-
PDF
- Split View
-
Views
-
Cite
Cite
Hisayoshi Suma, Hiroaki Tanabe, Tokuhisa Uejima, Shinya Suzuki, Taiko Horii, Tadashi Isomura, Selected ventriculoplasty for idiopathic dilated cardiomyopathy with advanced congestive heart failure: midterm results and risk analysis, European Journal of Cardio-Thoracic Surgery, Volume 32, Issue 6, December 2007, Pages 912–916, https://doi.org/10.1016/j.ejcts.2007.09.021
Close - Share Icon Share
Abstract
Background: To treat advanced heart failure due to idiopathic dilated cardiomyopathy, surgical ventricular restoration with mitral reconstruction was conducted and evaluated. Methods: In 95 patients (81 men, mean age: 54 years), New York Heart Association class III/IV was 44/51, and 33 patients (36%) were inotropic dependent preoperatively. Mitral regurgitation (≥2+) was noted in all patients. All patients underwent left ventriculoplasty (septal anterior ventricular exclusion in 38, partial left ventriculectomy in 57) and mitral reconstruction (repair 53, replacement 42). Fifty-two patients (55%) had concomitant tricuspid repair. Intra-aortic balloon pumping and left ventricular assist device was used in 24 patients and two patients, respectively. Results: Hospital mortality was 11.6% (11 of 95), with 6.6% (5 of 76) in elective and 31.6% (6 of 19) in emergency operations. The ejection fraction and cardiac index increased from 22.3 ± 6.3% to 27.2 ± 8.0% and from 2.3 ± 0.5 ml/m2/min to 2.8 ± 0.5 ml/m2/min, respectively (p < 0.001). The endodiastolic volume index, endosystolic volume index and diastolic dimension decreased from 232.9 ± 56.1 ml/m2 to 160.0 ± 49.8 ml/m2, from 178.9 ± 46.7 ml/m2 to 113.8 ± 44.7 ml/m2 and from 82.0 ± 9.0 mm to 68.9 ± 11.6 mm, respectively (p < 0.001). Late death occurred in 27 patients with 22 cardiac deaths. The mean NYHA class was 1.7 among the survivors. One-, 3- and 5-year survival rates were 72.8%, 61.4% and 50.5%, respectively. In the 62 patients who were non-inotropic dependent preoperatively, 1-, 3-, and 5-year survival rates (81.8%, 73.7% and 62.9%) were significantly better than the inotropic-dependent group (55.3%, 37.3% and 28.0%). Patients with mitral annuloplasty showed a significantly higher 5-year survival rate than patients with mitral valve replacement (59.6% vs 43.6%) in univariate analysis. By application of the exclusion site selection method, the two different ventriculoplasty procedures did not show significant difference in survival rates. Multivariate analysis showed that preoperative inotropes and old age were significant predictors for postoperative mortality. Conclusion: The selected ventriculoplasty in combination with mitral annuloplasty is a useful option for patients with an extremely dilated left ventricle in idiopathic dilated cardiomyopathy. Surgery should be considered before inotropic dependency occurs when prior medical treatment has failed.
1 Introduction
Current pharmacological therapy [1] and cardiac resynchronization therapy [2] have shown improved survival in patients with congestive heart failure. However, many patients still require heart transplantation, although this is not readily available due to a shortage of donors. Ventricular restoration by left ventriculoplasty has evolved to treat endstage ischemic and nonischemic dilated cardiomyopathy with medically refractory heart failure [3–7]. Whereas undersized mitral annuloplasty has been introduced with great enthusiasm [8], it is not effective enough in patients with an extremely dilated left ventricle [9]. Therefore, we need to downsize those dilated ventricles appropriately. Because we found a nonhomogeneous severity of interstitial fibrosis between the left ventricular free wall and the septum in idiopathic dilated cardiomyopathy [10,11], we introduced surgical exclusion site selection for the left ventriculoplasty procedure, which is the septal anterior ventricular exclusion (SAVE) [12] for bad septum cases and the modified partial left ventriculectomy (PLV) [13] when the lateral wall is the weakest part.
In this report, we evaluate our 7 years experience with the selected ventriculoplasty combined with mitral valve reconstruction for idiopathic dilated cardiomyopathy with congestive heart failure.
