-
PDF
- Split View
-
Views
-
Cite
Cite
Xavier Benoît D'Journo, Christophe Doddoli, Pierre Michelet, Anderson Loundou, Delphine Trousse, Roger Giudicelli, Pierre Antoine Fuentes, Pascal Alexandre Thomas, Transthoracic esophagectomy for adenocarcinoma of the oesophagus: standard versus extended two-field mediastinal lymphadenectomy?, European Journal of Cardio-Thoracic Surgery, Volume 27, Issue 4, April 2005, Pages 697–704, https://doi.org/10.1016/j.ejcts.2004.12.022
Close - Share Icon Share
Abstract
Objective: Controversy continues over the optimal extent of lymphadenectomy for the surgical treatment of Adenocarcinoma of the oesophagus. Methods: From 1996 to 2003, 102 transthoracic en-bloc esophagectomy were performed for adenocarcinoma. Based on the 1994 consensus conference of the International Society of Disease of Esophagus, 35 patients underwent standard lymphadenectomy whereas 67 underwent extended lymphadenectomy. Mortality, morbidity and long-term survival were reviewed in each group. Results: Extended lymphadenectomy increased the number of resected lymph nodes and improved the healthy/invaded lymph node ratio. It allowed to detect skip nodal metastasis in 36.4% of the N+ patients. Morbidity was higher following extended lymphadenectomy, with respect to pulmonary complications, and blood transfusions requirement (P=0.04). However, operative mortality was similar in both groups (9 vs. 11%). Overall disease-free survival was 28% at 5 years. Median of survival was higher in N0 than in N+ patients (55 months vs. 20 months; P=0.02). Extended lymphadenectomy was associated with an improving of disease-free survival when compared to standard lymphadenectomy (41 vs. 10% at 5 years; P≪0.05), especially in the subgroup of patients with a N0 disease (median of survival 44 months vs. 17 months; P=0.001). Based on multivariable analyses, predictive factors of recurrence affecting disease free-survival were the pT status (P=0.02), standard lymphadenectomy (P=0.05) and extracapsular lymph node involvement (0.04). Conclusions: These results indicate that extended 2-field lymphadenectomy is an important component of the surgical treatment of patients with adenocarcinoma of the oesophagus. It increases the likelihood of proper staging and affects patient outcome, while it does not enhance the operative mortality. However, extended lymphadenectomy increases non-fatal morbidity, especially the incidence of pulmonary complications and the need for blood transfusion.
1 Introduction
Surgery remains the cornerstone of the treatment of adenocarcinoma of lower third oesophagus in low-risk patient. Oesophagectomy is an important way to insure optimal palliation for dysphagia, to achieve local control of the tumour and finally to provide an accurate staging of the disease. Surgical treatment is based on standardized oncological principles focusing on the completeness of the resection: esophagectomy must be associated with a wide en-bloc dissection to ensure lateral and longitudinal clearances of the tumour into the mediastinum [1–3]. Indeed, a complete resection (R0) leaving no identifiable residual disease is considered as the most important determinant of survival [4,5].
Lymphadenectomy is also an important component of the completeness of the resection aiming to reduce local recurrence, and thereby to improve disease free-survival [5–8]. The counterpart is its potential to increase postoperative mortality and morbidity. Thus, controversy continues over the optimal extent of lymphadenectomy to be performed together with esophagectomy, sometimes even in the same surgical team. Some authors advocate minimal lymphadenectomy (i.e. one-field) and esophagectomy through a transhiatal approach to minimize procedure-related complications, considering mediastinal lymph node involvement as systemic disease with no hope for cure, thus narrowing the role of surgery to palliation [9–11]. Conversely, some surgical teams advocate a 3-field lymphadenectomy, including an extended dissection in the neck, with distinct survival benefit even in cases of tumours located in the distal oesophagus [3,4,12,13].
Between these 2 extreme and opposite views, it can still be hard to define the role of 2-field lymphadenectomy. Two-field lymphadenectomy implies a transthoracic approach to access upper mediastinal lymph node stations. The consensus conference of the International Society of Disease of Esophagus held in Munich in 1994, defined basis of radical esophagectomy considering four types of lymphadenectomy, among which standard and extended 2-field lymphadenectomies [14]. With standard lymphadenectomy, only lower mediastinal and upper abdominal node stations are removed. With extended lymphadenectomy, resection of the upper mediastinal nodes is added to the standard procedure. In the absence of prospective and randomised studies published to date, the aim of this study was to determine retrospectively the risk/benefit ratio of transthoracic standard versus extended 2-field lymphadenectomies for adenocarcinoma of the lower oesophagus.
