-
PDF
- Split View
-
Views
-
Cite
Cite
Tomaso Bottio, Vladimiro Vida, Massimo Padalino, Gino Gerosa, Giovanni Stellin, Early and long-term prognostic value of Troponin-I after cardiac surgery in newborns and children, European Journal of Cardio-Thoracic Surgery, Volume 30, Issue 2, August 2006, Pages 250–255, https://doi.org/10.1016/j.ejcts.2006.05.001
Close - Share Icon Share
Abstract
Background: Troponin-I (Tn-I) is a well-recognized early postoperative marker for myocardial damage in adults and children. The present prospective study was undertaken to investigate whether a postoperative Tn-I value higher than 35 μg/l is able to predict long-term outcome as it does in early postoperative course, after surgery for congenital heart defects (CHD). Materials and methods: Five hundred and twenty patients (median age 11 months; male 54.7%: 284 patients) undergoing congenital heart defect repair on cardiopulmonary by-pass were prospectively updated in our database including postoperative Tn-I values. Seventy of them (13.4%) (mean age 2.6 ± 5.8 months) (70/520) experienced low output syndrome in the early postoperative period. According to the complexity of their malformations, we have arbitrarily divided these patients into two groups: group A included atrial and ventricular septal defects (13 patients), while group B included hypoplastic left heart syndrome, atrio-ventricular canal, transposition of great vessels, tetralogy of Fallot, double outlet right ventricle, truncus arteriosus, total anomalous venous return, and other combined diseases (57 patients). These patients are the object of our study. We reviewed clinical, laboratory, and echocardiographic data performed in the immediate postoperative course (within 24 h) and in the follow-up. Results: In this study, 13 patients died (13/70 patients; 18.5%), 12 in group B and 1 in group A. In deceased patients, mean Tn-I value was 130 ± 175 μg/l (CK-MB 570 ± 280 μg/l). Conversely, survivors showed a lower mean Tn-I value (25.5 ± 28.9 μg/l; CK-MB 76 ± 86 μg/l). Overall, Tn-I peak value was higher than 35 μg/l in 19 patients (19/70; 27.2%); among these, 9 died (median Tn-I was 163 ± 186 μg/l), whereas in survivors it was 73.4 ± 37 μg/l (p = 0.37). The remaining four patients who died had a median Tn-I value of 21 μg/l. When Tn-I exceeded 35 μg/l (>100 μg/l in two cases), at echocardiogram a severely depressed cardiac function was evident. Nevertheless, at long-term follow-up (12 ± 6 months), the echocardiogram showed an enhanced cardiac performance with an ejection fraction of 70 ± 8.5% in all; none of these patients presented with worsened ventricular function. Conclusion: Cardiac Tn-I is a specific and sensitive marker of myocardial injury after cardiac surgery and it may predict early in-hospital outcomes. However, by long-term echocardiographic analysis, cardiac Tn-I value looses its prognostic significance and therefore it is not a predictor of long-term ventricular dysfunction.
1 Introduction
Troponin-I (Tn-I) and Troponin-T plasma concentrations are well established, specific, and sensitive markers of myocardial injury in adult and young patients [1–5]. Taggart et al. [6] have shown that age inferior to 12 months and ischemic time are associated with elevated Tn-I levels after cardiac surgery. Therefore, preoperative and perioperative elevation of cardiac Tn-I are usually considered predictors of mortality and morbidity also in infants and children [7,8]. Immer et al. [8] calculated a positive predictive value of 100% and a negative predictive value of 93% of perioperative complications when Tn-I value exceeds 35 μg/l during the first 24 postoperative hours. However, most of these studies [6–9] correlate the early postoperative outcomes to Tn-I postoperative values, concluding that Tn-I seems to be a reliable marker in the prediction of early complications.
The present prospective study was undertaken to investigate whether a postoperative Tn-I value higher than 35 μg/l, associated to high risk of early potential adverse major cardiac events, it has a long-term outcome predictive value as in the early perioperative period, after surgery for congenital heart defects (CHD).
