-
PDF
- Split View
-
Views
-
Cite
Cite
Charalambos Zisis, Alexandra Guillin, Laurent Heyries, Pascal Lienne, Xavier-Benoit D’Journo, Christophe Doddoli, Roger Giudicelli, Pascal-Alexandre Thomas, Stent placement in the management of oesophageal leaks, European Journal of Cardio-Thoracic Surgery, Volume 33, Issue 3, March 2008, Pages 451–456, https://doi.org/10.1016/j.ejcts.2007.12.020
Close - Share Icon Share
Abstract
Objective: To examine retrospectively the patients of our department who had a self-expandable totally covered metal stent placed for oesophageal leak. Methods: Patients hospitalised in our department for oesophageal cancer and/or oesophageal perforation between 2004 and 2006. All medical records were retrospectively reviewed. Seventy-two patients underwent oesophageal resection for oesophageal cancer and 16 were managed for oesophageal perforations. Results: Eight out of 72 patients submitted to resection for oesophageal cancer had postoperative leaks, while one patient developed tracheo-oesophageal fistula (TEF) due to prolonged mechanical ventilation. Six of them had stent placement in first intention, whereas two received the procedure after an unsuccessful repeat operation. The mean stent placement time was 18.4 days (SD = 15.2 days), whereas the median was 14 days. The leak was managed efficiently by the stent in seven patients, whereas two patients needed repeat operations (one with TEF). The mean stent removal time was 56.8 days (SD = 30.5 days) and the median was 40 days. None developed anastomotic stricture. On the other hand, three out of 16 patients with perforation had a stent, two of them for Boerhaave syndrome and one for iatrogenic rupture after bariatric surgery. One of them required the stent 17 days after surgical repair with excellent results, while the other two patients had the stent placed immediately, but still needed thoracotomy to control the leak. Conclusions: Stent placement can prove very useful in the management of post-oesophagectomy anastomotic leaks, but its contribution needs to be evaluated with caution in cases of oesophageal perforations or TEF. Larger series and prospective comparative clinical trials could eventually clarify the role of stents in clinical practice of surgical patients.
1 Introduction
After the establishment of stent use as palliative treatment for malignant oesophageal strictures [1–3], there have sporadically been publications concerning stent placement for managing oesophageal leaks or perforations [4–9].
The material of these publications is limited in a few cases, and is not homogenous. Decision-making for stent placement concerns different pathological entities ranging from postoperative oesophageal leak to oesophageal perforation (iatrogenic or spontaneous). This practice is feasible in well-equipped hospitals and is performed by gastroenterologists or even by thoracic surgeons familiar with such interventional endoscopic procedures. It has been applied as a simpler, less invasive and better tolerated by the patient alternative, but the exact indications remain to be defined.
Especially in intrathoracic anastomotic leakage after oesophageal resection, a potentially lethal complication with high mortality rates reaching 60–70% in older series and approximating even 35% in more recent studies [10], imminent surgical intervention had traditionally been considered as the gold standard in its management. As advocates of a more conservative approach have appeared in the literature during the last decade, stent placement is starting to be proposed as an option.
Regarding oesophageal perforation, a life-threatening entity with mortality rates up to 30% with surgical treatment, conservative treatment remains controversial while sporadic cases with good outcome after prosthesis insertion have been reported [4–6].
Based on this reported experience, we have performed stent placement for the last 3 years, in some cases of postoperative oesophageal leaks or benign oesophageal perforation in order to avoid major surgical manoeuvres. This experience is reported here in order to detect the clinical, surgical, and prognostic features of these particular cases.
2 Patients and methods
In this retrospective observational study, all medical records of the patients hospitalised in our department for oesophageal cancer and/or oesophageal perforation between January 2004 and December 2006 were reviewed. During this period, 72 patients underwent oesophageal resection for oesophageal cancer, and 16 patients were managed for oesophageal perforations. Following oesophageal resection, nine out of 72 patients were treated for postoperative oesophageal leaks with stent placement (Table 1 ). In the same period, three out of 16 patients with benign oesophageal perforation had a stent included in their treatment strategy, either as a first option or after surgery.