2 Materials and methods
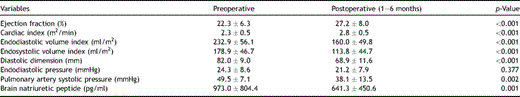
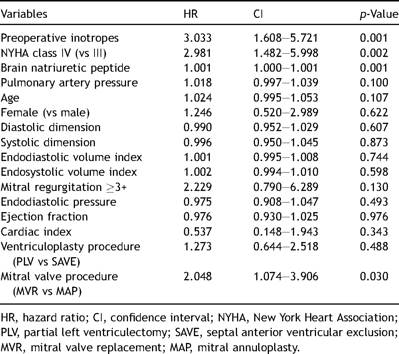
From 1999 to 2006, 95 patients were enrolled as candidates for the procedure at the Cardiovascular Institute Hospital and Hayama Heart Center in Japan. The diagnosis of idiopathic dilated cardiomyopathy was made via the patient’s history, echocardiography, cardiac magnetic resonance imaging and myocardial biopsy by excluding any specific cardiomyopathies and other nonspecific cardiomyopathies. There were 81 men and 14 women with a mean age of 54.2 ± 12.3 years. Preoperative New York Heart Association (NYHA) classes III and IV heart failure was noted in 44 patients (46%) and 51 patients (54%), respectively. Thirty-three patients (36%) were inotropic dependent before the operation. Preoperative variables are shown in Table 1 . The mean preoperative peak VO2 was 11.5 ± 3.9 ml/min/kg in 28 patients who were able to perform the exercise test.
All patients had nonstenotic coronary arteries in coronary arteriograms and no episodes of previous myocardial infarction. All patients had been treated with ACE inhibitors, ß blockers and other medications for heart failure once prior to the operation, but they were shown to be ineffective. Mitral regurgitation (2+ or more) was noted in all patients, and 75 patients (79%) had shown a mitral regurgitation of 3 or 4+. Tricuspid regurgitation was noted in 52 patients. Mitral annuloplasty with an undersized ring (generally 26 mm Carpentier-Edwards Physio Annuloplasty Ring) was our primary choice for the mitral procedure, whereas mitral valve replacement with a biological prosthesis was common in the early period of our experience. Indications for ventriculoplasty were refractory heart failure in NYHA class III or IV, and a dilated left ventricle with a diastolic dimension larger than 75 mm. The choice of procedure, whether SAVE or PLV, was assessed by preoperative echocardiography, cardiac magnetic resonance imaging and an intraoperative volume reduction test [3] to find the weakest area. The basic concept of the volume reduction test is that changes in left ventricular wall motion and thickness can be seen by transesophageal or epicardial echocardiography during the operation when the left ventricle has been decompressed through cardiopulmonary bypass because wall tension has decreased. By decompressing the left ventricle, we were able to see that contraction and wall thickness increased if the area is viable. Then, when the septum was akinetic and the lateral wall was viable through the evaluation, the SAVE procedure was indicated. On the contrary, if the posterolateral wall was thin and akinetic, and the septum was relatively good, PLV was chosen for the ventricular reduction. The surgical procedure of the SAVE operation has been described previously [12]. The criteria for choosing the ventricular procedure, SAVE or PLV, may not clearly be made by absolute values on MRI or echography. Our procedure was selected by eyeball judgement based on MRI or echo findings to detect the most akinetic and thinnest wall in the left ventricle. The Dobutamine stress echo test was conducted when the patient was non inotropic dependent and at no risk of ventricular arrhythmia preoperatively. Generally, the findings of the Dobutamine echo test reinforced our decision in the choice of procedure.
Another important aspect is how good the remaining myocardium after the ventriculoplasty is. Again, there is no absolute determiner in the preoperative examination to make sure that the remaining muscle will be powerful enough. However, in our opinion, first, an entirely akinetic left ventricle without any increase in wall thickness at the systolic phase by preoperative MRI or echography, and second, remarkable pulmonary artery hypertension without significant mitral regurgitation, should both be contraindicated for this surgical intervention.