2 Materials
2.1 Population
Between January 2001 and August 2003, 520 patients with a mean age of 5.4 ± 10.6 years (median age 11 months; range 0–55 years; 284 males, 236 females) underwent CHD repair on cardiopulmonary by-pass at our institution. These patients consecutively entered our database and follow-up. For the entire series, postoperative Tn-I blood value was recorded.
According to the complexity of cardiac defects, the patients were arbitrarily divided into two groups:
group A: 208 patients (40%) (in-hospital mortality 1/208: 0.5%), who underwent correction of simple cardiac defects (atrial septal defect and ventricular septal defect);
group B: 312 patients (60%) (in-hospital mortality 13/312: 4%) with complex CHDs: hypoplastic left heart syndrome, atrio-ventricular canal, transposition of great vessels, tetralogy of Fallot, double outlet right ventricle, truncus arteriosus, total anomalous venous return, and other combined diseases.
One hundred and four patients (20%) had undergone previous palliative and/or corrective cardiac operations. For the entire population, the mean length of ICU stay was 2.2 ± 3 days (median 1 day). Overall, 14 patients died (in-hospital mortality 2.7%). Prolonged ventilation (15.3% incidence), due to either cardiac or respiratory failure, and acute renal failure (16.5% incidence) were the prevalent postoperative complications. In 3.6% of patients a permanent neurological dysfunction occurred in the postoperative period. Other complications (such as bleeding requiring sternal revision, pulmonary and blood infections) were observed in 5.2% and 3.7% of patients, respectively.
2.1.1 Study group
Among these 520 patients, 70 (13.4%, mean age 2.6 ± 5.8 months; 43 males and 27 females) presented with perioperative low cardiac output syndrome (defined as: dopamine, i.v. infusion with dosage >5 mcg/(kg min), enoximone, i.v. infusion with dosage >5 mcg/(kg min), during more than 24 postoperative hours). This group of patients is the object of our study. The majority of them (57; in-hospital mortality 12/57: 21%) were in group B, whereas 13 patients in group A (in-hospital mortality 1/13: 7.7%). Thirteen patients died perioperatively and eight of them underwent postmortem examination.
3 Methods
Data collection included demographic data, type of congenital cardiac defect, type of surgical repair, cardiopulmonary by-pass time (CPB), cross-clamping time, complications, length of ICU stay, in-hospital and long-term clinical and echocardiographic outcomes. Blood samples were taken postoperatively at the admittance in ICU, and every 12 h thereafter.
The following tests were used to detect potential myocardial injury:
Tn-I (upper limit set at 0.6 μg/l);
CK activity (upper limit set at 136 U/l);
CK-MB activity (upper limit set at 4 μg/l).
For each patient, we considered only the highest Tn-I value. Cardiac function was evaluated by physical examination, ECG, and echocardiogram. Echocardiographic follow-up was performed before discharge, at 3, 6, 12 months after the procedure or whenever required by the clinical situation, in our outpatient clinic.
Duration of CPB, aortic cross-clamping time, circulatory arrest time, were prospectively collected (Table 1 ). All the procedures were performed with the aid of moderate hypothermic CPB. Diastolic cardiac arrest was achieved by an antegrade infusion of hematic cardioplegic solution. Both in neonates and older patients, cardioplegic aortic root infusion was repeated at 20–30 min. When performing circulatory arrest, the core temperature was lowered to 18 °C, adjusting the hematocrit to 18% in the cooling phase and 30% during re-warming. All the patients were transferred to ICU for postoperative care.
End-point of this study was the occurrence of one of the following cardiac events during the follow-up: (1) perioperative mortality and morbidity, (2) cardiac-related death, and (3) heart failure requiring specific medical therapy and readmission to ICU, particularly in those patients with a Tn-I value >35 μg/l.
For each patient, survival free from cardiac events was considered from the hospital discharge to the occurrence of the first cardiac event, or to the end of the study.