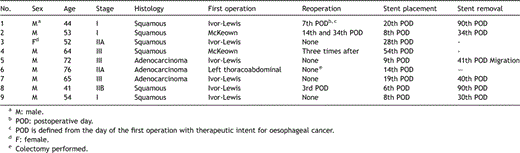
Demographics and manipulations in patients with oesophageal cancer
The postoperative oesophageal leak patient mean age was 57.9 ± 12.1 years, whereas the median was 54 years. Comorbidity of postoperative oesophageal leak patients is quoted in Table 2 .
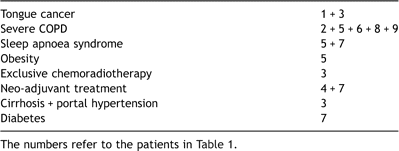
Existing comorbidity in the preoperative setting of cancer patients
2.1 Concerning benign oesophageal perforation patients
Two patients presented to the Emergency Department with dorsal thoracic pain after violent vomit (patients No. 1 and 2 of Table 3 ), and the examinations performed on admission documented perforation in the left border of the lower third of the oesophagus in both patients.
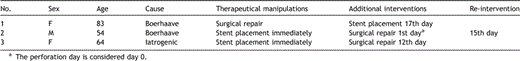
Demographics and manipulations in patients with oesophageal perforation
Patient No. 3 of this series (Table 3), who suffered from morbid obesity and type II diabetes mellitus, had had a laparoscopic bariatric procedure for gastroplasty performed, and a fistula of the middle to the inferior third of the oesophagus was confirmed on the 1st postoperative day on contrast swallow and oesophagoscopy.
Our practice was to insert prosthesis as soon as a leak was diagnosed. The patients were assessed by water-soluble contrast swallow and/or contrast application under endoscopic guidance. Usually the latter was the diagnostic procedure of choice for postoperative oesophageal leak patients, due to lack of patient cooperation within the context of poor general condition. A thoracic and abdominal CT-scan was always part of the diagnostic work-up. Leak occlusion confirmation was performed immediately after the manoeuvre by contrast application through the endoscope and, when possible, by contrast swallow.
The stents were inserted with flexible oesophagoscopy by an endoscopist gastroenterologist. The stents were self-expandable totally covered metal stents (SEMS) (Ultraflex – Boston Scientific, Choostent or Hanarostent SHIM with Anti-Reflux Valve to avoid gastro-oesophageal reflux). Informed consent for stent placement was obtained from the patients or their legal representatives following explanations on the procedure character, risks and performance evidence uncertainty.
At the same time, all patients were under broad-spectrum antibiotics, nutritional support and sufficient mediastinal drainage.
The stent extraction, after an uncomplicated course with restoration of the patients’ feeding capacity and adequate nutritional status, was scheduled 3–8 weeks later.
3 Results
3.1 Postoperative oesophageal leak management
The tumour was located in the cardia in two patients (22%), both with adenocarcinoma submitted to Ivor-Lewis resection, in the inferior third of the oesophagus in five patients (55.5%), and in the middle third in two patients, both with squamous carcinoma submitted to McKeown procedure. All patients had oesophagogastrostomy performed with gastric tube and gastric pull-up. The resection margins of the specimens were clear.
All nine postoperative oesophageal leak patients had eventful postoperative course. Many serious respiratory complications developed, devastating for the patients (Table 4 ). The respiratory compromise of these patients led to extensive diagnostic work-up in an intensive care investigational methodology setting. Stent placement was performed as specified in Table 1, as soon as a leak was confirmed, except for patients No. 1 and 8 that surgical repair was firstly attempted postoperatively when the leak was diagnosed, and a stent was inserted after the leak’s recurrence.
