-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas Schachner, Christina Steger, Simone Heiss, Nikolaos Bonaros, William Sterlacci, Guenther Laufer, Johannes Bonatti, Paclitaxel treatment reduces neointimal hyperplasia in cultured human saphenous veins, European Journal of Cardio-Thoracic Surgery, Volume 32, Issue 6, December 2007, Pages 906–911, https://doi.org/10.1016/j.ejcts.2007.09.015
Close - Share Icon Share
Abstract
Background: Paclitaxel exerts antiproliferative properties by stabilizing microtubuli of the cell. The substance is in clinical use for drug-eluting coronary stents. We aimed to test the hypothesis that paclitaxel treatment can reduce neointimal hyperplasia in cultured human saphenous veins and thus might be useful for local pharmacologic treatment of vein grafts prior to coronary artery bypass grafting (CABG). Methods: The remnants of saphenous veins from 13 patients undergoing CABG were collected. The development of neointimal hyperplasia was induced using an established organ culture model (incubation time 2 weeks). In the treatment group, paclitaxel was added to the culture medium at different concentrations. Results: Veins treated with 1 μmol/l paclitaxel showed a median increase of intimal thickness of 2 μm (range −76 to 46) above baseline levels, whereas untreated control veins increased by 15 μm (range −3 to 142) (p = 0.022). Treatment with 10 μmol/l paclitaxel resulted in a lower intimal thickness growth of 1 μm (range −82 to 212) above baseline levels (p = 0.035 vs controls). Treatment with 25 or 50 μmol/l paclitaxel did not further inhibit intimal hyperplasia. The neointimal amount of the contractile protein smooth muscle actin (SMA) in paclitaxel 1 μmol/l treated veins was significantly higher than baseline values (p = 0.037). The cytoskeletal protein desmin was predominant in the media, whereas it was less frequently found in the intima, and we observed no difference between controls and paclitaxel treated veins. The proliferation marker ki-67 was occasionally present in the circumferential media, whereas it was almost absent in both the (inner) longitudinal media and the intima. Elastic fibers were present in the media and intima before and after organ culture without significant differences between the groups. Collagen fibers (Masson’s trichrome) were found abundantly (80%) in the inner longitudinal media, less commonly (20%) in the outer circumferential media, and they were absent in the intima without difference between the groups. Conclusion: Local paclitaxel treatment reduces neointimal hyperplasia in cultured human saphenous veins, without changing the amount of elastic or collagen fibers. Paclitaxel treatment leads to an increased amount of the contractile protein SMA and thus might have a therapeutic potential for the prevention of vein graft disease.
1 Introduction
The fight against vein graft disease remains a challenge in peripheral vascular and coronary artery bypass surgery (CABG). Despite advances in the field of venous allografts and vascular xenografts, venous autografts are at present undoubtedly superior [1]. Intimal hyperplasia is the first significant pathophysiological change in arterialized veins and it is thought to ease the consecutive process of vein graft atherosclerosis that leads to stenosis and graft occlusion [2–4]. Different strategies have been proposed as adjuncts to a careful surgical technique, most of them in experimental settings. On the one hand gene therapy is a currently investigated method, and on the other hand pharmacologic interventions remain interesting options to prevent vein graft disease [5–10]. Pharmacologic treatment of vein grafts can be performed with substances that are already in clinical use and local therapy of the veins reduces the risk of side effects [6].
Paclitaxel (Taxol) is an antineoplastic diterpenoid that inhibits cell division and migration by a formation of abnormally stable microtubules. Local paclitaxel treatment has been shown to inhibit neointimal hyperplasia in injured rabbit carotid arteries and stented porcine coronary arteries [11,12]. Furthermore, paclitaxel coated stents are successfully implanted in patients with coronary artery disease [13,14].
We chose paclitaxel because the biochemical effect of this substance is irreversible for the cells treated one time. This was also nicely demonstrated in experiments by Axel et al. who found a prolonged growth inhibitory effect even after single dose paclitaxel application in smooth muscle cell culture [15]. For a potential surgical application either a carrier to prolong the effect of a drug or a long lasting (irreversible) biochemical action is required.