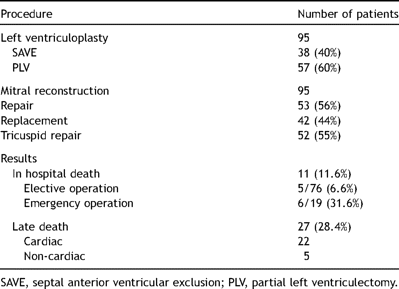
All patients underwent the left ventriculoplasty (SAVE 38, PLV 57) combined with a mitral reconstruction (repair 53, replacement 42). Fifty-two patients (55%) had a concomitant tricuspid repair (Table 2 ). Intra-aortic balloon pumping was used in 24 patients and a left ventricular assistance device was used in two patients. When biventricular-pacing devices became available in Japan, we used systematically implanted devices perioperatively in 15 patients who joined our study in the later stage to support postoperative hemodynamics on the criteria that the Japanese health insurance had presented. The criteria was congestive heart failure (NYHA III or IV) with a QRS duration >130 ms and an ejection fraction <35%. We did not use other perioperative criteria, as their use did not depend on the procedure type.
After the patients had recovered from the operation, ß-blockers, ACE inhibitors and other medication for heart failure were reinstituted. In all of the patients, amiodarone was prescribed perioperatively. In cases where ventricular tachycardia or fibrillation occurred postoperatively, a new class III drug, nifekalant (available only in Japan), was administered intravenously to avoid reinitiation of the arrhythmia. All patients were followed up with annual admissions for a detailed examination.
2.1 Statistical analyses
Continuous variables are expressed as the mean ± SD. Cumulative survivals were calculated by the Kaplan–Meier estimation with the date of operation and the date of the most recent follow-up. In comparisons between the two groups, p values were obtained by the χ2 test or paired t-test. The differences in the survival rate were determined by log-rank analysis. A multivariable analysis of independent factors was performed with the Cox proportional hazard model analysis.
3 Results
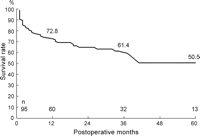
Hospital mortality was 11.6% (11 of 95), with 6.6% (5 of 76) in elective and 31.6% (6 of 19) in emergency operations. Changes of variables before and after the operation at 1–6 months are shown in Table 1. Among 27 late deaths, there were 22 cardiac deaths due to heart failure or sudden death (Table 2). The mean NYHA class improved from 3.5 to 1.7 in the survivors. One-, 3-, and 5-year survival rates were 72.8%, 61.4% and 50.5%, respectively (Fig. 1 ). In patients having the procedure before inotropic dependency (n = 62) and after inotropic dependency (n = 33), 1-, 3- and 5- year survival rates were significantly better in the former group, as shown in Fig. 2 . Also, patients having mitral annuloplasty have shown a significantly better survival rate than those with mitral valve replacement (Fig. 3 ).
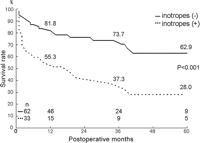
Five-year survival curves in patients with and without inotropic support before surgery.
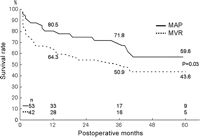
Five-year survival curves in patients with mitral annuloplasty (MAP) and mitral valve replacement (MVR).
Preoperative variables and surgical results in the SAVE and PLV procedure groups are shown in Table 3 , and the results were not significantly different between the two groups.

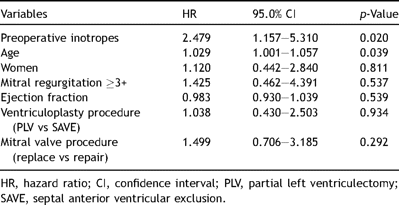
Univariate risk analysis has shown that preoperative inotropes, NYHA class IV, a higher preoperative brain natriuretic peptide level and mitral valve replacement (vs mitral annuloplasty) are significant risk factors for death after surgery (Table 4 ). Concerning the mitral procedure, however, it was not a fair comparison because the mitral valve replacement group included more NYHA class IV, larger left ventricles and was in the early period of our surgical experience. Multivariate analysis showed that preoperative inotropes and old age were significant predictors for postoperative mortality (Table 5 ).