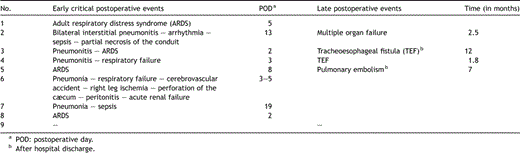
3.2 Postoperative course of leak patients
Patient No. 1 (Tables 1 and 4) was submitted to anastomosis with the hand-sewn method in two layers after a malfunction of the stapling device. On the 5th postoperative day (POD), pneumonia of the right upper lobe was developed, and the patient was re-intubated, whereas the endoscopy revealed looseness in one third of the anastomotic line. A repeat operation was performed with reconstruction of the mediastinal oesophagogastric anastomosis, and the patient’s clinical improvement permitted assisted ventilation to be discontinued on the 15th POD with normal endoscopy. The fistula recurred on the 20th POD with saliva drainage from the chest tube and positive blue test, and was managed with stent placement, without other pitfalls in the postoperative course.
Patient No. 2 (Tables 1 and 4) presented an air leak in the thoracic drainage on the 7th POD and subcutaneous emphysema. After stent placement and radiological confirmation, the leak stopped and the subcutaneous emphysema disappeared. On the 13th POD the patient manifested fever, pulmonary infiltrates and sepsis, and was submitted to a repeat operation, during which partial resection of the gastric conduit and cervical oesophagostomy were performed, as necrosis of the upper 5 cm of the gastric conduit was confirmed. The patient underwent a second intervention on the 34th POD, but finally succumbed to multiple organ failure 2.5 months after the initial operation.
In patient No. 3 there was an anastomotic leak without collection confirmed on the 28th postoperative day at the diagnostic investigation for persistent dysphagia of the patient. She had good fistula cicatrisation following stent placement, and was discharged home. She further denied extraction of the stent, and developed a TEF 12 months later, successfully managed with Y-tracheobronchial prosthesis. Ultimately, she passed away 13.5 months after oesophageal resection.
Patient No. 4 of the series had oesophageal prosthesis placed on the 54th POD, when he presented tracheoesophageal fistula (TEF), but 10 days later, the TEF recurred, and surgery was performed with combined tracheal and oesophageal prostheses. Two months later further surgical procedures were performed twice, but the patient passed away 7.5 months after the initial operation.
In patient No. 5 circumferential looseness in a quarter of the anastomotic line communicating with the pleural cavity was revealed on the 9th postoperative day and stent was inserted. Migration of the stent occurred with good fistula cicatrisation on the 41st POD.
Patient No. 6 was re-intubated for severe respiratory compromise on the 3rd POD, and a cascade of major complications followed (Table 4). On the 14th POD lateral fistula of one fourth of the circumference was documented and a stent was placed. On the 28th POD, cæcum perforation and peritonitis were managed by right colectomy. After a stormy course, the patient died on the 40th POD.
In patient No. 7 a contained leak was diagnosed on the 7th postoperative day, but stent insertion was performed on the 19th day postoperatively, when pneumonia with rapid clinical deterioration was radiologically documented. The signs of sepsis were totally reversed 4 days later and further postoperative course was without complications.
Patient No. 8 presented signs of systemic sepsis and acute respiratory failure on the 2nd postoperative day (fever 40 °C with ARDS), and he was re-intubated, whereas endoscopy demonstrated anastomotic dehiscence of 2 mm that needed a repeat operation on the 3rd postoperative day. Intercostal muscle positioning was performed, but an oesophagopleural fistula re-appeared 3 days later. A stent was placed with satisfactory results and significant clinical amelioration, while the patient was discharged 2 months after the initial operation, after repositioning the stent twice.
Patient No. 9 demonstrated intrapleural contrast leakage on the 7th POD, without apparent mucosal damage on endoscopy. Prosthesis was placed and the patient retained good clinical status and started per os alimentation on the 12th POD with a satisfactory postoperative course.
3.3 Statistical analysis of postoperative oesophageal leak patients
Continuous variables are expressed as mean (standard deviation) or median (interquartile range), while categorical variables are expressed in absolute and relative frequencies. The cumulative survival rate was graphed using the Kaplan–Meier method. Life table analyses were used to calculate the cumulative survival rate (standard errors) for specific time intervals. The follow-up of all patients was complete.