We aimed to test the hypothesis that paclitaxel treatment can reduce neointimal hyperplasia in cultured human saphenous veins, an established in vitro model of vein graft intimal hyperplasia of human saphenous vein patches. In addition we wanted to investigate if paclitaxel treatment alters the amount of the contractile protein smooth muscle actin, the cytoskeletal protein desmin, the proliferation marker ki-67, and the interstitially localized elastic fibers and collagen fibers.
2 Patients and methods
2.1 Tissue preparation
Only the superfluous remnants of dilated saphenous veins after coronary bypass surgery, determined for waste, were used after informed consent of patients was obtained. The study was approved by the local institutional review board.
Segments of saphenous veins were collected from 13 patients. The veins were transported in serum-free RPMI 1640-medium (supplemented by l-glutamine and NaHCO3) containing 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml amphothericin B, 25 μg/ml gentamicin and 4 U/ml heparin (all Sigma-Aldrich Inc., Germany) from the operating room to the laboratory. Under sterile conditions after removing gently the adhering tissues, the veins were cut into rings of 0.8 cm length and opened longitudinally. Afterwards the flat vessel pieces, belonging to the control and treatment group, were cultured for 14 days with the intimal surface facing up.
The patients’ characteristics are listed in Table 1 .

Patient characteristics of 13 CABG patients from whom tissue samples of the saphenous veins were obtained
2.2 Organ culture
Each vein was divided into six groups: group 1 (baseline) was part of the vein without any treatment or incubation time which was immediately fixed in 4% phosphate-buffered formaldehyde followed by dehydration and paraffin-embedding; group 2 (control group) received 10 ml of medium and was cultured without paclitaxel treatment.
Group 3, 4, 5 and 6 were the treatment groups, cultured with 9 ml of culture medium and 1 ml of paclitaxel at different concentrations: group 3 received paclitaxel at a concentration of 1 μmol/l, group 4 at a concentration of 10 μmol/l, group 5 at a concentration of 25 μmol/l, and group 6 at a concentration of 50 μmol/l paclitaxel.
The culture medium contained RPMI 1640 (with l-glutamine and NaHCO3 inside, Sigma-Aldrich Inc., Germany) supplemented by 30% Fetal Bovine Serum Mycoplex (PAA Laboratories GmbH, Austria), 100U/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml amphothericin B, 25 μg/ml gentamicin and 4U/ml heparin (all Sigma-Aldrich Inc., Germany).
Paclitaxel (Ebetaxel™) 6 mg/ml stock solution (Ebewe Pharma, Austria) was diluted with 0.9% NaCl to the desired concentrations of 1, 10, 25 and 50 μmol/l.
The control group and the paclitaxel treated veins were incubated for 14 days at 37 °C and 5% CO2 with a medium exchange every 2–3 days.
After incubation the vein pieces were fixed with 4% phosphate-buffered formaldehyde over night, dehydrated and paraffin-embedded.
2.3 Histology and morphometry
For measurement of the intimal and medial thickness, slides of 4 μm thickness were stained by hematoxylin-eosin and elastica stain and examined under the microscope (Axioplan, Zeiss Inc., Germany). All vessels were digitally photographed at 10-fold magnification using a digital camera (Canon Power Shot G5) adjusted to full zoom.
Measurements at 30 μm-distances were performed over the whole circumferential length.
For analysis Image J 1.32j-software for Java (National Institutes of Health, USA) was used.
The mean value of each section was regarded as representative.
Masson’s trichrome staining was performed to investigate the quantity of collagen fibers. The positively stained area was quantified in the intimal hyperplasia, inner longitudinal media and outer circumferential media.
Elastica staining was used to visualize the elastic fibers. The positively stained area was quantified in the intimal hyperplasia, inner longitudinal media and outer circumferential media.