4 Discussion
Nontransplant cardiac surgery for congestive heart failure has gained enormous attention over the last decade [3]. Endoventricular circular patch plasty [4,5] has been widely accepted in treating postinfarction ischemic cardiomyopathy, whereas the partial left ventriculectomy has failed to show optimal results for nonischemic cardiomyopathy, as shown by the Cleveland Clinic [6]. They reported a 3-year study of PLV in 62 patients with idiopathic dilated cardiomyopathy who were listed for heart transplantation. One- and 3-year survival rates were 80% and 60%, and event-free (cardiac transplantation, ventricular assistance device, class IV heart failure, or death) rates were 49% and 26%, respectively. The ejection fraction increased from 16% to 31.5% early after the surgery and stayed nearly the same at the end of 3 years. The left ventricular diastolic dimension and end-diastolic volume index both decreased without significant redilatation at 3 years. Elevated pulmonary artery pressure and low peak oxygen consumption were found to be preoperative high risk factors. Consequently, they abandoned the PLV procedure due to a poor postoperative outcome. However, they delivered an important message by showing that one quarter of PLV patients can survive without cardiac events at 3 years. If we can find a proper method to select those patients, a number of individuals on the cardiac transplant list can be successfully saved.
Undersizing mitral annuloplasty [8] has shown favorable results because of its low operative risk with symptomatic relief, but its effect on longevity remains controversial [14]. Recently we found that the left ventricular volume is an important determinant of the surgical outcome in mitral annuloplasty for a dilated left ventricle [9]. Therefore, we need an effective procedure to reduce the left ventricular volume in cases of excessively dilated left ventricles in patients with idiopathic dilated cardiomyopathy associated with mitral regurgitation. In advanced idiopathic dilated cardiomyopathy, the extent of interstitial fibrosis and the decreased myocardial contraction area in the left ventricle are not uniform [10,15]. The consequence of this was a site-selected exclusion of the left ventricle, either the lateral wall or the septum, for left ventricular downsizing. The return of heart failure after the surgery may have been caused by (1) a recurrence of mitral regurgitation, (2) a remaining dilated lesion of the left ventricle, (3) too small a left ventricle caused by excessive excision and (4) removal of the kinetic area while retaining poorly contractile and more fibrotic regions. Therefore, introduction of the SAVE procedure for bad septum cases and application of PLV for a poor lateral wall with viable septum cases could be reasonable steps for improving surgical results.
Because the procedure described here is mixed with the left ventriculoplasty associated with mitral and tricuspid reconstruction and biventricular pacing, it is difficult to demonstrate the isolated effect of each procedure on ventricular function. However, as previously described [9], patients with an extremely dilated left ventricle (such as a left ventricular diastolic dimension ≥75 mm or an endosystolic volume index ≥150 ml/m2) have shown a poor postoperative outcome after mitral valve reconstruction alone, demonstrating that the ventricular reduction must be combined. We also previously reported an isolated positive effect of the ventricular reduction procedure [16], and that the patient described in the report is now still alive and well with NYHA class I eight years after the operation. For these reasons, we believe the ventriculoplasty is important in treating an extremely dilated left ventricle due to idiopathic dilated cardiomyopathy.
The 3-year survival rate of 61.4% in our series and 73.7% in patients without preoperative inotropes indicate that our surgical strategy is acceptably effective in treating patients with medically refractory advanced heart failure due to idiopathic dilated cardiomyopathy. As we found in uni- and multivariate risk analyses, the surgical procedure should be considered before the patient becomes inotropic dependent. This is compatible with the results of Frazier and colleagues [17]. They compared PLV results in two groups of 21 patients, in whom the mean duration of heart failure was either 60 months or 26 months. They found that a shorter preoperative history of heart failure allowed a better postoperative improvement of ventricular function.
In conclusion, the selected ventriculoplasty, in combination with mitral annuloplasty, is a useful option for patients with an extremely dilated left ventricle in idiopathic dilated cardiomyopathy. Surgery should be considered before inotropic dependency occurs when prior medical treatment has failed.
References
- mitral valve insufficiency
- intra-aortic balloon pumping
- annuloplasty of mitral valve
- primary idiopathic dilated cardiomyopathy
- congestive heart failure
- mitral valve replacement surgery
- ventricular assist device
- diastole
- heart ventricle
- hospital mortality
- preoperative care
- reconstructive surgical procedures
- risk assessment
- surgical procedures, operative
- survival rate
- survivors
- heart
- mortality
- inotropic agents
- cardiac index
- ejection fraction
- left ventricular dilatation
- older adult
- new york heart association classification
- univariate analysis
- advanced heart failure
- medical management