The median survival time was 7 months (interquartile range: 3.5–7.5 months). The mean survival time was 9.0 months (SD = 10.4 months) and ranged from 1.4 to 35 months.
Five patients (55.6%) died with a median survival time of 7 months and mean 6.4 (SD = 4.8 months). The mean age for patients who survived was 51.0 (SD = 10.9 years), while for those who died was 63.4 (SD = 10.9 years).
The cumulative survival rate (Fig. 1 ) for the first 3 months was 77.8% (SE = 13.8%), for 6 months 77.8% (SE = 13.8%) and for 12 months 43.2% (SE = 19.8%).
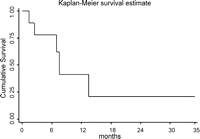
Kaplan–Meier survival estimates for patients with oesophageal cancer.
The mean stent placement time was 18.4 days (SD = 15.2 days), whereas the median was 14 days.
The mean stent removal time was 56.8 days (SD = 30.5 days) and the median was 40 days.
3.4 Stent removal
Three stents of postoperative oesophageal leak patients were not removed because of the complicated patient course. The stent of patient No. 3 (Table 1) was not removed due to the patient’s reluctance. Patient No. 4 presented TEF that needed complementary manipulations for stent placement and three repeat operations with eventual dismal outcome. Patient No. 6 died 25 days after stent placement, because of other complications non-related to the stent.
Patient No. 2 (Table 1) had the prosthesis removed at the second re-intervention because of gastric conduit necrosis. Patient No. 5 had migration of the stent, whereas the patients 1, 7, 8 and 9 had the stent removed on an outpatient basis as quoted in Table 1. All these patients did not present stricture formation or dysphagia during follow-up.
3.5 Benign oesophageal perforation management
Patient No. 1 (Table 3), having a medical history of angina, underwent a left posterolateral thoracotomy with direct suture and reinforcement with intercostal muscle buttressing. On the 17th postoperative day, a fistula was localised on the control oesophagraphy and a stent was placed. The postoperative course was uneventful thereon, and 28 days later she was discharged home. Forty-two days after placement, the stent was removed, and the endoscopic evaluation confirmed satisfactory cicatrisation of the fistula, whereas the patient’s nutritional status was totally restored by per os alimentation. Four months after stent removal the patient is doing well in good performance condition.
For patient No. 2, who was in perfect general condition, a more conservative approach was chosen, and a SEMS was placed immediately. The following day, surgical treatment was chosen as clinical signs of deterioration started. Left posterolateral thoracotomy was performed, the stent was removed, the perforation was sutured and a pedicle of intercostal muscle was positioned. The patient developed ARDS between the 2nd and the 5th POD and furthermore a left pleural cavity empyema, for which he underwent repeat thoracotomy for decortication on the 15th POD. Though clinical status progressively ameliorated, oesophagraphy showed a remnant fistula, for which a clip application was performed endoscopically. Since then, the patient progressively recovered; he was discharged after 20 days, and is doing well 14 months later.
Patient No. 3 had an endoprosthesis positioned as soon as the diagnosis was made. Twelve days later, a persistent leak was documented by oesophagraphy, and surgical repair was performed by vascularised pedicle buttressing at perforation site. The postoperative course was uneventful, and the patient is doing well.
All patients did not present stricture formation or dysphagia during follow-up.
4 Discussion
Stent placement in postoperative leaks after oesophageal surgery, as well as in iatrogenic or spontaneous perforations, remains a controversial option. It is a newly applied technique, which started in malignant inoperable oesophageal obstructions and has evolved in parallel with stent production technology and disposal development.