2 Materials and methods
2.1 Patients
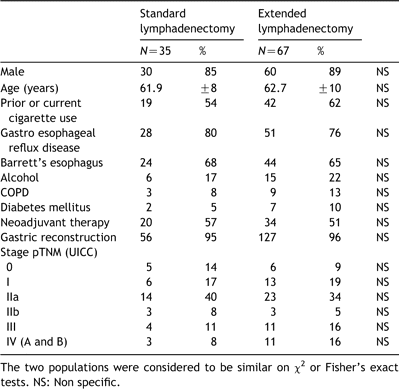
From January 1996 to October 2003, 102 transthoracic esophagectomies were performed for adenocarcinoma of the lower oesophagus, classified Siewert I and II. There were 12 women and 90 men. All the 102 tumours were considered accessible for surgical treatment. The technique of lymphadenectomy was left at surgeon discretion. Based on the definition of the consensus conference of the International Society of Disease of Esophagus, 35 patients underwent standard two-field lymphadenectomy whereas 67 underwent extended two-field lymphadenectomy. The two groups were similar with respect to various clinical or pathological characteristics (Table 1). Two third of the patients had a history of tobacco and alcohol abuse. Two third were known to have a previous history of chronic gastro-oesophageal reflux disease or Barrett metaplasia. All patients were clinically staged on the basis of oeso-gastroscopy, chest and abdominal ct-scans and routine endoscopic ultrasonography findings, according to the latest version of the UICC classification [15]. Neoadjuvant radio-chemotherapy (5FU-Cisplatin- 45Gy) was done in half the cases of advanced tumour (usT3N+).
Medical and surgical complications were recorded. Respiratory complications were defined by all medical events concerning lung parenchyma (i.e. pneumonia, airway congestion, atelectasis, acute lung injury, acute respiratory distress syndrome) in the absence of surgical complications requiring reoperation. Surgical complications included anastomotic leakage, laryngeal paralysis, chylothorax, pleural effusion, empyema and bleeding. Hospital mortality was defined as all death occurring during hospital stay (≪ and ≫30 days).
2.2 Surgery
102 transthoracic esophagectomies were performed. The surgical technique consisted of Ivor Lewis procedures in the majority of the cases (97%) with a gastric tube reconstruction in the posterior mediastinum and an intrathoracic anastomosis. All resections were carried out by the same approach involving an initial midline laparotomy for mobilization of the stomach followed by a right-sided posterolateral thoratomy allowing resection of the oesophagus and mediastinal lymphadenectomy. Three patients required cervicotomy with a cervical anastomosis with a colic interposition due to previous gastric surgery. The intrathoracic anastomosis was performed with a circular stapler. Right thoracotomy included one-lung ventilation obtained with endo-bronchial intubation. In all cases, a complete esophagectomy with resection of the upper third of the stomach was performed.
Definitions of lymphadenectomy were based on the 1994 consensus conference of the International Society of Disease of Esophagus [14]. At standard two-field lymphadenectomy (n=35), only the lower mediastinal and upper abdominal nodes were resected. In summary, standard two-field lymphadenectomy included the paracardial, lesser curvature,celiac and left gastric artery in the abdominal field, and lower mediastinal as well as subcarinal nodes in the thoracic field. At extended lymphadenectomy (n=67), resection of common-hepatic-artery and splenic artery nodes in the abdominal field,and the right and left upper mediastinal nodes in the thoracic field were added to the standard procedure. In the chest, posterior mediastinectomy was performed en-bloc together with the oesophagus, the mediastinal pleura, the azygos vein arch, fat mediastinal tissues, and all lymphatic tissues including the thoracic duct, subcarinal and peribronchial lymph node stations. All operations were performed by the same 4 senior surgeons of a single institution along the period, among whom 2 performed extended lymphadenectomy routinely. Thus, the extend of lymphadenectomy and, in turn, the definition of the two groups of patients submitted to comparison, reflected surgeon's preference and conviction.