2.4 Immunohistochemistry
Immunohistochemistry was carried out on paraffin-embedded sections, using three different antibodies: alpha smooth muscle actin antibody (1A4; ab 7817; Abcam, Cambridge, UK), monoclonal mouse anti-human desmin (Clone D33, Code NO. M0760; Dako Inc., Glostrup, Denmark), and rabbit polyclonal to Ki67 – proliferation marker (ab 833; Abcam, Cambridge, UK).
The Elite Universal Vectastain ABC Kit (PK-6200; Vector Laboratories Inc., Burlingame, California) based on a biotin labeled secondary antibody was applied according to the instructions. The activity of endogenous peroxidase was blocked with 3% H2O2 in methanol for 10 min.
Ki67 needed microwave antigen retrieval with 10 mM citrate buffer pH 6.0.
The sections were visualized by Vector NovaRED Substrate Kit for Peroxidase (DK-4800; Vector Laboratories Inc., Burlingame, California) and counterstained by hemalaun.
The results were quantified by an experienced pathologist by counting the percentage of positively stained cells out of all cells found in the neointima, inner longitudinal media or outer circumferential media. Staining intensity at least two times stronger than the background was taken into consideration.
The periadventitial tissue of the veins was found to be (at least partially) removed during surgery. Thus histological analysis was performed on the intima and inner longitudinal and outer circumferential media.
2.5 Statistical analysis
The SPSS for Windows statistical software package (SPSS 10.0) was used for analysis. Neointimal and medial thickness is given as median and range. The difference of intimal thickness and immunohistochemical staining intensities between the groups was calculated using the Mann–Whitney U-test. The bivariate correlation between continuous variables was calculated using Spearman’s rho correlation coefficient. A p value of less than 0.05 was regarded as statistically significant.
3 Results
3.1 Association between clinical data and pre-existing intimal thickness
We found no significant association between the (pre-existent) baseline intimal thickness or the growth rate of intimal thickness and age, gender, triglyceride levels, cholesterol levels, and diabetes mellitus (p = n.s.).
3.2 Morphometry
The baseline segments of human saphenous veins from 13 patients showed a wide range of pre-existent (neo-) intimal hyperplasia with an intimal thickness of 19 (range 5–114) μm. The intimal hyperplasia contained circumferentially orientated vascular smooth muscle cells (SMC). The media contains an inner layer of longitudinally orientated SMCs and an outer layer of circumferentially orientated SMCs.
After 2 weeks of organ culture the intimal thickness in untreated control veins (n = 13) was 51 (range 9–155) μm. (Fig. 1 ).
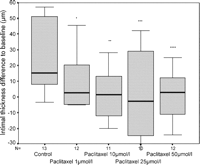
Neointimal growth of cultured human saphenous vein in comparison to baseline levels. All asterisks mark significance levels compared with controls. *p = 0.022, **p = 0.035, ***p = 0.03, ****p = 0.011.
Veins treated with 1 μmol/l paclitaxel in the culture medium (n = 12) showed a median increase of intimal thickness of 2 μm (range −76 μm to 46 μm) from baseline levels, whereas untreated control veins showed an increase by 15 μm (range −3 μm to 142 μm) (p = 0.022; Fig. 1). The treatment with 10 μmol/l paclitaxel (n = 11) resulted in a lower neointimal growth of 1 μm (range −82 μm to 212 μm) above baseline levels (p = 0.035 vs controls). Treatment with 25 μmol/l paclitaxel (n = 10) resulted in a decrease of intimal thickness of minus 3 μm (range −90 μm to 42 μm) from baseline levels (p = 0.03 vs controls). Treatment with 50 μmol/l paclitaxel (n = 12) resulted in an intimal growth of 3 μm (range −66 μm to 25 μm) above baseline levels (p = 0.011 vs controls).