Tn-I concentrations were determined by a fluorometric enzyme immunoassay analyzer (Stratus CS, Dade Behring) with a functional sensitivity of 0.03 μg/l; the cutoff level was 0.08 ng/ml.
3.1 Statistical analysis
The prevalence of risk factors (age, cardiac defect) and the cumulative incidence of perioperative, in-hospital and early and long-term follow-up cardiac complications (CPB time, cross-clamping time, circulatory arrest, ICU stay, Tn-I, CK-MB, and EF at echocardiogram), for the two groups, were compared with the Fisher’s exact test for categorical variables and t-test for continuous variables.
Results are reported as mean ± standard deviation in text and tables. Statistical significance was defined as a p-value less than 0.05.
4 Results
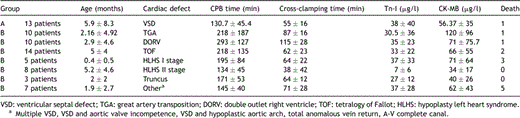
The clinical characteristics of the patients are reported in Tables 1 and 2 . Thirteen of 70 patients died during the postoperative period, accounting for a study group mortality of 18.5%; mean ICU stay was 16 ± 14.5 days.

4.1 Postoperative outcomes
The 13 patients who died showed a mean Tn-I of 130 ± 175 μg/l (median value 72 μg/l; range 15–548.2 μg/l) (CK-MB 570 ± 280 μg/l), while it was 25.5 ± 28.9 μg/l (median value 16.7 μg/l; range 0.22–150 μg/l) in the survivors (p = 0.05) (CK-MB 76 ± 86 μg/l). Six patients had a postoperative Tn-I >100 μg/l; among these, only two survived (Tn-I was 150 and 124 μg/l, respectively).
Tn-I was higher than 35 μg/l in 19 patients (27%; median age 2.76 months), while it was lower in the remaining 51 patients, within the range of 20–34 μg/l in 19, between 10 and 19 μg/l in 20, and 1–9 μg/l in 10; only two patients (0.3%) had a postoperative Tn-I value lower than 1 μg/l.
Among those with a Tn-I >35 μg/l (high-risk group, 19 patients), we observed 9 deaths (47%). Mean value of Tn-I observed in these patients who died was 163 ± 186 μg/l (median Tn-I 91 μg/l; range 55–548.2 μg/l) (CK-MB 258.75 ± 248.19 μg/l). Among survivors (10 patients), Tn-I mean value was 73.4 ± 37 μg/l (median value 67 μg/l; range 35–150 μg/l) (CK-MB 68.18 ± 39.34 μg/l) (p = 0.16).
Among those with a Tn-I ≪35 μg/l (low-risk group), 4 died (4/52: 7.7%). Median Tn-I was 22 μg/l (range between 15 and 32 μg/l) (CK-MB 28.84 ± 11.58 μg/l).
4.2 Operative data
The high-risk group had a significantly longer CPB time than the low-risk group (272 ± 164 min vs 150 ± 67 min; p = 0.001). The patients who died had a mean CPB time and ICU stay significantly longer than survivors (p ≤ 0.05), while mean cross-clamping time was comparable (93 ± 40 min vs 68 ± 26 min; p = 0.45).
Circulatory arrest was employed in 26 patients. Among these, 4 patients died (15.4%: median arrest time of 26.5 min, range 5–91 min). In the 22 survivors, the median arrest time was 28.5 min, ranging 3–65 min (p = 0.76).
4.3 Autopsy
The postmortem examination was performed in the majority of dead patients (8/13: 61%). Massive myocardial damage was observed in seven autopsies with areas of focal myocardial hemorrhage in four cases; lung infarction with sign of multiorgan failure was observed in the remaining case (Table 3 ).

4.4 Long-term survival and echocardiographic follow-up
The mean follow-up time was 12 ± 6 months (range 1–23 months). The follow-up was 100% complete (Table 4 ).