Pyloroplasty and feeding jejunostomy were performed in all patients. Pathologic examinations were recorded for all patients and tumours were classified using the latest UICC TNM classification. Charting of the lymph nodes was done, using a database, counting the number of resected and invaded nodes in the specimen and in lymph nodes separately taken and identified at surgery. The healthy/invaded lymph node ratio was then established.
2.3 Statistical analysis
The medical records of all patients who underwent oesophagectomy for a malignant disease were reviewed. Patient charts were identified by screening of a database into which data were entered prospectively for any patient undergoing surgery for thoracic malignancy at our department. χ2 or Fisher's exact tests were used to compare categorical data. Student's t-test was used for continuous data. P values below 0.05 were considered to indicate statistical significance. All patients were seen at the outpatient clinic at intervals of three to four months during the first two years and every six months. For patients lost to medical follow-up, missing data were obtained by consulting the City Hall registry. Survival was measured from the date of operation and survivorship calculated according to the Kaplan–Meier method, including the operative mortality. Survival curves were calculated from the time of surgery to death from any cause or to the time of the last follow-up visit (at which time data were censored). Disease-free survival was counted up to the time of a first relapse or death from any cause or the time of the last visit without a previous relapse. Survival and disease free survival curves were constructed by the Kaplan–Meier method, and the log-rank test was used to determine significance. Predictors affecting survival and disease-free survival were obtained by univariable and multivariable analysis. Cox regression was employed to determine variables to be included in the multivariable analysis using P values below 0.2. P values below 0.05 were considered to indicate statistical significance in multivariable analysis.
3 Results
3.1 Lymph node dissection
Extended 2-field lymphadenectomy increased the mean number of resected lymph nodes when compared to standard 2-field lymphadenectomy (17.2 vs. 4.9; P=0.001). Extended lymph node dissection allowed to perform a more complete staging since the number of sampled lymph node stations was increased (Table 2). Extended lymphadenectomy also improved the healthy/invaded lymph node ratio (0.07 vs. 0.1; P=0.6). Lymph node involvement was found in 30% of the patients treated with extended lymphadenectomy and in 20% the patients treated with the standard procedure, but this difference did not achieve statistical significance. At whole, 28 patients had lymph node involvement located in the abdominal field (n=6; 21%), or in the mediastinal field (n=7; 25%) or in both fields (n=15; 53%). Thus, extended lymphadenectomy allowed a more accurate definition of the lymph nodes status in 25% of the patients: mainly those who presented with invaded lymph nodes confined to the mediastinum. Furthermore, 8 patients out 22 N+ patients (36.4%) who received extended 2-field lymphadenectomy presented with ‘skip’ nodal metastases to the upper mediastinum or the celiac nodes without invasion of the peritumoral nodes.
3.2 Mortality and morbidity
Data on mortality and morbidity are summarized in Table 3. Medical complications were significantly more frequent following extended lymphadenectomy when compared to standard lymphadenectomy (61 vs. 31%; P=0.004), and especially respiratory complications that appeared as the most common complications (49 vs. 25%; P=0.02). Pneumonia, atelectasis and ARDS represented the majority of the medical morbidity observed in both groups. Atrial arrhythmia was more frequent after extended dissection (10 vs. 0%; P=0.04). Surgical complications were increased in the extended lymphadenectomy group (14 vs. 2%; P=0.02) and especially pleural effusions requiring a chest tube drainage and chylothorax. Lengths of stay in the intensive care unit (11 vs. 8 days; P=NS) or overall in-hospital stay (30 vs. 26 days; P=NS) were similar in both groups as well as postoperative mechanical ventilation times (20 vs. 15h; P=NS). Postoperative blood transfusion needs were significantly higher following extended lymph node dissection (3.4 vs. 1.6 units; P=0.04).
