-
PDF
- Split View
-
Views
-
Cite
Cite
Hideki Itano, The optimal technique for combined application of fibrin sealant and bioabsorbable felt against alveolar air leakage, European Journal of Cardio-Thoracic Surgery, Volume 33, Issue 3, March 2008, Pages 457–460, https://doi.org/10.1016/j.ejcts.2007.12.036
Close - Share Icon Share
Abstract
Objectives: The combination of fibrin sealant and bioabsorbable sheet is known to provide a better sealing effect on alveolar air leakage compared to the single use of fibrin sealant. However, there is little evidence that reveals the optimum techniques for their combined application. Presently we developed a novel Rub + Soak B method that comprises the attachment of thrombin-impregnated sheet to the fibrinogen-rubbed lung tissue area. This study aimed to evaluate its sealing effect compared to various combined application techniques. Methods: Experiment I: The viscosity and osmolality of each fibrin sealant component were measured. Experiment II: Pleural defects produced by electrocauterization in retrieved swine lungs (n = 24) were covered with fibrin sealant and polyglycolic acid felt by using the following five techniques: concomitant spraying of fibrinogen and thrombin solutions over the pleural defect area (Group I, Control); rubbing the thrombin solution on the area, attaching the felt soaked in the fibrinogen solution, and applying the remaining thrombin and fibrinogen solutions (half the original quantity) alternately to the area (Group II, Rub + Soak A); rubbing the fibrinogen solution on the area, attaching the felt soaked in the thrombin solution, and applying the remaining fibrinogen and thrombin solutions alternately to the area (Group III, Rub + Soak B); rubbing the fibrinogen solution on the area, attaching the dry felt, and spraying both the remaining solutions concomitantly (Group IV, Rub + Spray); and spraying both the solutions, attaching the dry felt, and respraying the remaining solutions over the area (Group V, Spray Sandwich). The minimum seal-breaking airway pressure was compared among the groups. Samples were histologically assessed. Results: Experiment I: The fibrinogen solution was 34.8 times more viscous and had 3.5 times higher osmolality than the thrombin solution. Experiment II: The seal-breaking pressure was significantly higher in Group III than in Groups I, II, and V (p < 0.05). Histologically, clot penetration into the tissue was significant in Group III. Conclusions: The novel Rub + Soak B technique was the most effective and reasonable combination technique wherein the sealing mechanism was supported by the physical properties of the fibrin sealant components.
1 Introduction
Postoperative air leakage remains a major cause of morbidity after lung resection. It leads to prolonged chest tube drainage time that is associated with pain and immobilization and consequently increases the patient’s risk of the development of empyema and other serious complications [1]. Fibrin sealant has been widely used since the early 1980s as an effective tool to seal pulmonary air leakage in lung surgery [1–4]. Thoracic surgeons, however, have often found that fibrin sealant used for pulmonary parenchymal defects could not effectively seal air leakages. To overcome this drawback, the application of fibrin sealant in combination with synthetic bioabsorbable sheet has been developed as a more effective tool for the control of alveolar air leakage [5–7]. Some studies have experimentally shown that this combination provides a better sealing effect than the fibrin sealant alone [6,7], and this combination is presently being used to a great extent in pulmonary surgery. A variety of techniques for the combined application of these two materials have been reported along with their efficacy [7–10]; however, thus far, there are no comparative studies of these combined applications that reveal the technique with an optimum sealing effect. Presently, we have developed a novel Rub + Soak B technique that involves rubbing the lung tissue with fibrinogen solution and sheet impregnation with thrombin solution, followed by attaching the sheet to the rubbed area. The present experimental study was designed to compare this method with the various combination techniques in order to clarify the optimum application for controlling alveolar air leakage.
2 Materials and methods
2.1 Experiment I
The fibrin sealant used in this study was manufactured by CSL Behring Co. (Beriplast P® Combi-Set; Tokyo, Japan). The viscosity of each component of the fibrin sealant and normal saline was measured in three samples by using an oscillating viscometer (Digital Viscomate VM-100A; CBC Co., Ltd., Tokyo, Japan). The osmolality of each component of the fibrin sealant and normal saline was also measured identically by using Osmometer 3D3 (Advanced Instruments, Inc., Norwood, MA, USA).