Ejection fraction at echocardiogram according to postoperative Tn-I peak value
Concerning the EF, those patients with Tn-I value higher than 100 μg/l showed a significantly reduced cardiac function in comparison with all others of the Tn-I sub-groups, while only the patients with a mean Tn-I value ≪9 μg/l had significantly better cardiac performances. Moreover, dividing the patients in sub-groups according to the Tn-I value (Table 4), we observed that a difference >15–20 μg/l of Tn-I was enough to make the cardiac function significantly worse.
Among the high-risk group, echocardiography showed severe cardiac failure (ejection fraction lower than 20%) in the nine deceased patients. In survivors, mean postoperative ejection fraction was estimated to be 35.7 ± 8.5%. In the long-term, ventricular function improved to normality with normalization of ejection fraction in all patients. Despite a postoperative Tn-I >100 μg/l, two patients, showing a severely depressed cardiac function in ICU and at discharge, at 12 months control, presented with a left ventricle ejection fraction within normal limits (LVEF 50–70%).
Among the low-risk group, 2 out of 4 dead patients showed a very low ejection fraction (30%). However, the long-term bidimensional echocardiography showed improvement (mean ejection fraction 71.6 ± 8.9%) in all but three patients, in whom LVEF at discharge was higher than 50%. There was no late death at follow-up.
All patients with a postoperative Tn-I value greater than 20 μg/l showed a significantly better cardiac performance in the long term, while in patients with a postoperative peak value lower than 20 μg/l, in-hospital cardiac function was comparable to that observed at follow-up (Table 4).
5 Discussion
This study was undertaken with the aim of finding a long-term relationship between Tn-I blood levels measured after surgery and left ventricular performance, in a wide population of children undergoing surgery for CHD. Our hypothesis was that the close relationship between the Tn-I peak value and the degree of left ventricular cardiac dysfunction, detected in the early perioperative period, could reverse at long-term follow-up.
As previously reported [1,8,10,11], Tn-I plasma concentration is a specific marker of myocardial injury. Tn-I detects either presence or persistence of myocardial injury after cardiac surgery, and is able to discriminate between patients at higher risk of developing a clinically relevant cardiac failure from those with a good clinical outcome. A trend toward higher Tn-I value has been already observed in patients undergoing surgery for congenital cardiac defects, especially when the repair is undertaken in infancy and when cardiopulmonary by-pass is longer than 100 min [6,10–12]. As expected, analysis of our data shows a close relationship between complexity of the operation and length of CPB, and Tn-I serum level.
Immer et al. [8] have classified the patients undergoing cardiac surgery for congenital heart defects into two different risk groups: those at ‘low risk’, in whom Tn-I value was lower than 35 μg/l, and those at ‘high risk’ in whom Tn-I was higher than 35 μg/l. In their series of 54 children who underwent surgery for ASD and VSD closure (equivalent to our group A), and 19 who underwent repair for tetralogy of Fallot or double outlet right ventricle (equivalent to our group B), only 2 children died among the 19 patients with a postoperative Tn-I value higher than 35 μg/l. Both patients showed a Tn-I serum level above 100 μg/l (126 and 319 μg/l, respectively). This supports the findings reported by Taggart et al. [6] and Kirklin et al. [13], who considered Tn-I values higher than 100 μg/l as lethal. In our experience, among the six patients with a Tn-I exceeding 100 μg/l, mortality was four (66%). Survivors had a Tn-I level between 124 and 150 μg/l. Twelve months of echocardiographic evaluation showed a complete cardiac function recovery in both of them.
In our study group left ventricular ejection fraction was significantly worse in patients with Tn-I > 35 μg/l, versus those with Tn-I ≪35 μg/l. Moreover, concerning the patients with a postoperative peak Tn-I value ≪35 μg/l, only the patients in whom the Tn-I was within 1 and 9 μg/l (Tn-I) had a significantly better cardiac function, while for those with Tn-I >10 μg/l cardiac function was worse.
However, all survivors with a postoperative Tn-I value higher than 20 μg/l showed a significant improvement of cardiac performance at echocardiographic follow-up. On the contrary, although improved, cardiac function was comparable to that observed in the postoperative period in patients with Tn-I values lower than 20 μg/l.