3.3 Survival and recurrence
Overall and disease-free 5-year survival rates were 40 and 28%, respectively (Fig. 1). Median and 5-year survival rates were significantly higher in N0 patients when compared to N+ patients (48 vs. 23%; P=0.02). Extended lymphadenectomy was associated with a significantly higher 5-year disease-free survival when compared to standard lymphadenectomy (41 vs. 10%; P=0.01) (Fig. 2). In N0 patients, extended lymphadenectomy was associated with an improved disease-free survival when compared to standard lymphadenectomy (median of survival: 44 vs. 17 months; P=0.001), whereas no significant difference in median survival was observed in N+ patients (17 vs. 22 months; P=0.9) (Fig. 3). Among factors affecting disease free survival, 8 variables appeared with P values below 0.2 at univariable analysis and were selected for multivariable analysis: sex, type of lymphadenectomy, neoadjuvant therapy, pT stage, pN stage, number of invaded nodes, healthy/ invaded lymph node ratio and extracapsular lymph node involvement. Multivariable analysis allowed to discriminate 3 independent factors affecting significantly disease-free survival: pT stage (P=0.02), type of lymphadenectomy (P=0.05) and extracapsular lymph node involvement (P=0.04) (Table 4).
Follow up of patients allowed to determine the length before relapse and the location of recurrence. There was no significantly difference between standard and extended lymphadenectomy in terms of proportions of patients ‘free of disease’ (60 vs. 71%; P=NS), with locoregional (28 vs. 20%) or general recurrence (7 vs. 11%). The ratio of patients free of disease appeared higher according to the pN status and the type of lymphadenectomy (Table 5). For N0 patients, recurrence was 24% with extended lymphadenectomy and 38% for standard lymphadenectomy (P=0.21). Location of relapse was similar in both groups. Locoregional recurrence involved chest (5%), abdominal (5%) and neck fields (2%). Metastatic recurrences included lung and liver metastases in 10% of the cases.
4 Discussion
Anatomy and biology explain why oesophageal cancer has frequent metastasis to the lymph nodes once the tumour involves the submucosal layer, and spreads widely from the neck to the abdomen despite tumour location, with early systemic dissemination of the disease. Unsurprisingly, invaded lymph nodes are present at surgery in 30–80% of the patients, depending on the accuracy of the preoperative staging. In more than 40% of the cases, the first site of disease recurrence is a lymph node station [17]. Finally, lymph node status appears as a strong prognosticator in most surgical series. However, controversy continues over (1) what constitutes optimal lymphadenectomy, and (2) whether lymphadenectomy offers any benefit in survival.
During the consensus conference of the International Society of Disease of Esophagus held in Munich in 1994 [6], it was agreed that extended mediastinal lymphadenectomy was required for supracarinal cancers. Such a consensus was not reached by the panellists for subcarinal cancers, in the absence of prospective and randomised studies for adenocarcinoma of the oesophagus. Although some authors have addressed the potential role of 3-field lymphadenectomy in the surgical treatment of adenocarcinoma of the oesophagus [5,17], this procedure is technically demanding in view of the high percentage of recurrent nerve injuries and respiratory complications. A recently published prospective randomised study suggests that transthoracic en-bloc oesophagectomy with standard 2-field lymphadenectomy offers better chances of cure than transhiatal oesophagectomy for adenocarcinoma of the oesophagus [10]. We focused our attention on whether or not the type of 2-field lymphadenectomy may impact on the surgical results, in terms of completeness of the resection, procedure-related morbidity and mortality and disease-free survival.
4.1 Completeness of the resection
Mediastinal lymphadenectomy appears as a basic requirement of the completeness of the surgical resection because mediastinal lymph node involvement is relatively frequent, in about 40% of the cases of subcarinal tumour [3–7]. The greater the number of lymph nodes removed, the greater the chances of obtaining a R0 resection [17,18]. Our study indicates clearly that in up to 25% of all patients, and in more than 35% of the N+ patients, standard 2-field lymphadenectomy would have lead to inadequate staging and, in turn, incomplete resection. On 28 N+ patients, 11 patients had regional positive nodes, 7 patients had regional and distant positives nodes and 10 patients (35%) had distant positive lymph nodes into the mediastinum with a skipping of regional lymph node station.