2.2 Animals
Male swines at 3–4 months of age and weighing 32–46 kg were used in this study (n = 24). After euthanizing these animals, their heart–lung blocks were retrieved and used for the experiments. All animals received humane care in accordance with the Japanese Government Animal Protection and Management Law.
2.3 Experiment II
A tracheal tube was inserted into the trachea, fixed, and then connected to a manometer. After clamping the right main bronchus, airway pressure was regulated by controlling the flow rate of nitrogen gas. A pleural defect of 20 mm × 30 mm in size with a depth of 1.0 mm was created at left S6 with the lung completely expanded at a positive airway pressure of 10 cm H2O by using an electric cautery in the coagulation mode with a power of 35 W (ICC 350-MR; Erbe, Tübingen, Germany). In each sample, only one defect was studied per lung. Pleural defects were covered using five different techniques in a randomized fashion with the left lung expanded and minimal air leakage from the pleural defect under an airway pressure of 6 cm H2O. The covered surface was maintained at rest for 5 min and then gently immersed in normal saline solution while keeping the airway pressure of 6 cm H2O. The airway pressure was gradually increased, and the minimum positive airway pressure that caused air leakage from the defect (seal-breaking pressure (SBP)) was recorded for each sample ( Video 1).
2.4 Fibrin sealant application
The Beriplast P® Combi-Set includes separate vials containing individual agents, which when combined form a fibrin clot. Fibrinogen (F) solution is a protein concentrate comprising fibrinogen; aprotinin, which is a fibrinolysis inhibitor; and factor XIII, which stabilizes fibrin by intermolecular cross-linkage. Thrombin (T) solution comprises thrombin reconstituted in calcium chloride solution. The mechanism of clot formation by fibrin sealant has been well described previously [11–13]. Bioabsorbable polyglycolic acid (PGA) felt used in this study was 0.15-mm-thick NEOVEIL® sheet (Gunze Co., Kyoto, Japan). We covered the pleural defects by the following five different combination techniques of PGA felt and fibrin sealant. In Group I (control group, n = 5), the entire amount of the F and T solutions was applied on the pleural defect area exclusively by spraying without using the PGA felt. In Group II (Rub + Soak A group, n = 5), the T solution was dripped and gently rubbed on the pleural defect area. Next, three pieces of PGA felt that were soaked in the F solution were attached to the area. The remaining T and F solutions (half the original quantity) were spread alternately over the area [7,8]. In Group III (Rub + Soak B group, n = 5; Video 1), the F solution was dripped and gently rubbed on the pleural defect area. Next, three pieces of PGA felt that were soaked in the T solution were attached to the area. The remaining F and T solutions (half the original quantity) were alternately spread over the area. In Group IV (Rub + Spray group, n = 5), the F solution was dripped and gently rubbed on the pleural defect area. Next, three dry pieces of PGA felt that were not soaked in any solution were attached to the area. The remaining T and F solutions (half the original quantity) were concomitantly sprayed over the area [9]. In Group V (Spray Sandwich group, n = 4), half the amount of the T and F solutions was concomitantly sprayed over the area. Next, three dry pieces of PGA felt were attached to the area. Finally, the remaining quantity of the T and F solutions was resprayed on the area. A total of 1.2 ml of each component solution and three pieces of 15-mm × 30-mm NEOVEIL® were used for the covering in each sample.
2.5 Histological examinations
Since the fibrin seal was broken during the SBP measurement, lung samples representative of Groups I, II, III, and V that were not used for SBP measurement were used to obtain histological specimens of the coverage sites. Five minutes after the sealing procedure, the whole left lung was fixed with 10% neutral formalin by immersion and gentle endobronchial injection of formalin until the lungs completely expanded. After fixation, each defect site was resected, embedded in paraffin, and processed to obtain 3-μm sections for hematoxylin–eosin (HE) and phosphotungstic acid hematoxylin (PTAH) staining as a typical demonstration technique of fibrin. The specimens were analyzed in the pathological division of our hospital.