It has long been believed that adult cardiomyocytes do not proliferate after birth, thus the myocardium in the necrotic area should be gradually replaced by collagen tissue resulting in cardiac dysfunction. Recently, Anversa and Kajstura [14] and Beltrami et al. [15] showed that adult cardiomyocytes could enter the cell cycle and increase the cell number, and that undifferentiated stem cells in the bone marrow may be transported to the heart differentiating into cardiomyocytes and vascular endothelial cells in a murine model [16]; similar finding has also been observed by Laflamme et al. [17]. Quaini et al. [18] showed that circulating stem cells can contribute to the re-population of solid-organ by observing a male chimerism in heart allografts from female donors, thus probably contributing to ventricular remodeling. The observation that circulating blood cells might colonize solid-organ tissues suggests that tissue regeneration and repair of injured or diseased areas are feasible [19]. According to our clinical experience, we can speculate that cardiac remodeling might also appear after a devastating myocardial damage (Tn-I value >100 μg/l). This appealing hypothesis may explain why patients with Tn-I >35 μg/l and cardiac dysfunction at discharge, showed a complete recovery at 12 months follow-up. However, mean Tn-I value in survivors was lower than that observed in children who died (25 μg/l vs 130 μg/l), confirming therefore the relationship between Tn-I and cardiac failure during in-hospital stay. Thus, as reported by other authors [6–9], also in our experience, Tn-I played an important role as a monitor of perioperative myocardial cell damage being an additional prognostic risk. In light of these results, we believe that a routine evaluation of postoperative Tn-I levels may be useful for safe clinical management, so as to identify high-risk patients, and optimize postoperative care.
As far as CPB time is concerned, Immer et al. [8] identified a significant correlation between peak Tn-I value and cardiopulmonary by-pass time. The longer the CPB time (>100 min), the higher the mortality risk; and the longer the ischemic time, the higher the Tn-I levels [6,8]. Our results also support these previous findings. Tn-I levels were in fact significantly higher in those patients in whom CPB time was longer. We believe that a cutoff time for CPB should be raised to 140 min, being our mean CPB time of 139 min, in low-risk patients (Tn-I ≪35 μg/l). Thus, although we think that a routine evaluation of the postoperative Tn-I in pediatric cardiac surgery is useful, we speculate that it is mandatory when CPB time is longer than 140 min.
Study limitations of this study are as follows:
the great variety of clinical conditions and surgical procedures;
the lack of blood samples for preoperative Tn-I value examination;
the lack of a time trends of postoperative Tn-I levels since only the peak value has been recorded.
However, in this study we believe results are significant because we have included a large population of patients who had undergone surgery for several different CHD, either simple or complex lesions.
6 Conclusion
In conclusion, this study confirms that cardiac Tn-I is a specific and sensitive marker of myocardial injury after pediatric cardiac surgery and it may be a predicting factor for early in-hospital outcome. According to the echocardiographic long-term analysis, cardiac Tn-I loses part of its prognostic power and it is unable to identify those patients with unsatisfactory cardiac recovery.
Acknowledgments
The study was supported by grants of Istituto Superiore di Sanità and Ministero Istruzione Università e Ricerca, Rome, Italy and by Regione Veneto, Venice, Italy.
References
- cardiopulmonary bypass
- hypoplastic left heart syndrome
- tetralogy of fallot
- truncus arteriosus
- echocardiography
- transposition of great vessels
- double outlet right ventricle
- congenital abnormality
- ventricular function
- cardiac surgery procedures
- ventricular dysfunction
- atrium
- congenital heart defects
- ventricular septal defect
- adult
- child
- depressive disorders
- follow-up
- heart ventricle
- newborn
- surgical procedures, operative
- survivors
- heart
- patient prognosis
- troponin i
- cardiac function
- ejection fraction
- myocardial injury
- depressed mood
- venous return
- surgery for congenital heart disease
- creatine kinase mb isoenzyme