Concerning postoperative oesophageal leak patients, our small experience was dictated by the severity of the cases, the perplexity of comorbidity factors, and the participation of many system disorders in the complicated postoperative course of these patients. As shown in the description of the above cases, the surgical management of oesophageal cancer engages patients with dismal prognostic factors (severe respiratory compromise, ethylism, cirrhosis, diabetes), which are further enhanced with neo-adjuvant treatment. Despite meticulous surgical technique, reduction in operating time, no need for perioperative transfusion, advances in anaesthetic management and postoperative care, the incidence of postoperative complications for these patients remains high. The most serious among such complications, and the main contributor to morbidity and mortality rates after oesophageal resection, is the development of postoperative oesophageal leak and pulmonary complications [11], which are related to each other. For that reason, clinical signs of respiratory compromise may imply leak. When postoperative oesophageal leak, even in oesophageal surgery high-volume experienced centres exceeds 10% [10], incidence in these medically borderline patients is significantly higher [12,13]. In such a patient population, repeat operations could be an additional contributing dismal prognostic factor. Stent placement seems to be an attractive option because of minimal interventional burden, relative simplicity, availability, and feasibility. Indeed, manipulations can take place even on the patient’s bed in the intensive care unit.
However, it is necessary to follow these patients closely regarding required diagnostic examinations (oesophagogram, endoscopy), as well as clinical features. Suspicion of remaining leakage must lead to alternative therapeutic options, mainly of surgical concern. Regulation and optimisation of all nutritional, immunological, cardiopulmonary, hepatic and renal functional parameters, as well as adequate pain control is the fundamental strategy for maintaining good results.
High in-hospital mortality rates (25%) have been reported in postoperative oesophageal leak patients managed with stents. Median survival time of the patients with postoperative oesophageal leak was 7 months, whereas the median survival of all patients submitted to oesophageal resection in our centre is 30 months with a 5-year survival of 42%. However, it is emphasised that the patients were severely ill at the time of stent placement [14]. This fact coincides with our experience from the postoperative oesophageal leak patient series as described above.
Concerning benign oesophageal perforation patients, excellent results have been published by Fischer et al. [15], with one death and only two additional surgical procedures (for empyema) in a series of 15 patients, eight out of whom make up a late treatment group, exclusively treated with self-expandable metal stents. Some authors designate the use of stents for late benign oesophageal perforation diagnosis, as in these cases surgery mortality rates equal those of a conservative approach [16]. At present, we have to be reluctant to recommend the use of stents as a primary therapeutic option, as current evidence is not adequate. Rapid surgical repair remains the gold standard of a successful therapeutic approach. However, stents can play a significant role as complementary weapons in the clinician’s armamentarium. Whenever a leak remains, prosthesis can be tried for occlusion.
All authors direct our attention to the fact that SEMS should always be extracted after healing, as severe long-term placement complications have been reported convincingly [17]. Characteristically, TEF development in patient No. 3 (Table 1) is clearly attributed to the stent overstaying. The ideal timing for metallic stent removal is not well defined and varies for different authors from 2 weeks to 4 months; however, it is safer for the stent to be removed within 2 months after placement [18,19]. A constant concern with stents is the likelihood of migration, as was the case with patient No. 5 (Table 1). Alternatively, self-expanding, covered, plastic oesophageal stents (SEPS) that have been proposed as cheaper, safe, easier to deploy, to be replaced and removed even after a long time period [9], have been implicated in an enormous rate of late SEPS dislocation reaching 37.5% (between 8 and 95 days after placement) [14]. As many corrective manipulations may be required as complementary interventions, the cost increases significantly. High rate of stent migration (25%) has also been reported recently with this type of stent and is recognised by the authors as the principle weakness of this treatment strategy [20].
Criteria for stent application in anastomotic leaks have been proposed, such as the dehiscence being less than 70% of the circumference, anastomotic line without ischaemic and vital elements, and effective internal or external drainage of the perianastomotic mediastinum [21]. A multidisciplinary approach involving the surgeon, endoscopist, and interventional radiologist is required, and surgical repeat exploration must be considered, if clinical improvement is not achieved with non-operative treatment [22].
Patient population heterogeneity remains a principal concern. Decision-making has to be individualised on the basis of the patient clinical context. We believe that a leakage confirmed on the three first postoperative days has to be managed surgically as the possibility of gastric necrosis or a significant technical error must be eliminated [23–25].
Whether postoperative oesophageal leaks and benign oesophageal perforations are to be included in stent use indications remains to be confirmed. Certainly, this technique is feasible and well promising, but it needs large series and long-term trial in order to be adequately validated.