2.6 Statistical analysis
Data were analyzed using one-way analysis of variance (one-way ANOVA) and Bonferroni–Dunn post hoc test (Stat View, SAS Institute Inc., Cary, NC, USA). Data were transformed with a logarithmic transformation, and the uniformity of variance among groups was confirmed by Bartlett test before the analysis of variance. All data are expressed as means ± SD.
3 Results
3.1 Experiment I
The viscosity of the F solution (49.8 ± 4.5 mPa S) was 34.8 times higher than that of the T solution (1.43 ± 0.04 mPa S) and 43.3 times higher than that of normal saline (1.15 ± 0.01 mPa S). The osmolality of the F solution (845 ± 14 mOsm/kg H2O) was 3.5 times higher than that of the T solution (243 ± 1.0 mOsm/kg H2O) and 2.9 times higher than that of normal saline (287 ± 1.7 mOsm/kg H2O).
3.2 Experiment II
The SBP required to produce air leakage from the covered pleural defect in the swine lung was significantly higher in Group III (57 ± 20 cm H2O) than in Groups I (19 ± 1.8 cm H2O), II (19 ± 4.1 cm H2O), and V (25 ± 16 cm H2O) (p < 0.05; Fig. 1 ).

The mean seal-breaking pressure (SBP) was significantly higher in Group III than in Groups I, II, and V (p < 0.05 for all).
3.3 Histological assessment
The layers of fibrin and PGA felt covering the pleural defects were observed by both HE and PTAH staining. Penetration of the fibrin clot into the lung tissue was not observed in Groups I, II, and V, but was significant in Group III (Fig. 2 ).
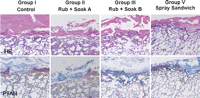
Histological findings of hematoxylin–eosin (HE) and phosphotungstic acid hematoxylin (PTAH) staining in Groups I, II, III, and V. Tissue penetration of the fibrin clot was significant in Group III, whereas it was not observed in Groups I, II, and V. The upper and lower panels show HE and PTAH staining, respectively. Magnification: 12×.
4 Discussion
By using the methodology of Morikawa and Katoh [7], we have developed a novel Rub + Soak B technique that utilizes the physical properties of each fibrin sealant component. It involves rubbing the lung tissue with one fibrin sealant component and sheet impregnation with the other component of the fibrin sealant, followed by attaching the sheet to the rubbed area.
Two factors influence the effectiveness of this combination sealing method; the degree of sealant attachment to the lung tissue and consistency of felt fiber integration into the clot layer with minimal retention of air spaces in the felt fiber. In our study, the best results were achieved with the Rub + Soak B method. This can be explained as follows ( Video 2). Fibrinogen is indispensable for the formation of a fibrin clot [11,12]. When the F solution is rubbed over the pleural defect area, as the first step of the covering technique, it penetrates the tissue. This step is important because permeation of the highly viscous F solution into the lung tissue by simple dripping or spraying is difficult [13]. The subsequent attachment of the thrombin-impregnated felt sheet over the fibrinogen-rubbed area enables direct tissue permeation of the less-viscous T solution that then reacts with the fibrinogen to form a fibrin clot in the tissue. This clot penetrates the tissue and functions as a secure foothold that fixes the sealing material to the lung tissue, thereby contributing to excellent adhesion strength. Histological findings of predominant tissue penetration of the fibrin clot in the Rub + Soak B group supports this mechanism (Fig. 2). Group IV demonstrated the second highest SBP, thus supporting the efficacy of fibrinogen rubbing as the first step (Fig. 1). The rubbing of the F solution with higher osmolality might also result in the absorption and removal of intervening substances, such as fluid or blood, at the base of the micro recesses of the lung surface; this might produce an improved sealing effect [14].