4.2 Mortality and morbidity
Medical and surgical complication rates increased with the extent of lymphadenectomy but this fact did not lead to a higher post-operative mortality. Respiratory complications remain the leading cause of morbidity after esophagectomy, mainly due to pneumonia and ARDS, and are increased with extended procedure. In our study, extended and standard lymphadenectomy patient groups included both a right thoracotomy and differed only by the extent of lymphadenectomy. Many hypotheses can be postulated to explain differences of respiratory morbidity between these two types of lymphadenectomy. On one hand, mediastinal lymph node dissections may cause injuries to the bronchial arteries and nerves, the tracheobronchial tree itself, and both recurrent nerves [25]. On the other hand, thoracotomy includes one-lung ventilation, which favours capillary permeability due to alveolar stress, and in turn pulmonary oedema. Mediastinal lymphadenectomy impairs the lymphatic backflow and adds to pulmonary oedema. In addition, surgical complications were more frequent with extended lymphadenectomy, mainly chylothorax or pleural effusion requiring chest tube drainage. Finally, blood transfusion needs were increased by extended dissection. It is quite likely that more blood transfusions affect respiratory status and lead to development of pulmonary complications.
4.3 Effect on free-disease survival
Despite its retrospective design, our study presents with 2 characteristics reinforcing the meaning of its results: the prospective compilation of the data, and the comparison of 2 groups of patients treated concurrently that proved to be homogeneous regarding to the main clinical variables. Differences in overall and disease-free survival rates between the two types of lymphadenectomy were found to be statistically significant. Moreover, extended lymphadenectomy was found as an independent prognosticator of improved disease-free survival at multivariable analysis, suggesting a beneficial effect of the technique. The subgroups analysis, however, showed that the difference was due to the dramatic dissimilarity in survival observed in N0 patients according to the type of lymphadenectomy. This last observation may be interpreted contradictorily. The first explanation is artefactual, and related to a stage migration effect, also known as the ‘Will Rogers’ phenomenon [20]. The second attributes a therapeutic efficiency to the removal of apparently healthy lymph nodes.
There is no doubt that lymph node dissection contributes to the accuracy of the final staging: because of an incomplete lymph node dissection in the ‘standard’ group, some patients with more advanced lymph node disease have not been detected, since some of the mediastinal lymph nodes have not been removed and consequently not examined. Indeed, lymph node involvement rate was 20% with standard lymphadenectomy while this rate was up to 30% with the extended procedure. The incidence of invaded nodes was increased in both surgical fields (i.e. abdominal and mediastinal) with extended lymphadenectomy (15 and 24%, respectively) compared to standard lymphadenectomy (8 and 14%, respectively). Extended lymphadenectomy clearly decreases the proportion of ‘false-negative’ patients regarding their N status. Standard lymphadenectomy, in turn, would have result in a loss of chance to be cured for those patients who finally had an unknown residual disease after surgery, and probably did not receive any form of adjuvant therapy [22].
Obviously, the total numbers of invaded lymph nodes as well as their location have important prognostic and therapeutic implications. Skinner demonstrated that survival rate was closely related to the lymph node status and the number of invaded nodes [2]. Lerut and colleagues [17] also confirmed the importance of the number of resected and invaded lymph nodes. They found that the number of positive nodes and the number of resected nodes exercised a linear effect in the Cox regression analysis of survival, with increasingly worse survival as the number of resected and involved nodes increased. The healthy/invaded lymph node ratio is thus a marker of the quality of lymphadenectomy, and has been disclosed as a prevailing prognosticator. It is now well established that a healthy/invaded lymph node ratio up to 0.1 or a number of positive nodes up to 4 nodes allows to select a subgroup of N+ patients eligible for adjuvant therapy because of a higher risk of relapse [3,7,16,21]. Extracapsular tumour extension within an involved lymph node is also an important determinant of survival [23]. Our findings are in line with these statements since the number of resected nodes, the number of invaded nodes and extracapsular spreading affected disease free-survival at Cox regression analysis.
Another concern is the frequency, up to 50%, of a micrometastatic disease detected with the use of immunohistochemistry or polymerase chain reaction (PCR) and reverse transcriptase PCR in otherwise healthy lymph nodes after routine histopathologic examination [19]. Occult nodal micrometastasis has been shown to be an independent prognosticator of survival. It remains speculative that the technique of lymphadenectomy by itself may impact on survival. This hypothesis, however, deserves further investigations.