3.3 Immunohistochemistry (SMA, desmin, ki-67)
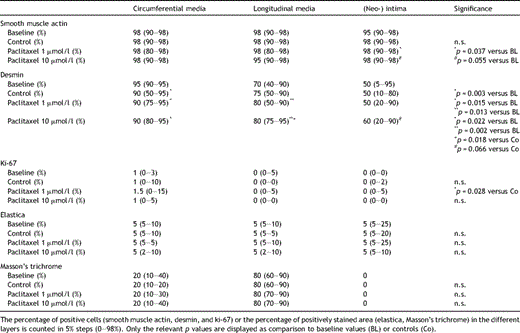
Smooth muscle actin (SMA) was present abundantly in all layers of the vascular wall of veins of baseline, control, and paclitaxel treated groups. In the longitudinal media and in the circumferential media no difference was found between the groups. In the (neo-) intima of paclitaxel 1 μmol/l treated veins the SMA amount was significantly higher compared with baseline values (p = 0.037). No difference was noticed between the other groups regarding the SMA positivity (Table 2 ) (Fig. 2 ).
Different amounts of smooth muscle actin (SMA), desmin, and the proliferation marker ki-67 in the different layers of cultured human saphenous veins
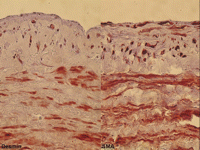
Immunohistochemical staining of paclitaxel (1 μmol/l) treated human saphenous veins after 2 weeks of organ culture. The desmin amount (left panel) is highest in the outer circumferential media and lower in the inner longitudinal media and intima. The SMA amount (right panel) is high in all layers of the vascular wall.
Desmin was found abundantly in the (outer) circumferential and (inner) longitudinal media, whereas it was less frequently found in the intima. In all three groups of cultured veins (controls, paclitaxel 1 μmol/l, paclitaxel 10 μmol/l) significantly less desmin was found compared with baselines, whereas no difference between controls and paclitaxel treated veins was found. (Table 2) In the longitudinal media the amount of desmin-positive cells was increased in paclitaxel-treated (1 + 10 μmol/l paclitaxel) veins compared with both baseline values and controls. In the intima, no significant difference of the amount of desmin positive cells was found between the groups (Fig. 4 ).
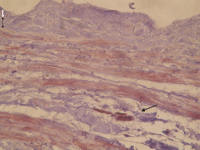
Immunohistochemical stain for ki-67 of a human saphenous vein after 2 weeks of organ culture without paclitaxel treatment (control group). Note the positive staining in the outer circumferential media (black arrow), whereas in the inner longitudinal media (marked by broken black arrow) and in the intima (white arrow) ki-67 positive cells were almost absent (40-fold magnification).
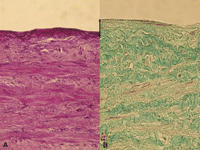
Elastica stain (A) of a human saphenous vein after storage in transportation medium without consecutive organ culture (baseline group). Elastic fibers are found in the whole vascular wall without differences between the groups. Panel B shows untreated human saphenous vein after 2 weeks of organ culture (control group). Collagen fibers (Masson’s trichrome stain, B) are predominately localized in the inner longitudinal media (only intensive green color is counted positive), to a less extent in the outer media. In the neointima that increased during organ culture (marked by black arrow) no collagen fibers are noted. (light green color does not count positively). For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
The proliferation marker ki-67 was present in the circumferential media, whereas it was hardly ever found in either the longitudinal media or in the intima. (Fig. 3) Interestingly ki-67 was more often found in the media of paclitaxel 1 μmol/l treated veins compared with controls. Between the other groups no difference in the ki-67 positivity was noticed (Table 2).
3.4 Elastica and Masson’s trichrome stain
Elastic fibers were present in the venous media and intima before and after organ culture. There were no significant differences in the amount of elastic fibers between the groups (Table 2).
Collagen fibers, stained by Masson’s trichrome, were found abundantly in the inner longitudinal media, whereas there was a lower amount in the outer circumferential media. Typically no Masson’s trichrome positive areas were found in the neointima and there were no differences in the staining intensity between the groups (Table 2).
4 Discussion
The first phenomenon which we observed in human saphenous veins was their pre-existent intimal hyperplasia which ranged from 5 to 114 μm. One may speculate if this condition influences their longevity, which was however not the content of this study. In 12/13 control veins, the intimal thickness almost doubled compared with pre-existing baseline values. Interestingly in one vein the intimal thickness slightly decreased from 56 to 53 μm during 2 weeks of organ culture. This demonstrates a variety of reactions of human saphenous veins in organ culture.