When the thrombin-impregnated felt is attached to the fibrinogen-rubbed area, the F solution is partially soaked up by the lining layer of the felt sheet through capillary action. This absorbed fibrinogen interacts with the thrombin in the felt fibers forming a fibrin clot that fills the micro spaces in the lower layer of the felt, thereby leaving minimal air spaces. Immediately after the remaining F solution is applied over the felt, the fibrinogen is easily absorbed and forms a clot with the thrombin in the felt fibers that fills the felt microstructure. The fibrin clot attaches to the first clot in the lower layer, and the felt fiber eventually integrates into the clot layer with minimal retention of air spaces. Simultaneously, this fiber-integrated clot layer firmly attaches to the pleural defect area via the abovementioned fibrin clot foothold. Thus, the components – the lung parenchyma, felt fiber, and fibrin clot – are completely integrated. Wet felt may have greater capillary attraction than dry felt because the microstructure size of the fabric decreases after fluid impregnation, and the air in these felt fibers is replaced completely by the subsequently formed fibrin clot. Therefore, sheet impregnation using the T solution might be a vital second step in achieving consistent integration of the felt into the clot layer.
To form a secure foothold for the adhesion of the sealing material into the tissue, both fibrinogen application and an adequate supply of thrombin to the tissue are necessary. The PGA felt impregnated with the T solution functions as a sponge-like retention device, and the entire amount of the T solution can efficiently react with fibrinogen with minimal spillage, given that the T solution can easily spill over due to its low viscosity. After the attachment of the thrombin-impregnated felt, the T solution can easily enter and permeate the tissue due to its low viscosity. Thus, the attachment of the thrombin-impregnated sheet is an essential third step to supply thrombin efficiently and directly to the tissue. Therefore, these three steps can be combined to constitute an essential mechanism for realizing the optimal sealing effect by utilizing the physical properties of each fibrin sealant component.
The concomitant spraying of two fibrin sealant components was developed to improve the sealing effect by achieving a more homogeneous and efficient mixture of the two components than that obtained by their sequential application [4,13,15]. However, the spray application of the two components over the dry PGA felt might result in rapid clot formation concurrent with the mixture of two sealant components, thereby leaving air spaces within the felt microstructure and causing an inadequate supply of thrombin to the tissue. Moreover, concomitant spraying over the lung tissue might result in insufficient tissue penetration of the F solution. Accordingly, in the combined application of a fibrin sealant and absorbable sheet, separate spraying of both the sealant components might be necessary for improved sealing.
It has been reported that fibrin sealants with higher concentrations of fibrinogen produce stronger clots [13,16,17], whereas those containing higher concentrations of thrombin form clots more rapidly [16]. Furthermore, factor XIII increases the tensile strength and stability of the clot [18]. Therefore, to achieve stronger fibrin clots, the concentration of fibrinogen should be higher. The addition of factor XIII to the fibrinogen concentrate is also essential for better cross-linking and clot strength. Consequently, as confirmed by Experiment I in the present study, the viscosity and osmolality of the F solution increase [13,18], whereas the T solution possesses considerably less viscosity and osmolality. These physical properties of each component of commercially available fibrin sealants were incidentally obtained while determining the optimal composition of products with the best adhesive effect in the single application of fibrin sealant without absorbable sheet [19]. The novel Rub + Soak B method benefits from these physical properties in the interaction between the sealant, absorbable sheet, and lung tissue to achieve an improved sealing effect.
Although the efficacy of this novel method in a comparable human clinical setting remains to be determined, it appears promising.
In conclusion, the novel Rub + Soak B method exhibited excellent withstand pressure against alveolar air leakage along with histological evidence of substantial tissue penetration of the fibrin clot. The findings suggest that it is the most effective and reasonable technique for the combined application of fibrin sealant and PGA felt to control alveolar air leakage. We believe that this technique can be developed as an important aid in lung surgery.
Acknowledgments
The author is grateful to Mr Shinohara, Ms Yamamoto, and Ms Taguchi for assistance with the experimental set-up and to Dr Sasaki for assistance with histological processing and assessment.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ejcts.2007.12.036.
References
Author notes
Presented at the 15th European Conference on General Thoracic Surgery, Leuven, Belgium, June 3–6, 2007.
This study was supported by CSL Behring Co.




