/ml and IL10 >30 pg/ml 12 h after surgery were found to be associated with a significant increase in the risk of acute renal dysfunction in the postoperative period (p < 0.017, OR 1.3 and 95% CI 1.0–1.7 for IL6 and p < 0.041, OR 1.2, 95% CI 1.01–1.4 for the IL10) (Tables 4 and 5 ).

Relation between renal dysfunction and IL6 levels (on arrival to ITU and 12 h after surgery).

Relation between renal dysfunction and IL10 levels (on arrival to ITU and 12 h after surgery).
ELISA analysis of the filtrate fluid using the same technique failed to identify any trace of either IL6 or IL10.
4 Discussion
IL6 is a type I inflammatory cytokine. It is secreted by smooth muscle cells, T cells and macrophages, and its secretion is controlled by IFN-beta and TNF-alpha. It is the main mediator of the acute phase response, regulates inflammation, induces B cell differentiation, activates T cells, induces T cell cytotoxicity and commits monocytes to differentiate macrophages. On the other hand IL10 is a major type II anti-inflammatory cytokine produced by B and T cells. It prevents type I cytokine synthesis, so would inhibit IL6 production, especially in macrophages and smooth muscle cells. It prevents the generation of Th1 cells while committing naïve Th0 cells to Th2, so it drives anti-inflammatory processes.
Acute renal failure is a well-known complication after cardiac surgery. The incidence varies between 1% and 15% [4,5]. In the 1990s the mortality of acute failure requiring filtration was quite high and varied between 70% and 90% [2], but during this decade, better outcome has been reported [5,6].
The preoperative patient characteristics most strongly associated with postoperative severe renal insufficiency included: age, gender, redo CABG, congestive heart failure, peripheral vascular disease, diabetes, hypertension, and preoperative intra-aortic balloon pump [7]. Operation type was also found to be a risk factor for ARF and to have an impact on mortality as well [8]. Lawman and colleagues found that 2.4% of patients having a CABG were filtered with a mortality 37.8%, 14.2% of patients having valve replacements were haemofiltered with a mortality rate of 60.9%. To avoid the effect of operation type on the outcome, only first time conventional CABG done on cardiopulmonary bypass was included.
Acute renal dysfunction after CABG is considered when creatinine levels rise above 176 μmol/l [9]. Acute renal failure does increase mortality and morbidity after CABG. Lassnigg and colleagues found that postoperative creatinine level correlate with 30-day mortality [10]. Mortality rates were found to be 2.6% in those with a drop of postoperative creatinine in contrast to 8.9% in those with an increase of postoperative creatinine.
Regarding the effect of haemofilter on the level of IL6 and IL10, we found that haemofilter has no effect on both IL6 and IL10 and neither was detected in the haemofiltrate. In a study of 20 paediatric cardiac operations, Brancaccio and colleagues found that haemofilter has no effect on IL6 [11]. This finding is similar to our findings where neither IL6 nor IL10 was detected in the filtrate. Another investigator who used polyamide filters and polysulfon filters in paediatric patients reported tiny amounts of IL6 in the filtrate particularly with polysulfon haemofilter; however they found no effect of either filter on IL10 level [12]. This finding again goes in line with ours where IL10 was also not detected in the filtrate.
Our study showed a trend towards reduction of both urea and creatinine in the filter group. On the other side there was a higher incidence of acute renal dysfunction with the use of haemofilter during cardiopulmonary bypass .The difference was not statistically significant (p value 0.07). Regarding the cytokines, the study showed the haemofilter has no direct effect on the level of both IL10 and IL6 and there was no difference in the levels of IL6 and IL10 between the two groups.
5 Conclusion
Haemofilter use during cardiopulmonary bypass has no protective effect on kidney function after conventional CABG operations. Its use was associated with increased incidence of acute renal dysfunction but this was not statistically significant. Haemofilters have no direct effect on the level of IL6 and IL10. High levels of IL6 and/or IL10 could predict the development of acute renal dysfunction after conventional CABG.
Presented at the 21st Annual Meeting of the European Association for Cardio-thoracic Surgery, Geneva, Switzerland, September 16–19, 2007.
Appendix A: Conference discussion
Dr A. El-Essawi (Braunschweig, Germany): I have three short questions. First, did you compare creatinine clearance or did you just stick to creatinine and urea? It's a bit weak.
Dr Prendergast: Absolutely. In fact, we have some more data. This was the data that we put in. One of the things that we’ve gone back to is to look at creatinine clearance. So I can’t present you that data, but it has been talked about by the group. But I agree with you that simple creatinine is a rather weak marker.
Dr El-Essawi: Postoperatively you stated differences in the incidence of renal insufficiency. Did you have any patients who required dialysis?
Dr Prendergast: No, none.
Dr El-Essawi: And could you imagine that the reason for higher levels of interleukins is the renal impairment and not the other way around?
Dr Prendergast: Of course, absolutely. It's simply an association.
Dr P. Totaro (Brescia, Italy): If I’m correct, in the nonhaemofilter group, your incidence of postoperative renal failure was about 10%?
Dr Prendergast: Yes.
Dr Totaro: Which is a bit higher, considering it is a group of elective patients.
Dr Prendergast: On good patients as well by our own inclusion criteria.
Dr Totaro: Does it reflect your normal incidence of postoperative renal failure, or do you have any idea or comments on this high percentage?
Dr Prendergast: We noticed that this was quite high and it doesn’t seem to reflect our usual incidence of renal failure. I think to some extent it's an arbitrary definition, adopting 200 micromolar. Perhaps it has something to do with the definition, but we accept that it's higher than we would expect.
Dr J. Horisberger (Lausanne, Switzerland): In a publication in 1996 by Journois, as well as eliminating TNF and reducing levels of bradykinin, they showed that after a 24-hour period, there was actually a decrease in the IL-1 and IL-6. Can you explain maybe why they have a different result?
Dr Prendergast: No, I couldn’t give you any useful explanation about that. I’m sorry.
Acknowledgements
Thanks to the staff of the perfusion departments of Manchester Royal Infirmary Hospital.