In our study, we found a significant inhibitory effect on intimal hyperplasia in cultured human saphenous veins with paclitaxel treatment at a concentration of 1 and 10 μmol/l. This inhibitory effect did not increase at higher paclitaxel concentrations. This is well in accordance with Herdeg et al. who showed a reduction of neointimal hyperplasia in balloon injured carotid arteries (rabbit) by endovascular application of paclitaxel at a concentration of 10 μmol/l [11]. Axel et al. have already earlier demonstrated a strong growth inhibitory effect of paclitaxel on cultured vascular smooth muscle cells at concentrations between 1 and 10 μmol/l [15]. Heldman et al. found a reduced neointimal hyperplasia in porcine coronary arteries using paclitaxel-coated stents compared with controls [12].
In our experiments the contractile protein smooth muscle actin was found abundantly in all layers of the veins. A significant increase of smooth muscle actin was found in the neointima of 1 μmol/l paclitaxel treated veins compared with initial baseline values. This effect of increasing this contractile protein could not be found at higher paclitaxel concentrations. In agreement Johnson et al. found high concentrations of smooth muscle actin in cultured human saphenous veins with no difference between freshly isolated and surgically manipulated veins [16].
We observed higher amounts of desmin in the media than in the intimal layer of the saphenous veins. After 2 weeks of organ culture the desmin intensity decreased in the outer part containing circumferentially orientated medial SMCs. Interestingly an increased desmin amount was present in the longitudinal media of paclitaxel treated veins. A decreased desmin amount is associated with the synthetic phenotype of SMCs, whereas a higher amount of this cytoskeletal protein is a typical feature of contractile SMCs [16].
In our study, we found hardly any ki-67 positive cells in the intima or in the longitudinal media. This is in accordance with Galea et al. who described a low amount of proliferating cell nuclear antigen in the wall of human saphenous veins [17]. In contrast to our findings Porter et al. describe a 45% rate of Bromodeoxyuridine labeled neointimal cells after 2 weeks of human saphenous vein organ culture [18]. However, we found the proliferation marker ki-67 in the outer circumferential media in the 1% range. Ki-67 is expressed during the G1, S, G2, and M-phase of the cell cycle. One may speculate that the saphenous veins after exposure to surgical trauma and an ischemic time in saline solution underwent an early activation phase during their storage in transport medium which preserves viability.
We noticed a constant amount of elastic fibers in the wall of the saphenous veins which was about 5%. In addition we found no effect of paclitaxel treatment on the presence of elastic fibers in cultured veins. Since the percentage of elastic fibers remained stable during organ culture, the absolute amount of elastic fibers increased with the formation of intimal hyperplasia. This is in agreement with Huang et al. who could show an increase of elastic fibers in neointimal hyperplasia of saphenous veins [19].
In our study. we observed a distinct microtopographical distribution of Masson’s trichrome positive areas within the vascular layers. In the inner media 4/5 of the interstitial area were positively stained for collagen fibers, compared with 1/5 of the outer media. In the neointima no Masson’s trichrome positive areas were detectable. Aguilera et al. found collagen type IV surrounding the media SMCs whereas it was completely absent in the neointima of human saphenous veins [20]. The positive staining areas for Masson’s trichrome in our experiments can of course indicate the interstitial matrix collagens type I + III in addition to the basement membrane collagen type IV.
We conclude that local paclitaxel treatment reduced neointimal hyperplasia in the human saphenous vein organ culture model, without changing the relative amount of elastic or collagen fibers. Paclitaxel treated veins showed an increased amount of the contractile protein smooth muscle actin and the cytoskeletal protein desmin. Thus paclitaxel might have a therapeutic potential for the prevention of vein graft disease.
Presented at the 21st Annual Meeting of the European Association for Cardio-thoracic Surgery, Geneva, Switzerland, September 16–19, 2007.