The concept of sentinel lymph node mapping and detection is an alternative concept to routine lymphadenectomy that may open the door to individualized therapy, allowing selective and modified lymphadenectomy targeting sentinel nodes for clinically N0 oesophageal cancer, while avoiding the side effects of an extensive dissection. Unfortunately, sentinel nodes in oesophageal cancer are multiple and are distributed widely from the cervical to the abdominal area. Furthermore, immunohistochemistry allows detecting twice more skip nodal metastasis than routine histopathology. Skipping of regional lymph node stations, as detected by routine histology, occurred in more than 35% of our patients, thus questioning the clinical relevance of such a concept for oesophageal adenocarcinoma [24].
5 Conclusions
Ours results suggest that extended 2-field lymphadenectomy is an important component of the surgical treatment of patients with adenocarcinoma of the oesophagus. It increases the likelihood of proper staging and affects patient outcome, while it does not enhance the operative mortality. However, extended lymphadenectomy increases non-fatal morbidity, especially the incidence of pulmonary complications and the need for blood transfusion. En-bloc oesophagectomy with extended 2-field lymphadenectomy appears as a reasonable compromise between extended surgical procedures with 3-field lymphadenectomy and minimally invasive resections through a transhiatal approach.
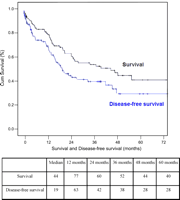
Survival and disease-free survival for all patients (Kaplan Meier method).
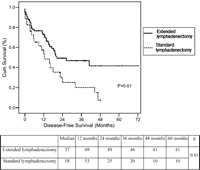
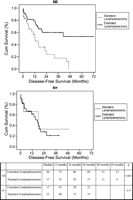
Disease-free survival according to pN status and lymphadenectomy type (Log Rank test, Kaplan Meier method). In N0 patients, extended lymphadenectomy was associated with an improved disease-free survival when compared to standard lymphadenectomy (median of survival: 44 vs. 17 months; P=0.001), whereas no significant difference in median survival was observed in N+ patients (17 vs. 22 months; P=0.9).
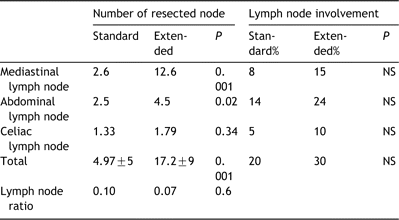
Number of resected node and ratio of metastases lymph node involvement
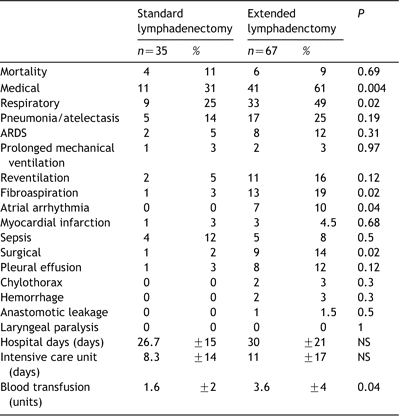
Length of stay, blood transfusion, mortality and morbidity in each group of lymphadenectomy
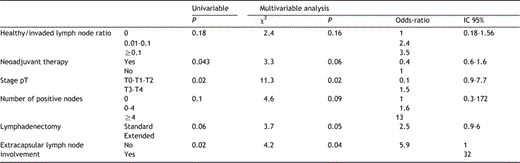
Predictive factors of recurrence affecting disease free-survival: Uni and multivariable analysis (Cox regression)
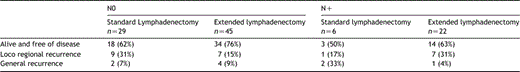
Free of disease and recurrence in each group of lymphadenectomy according to lymph node status
Appendix A. Conference discussion
Dr Walter Klepetko (Vienna, Austria): I would like to start off the discussion with two questions. I was struck by the low number of lymph nodes that you reported in the standard lymphadenectomy group. The mean number of 3 or 4 lymph nodes is really low. Can you comment on that?
Dr D'Journo: Standard lymphadenectomy was not based on the intent to improve survival but to minimize complications. Our study was not based on historical comparison but was based on the comparison of two groups concurrently treated in the same institutions. In our institution four senior surgeons routinely perform esophagectomy, and among the four senior surgeons, two performed extended lymphadenectomy and the two others performed standard lymphadenectomy. On the one hand, standard lymphadenectomy was performed to minimize complications without the intent to improve outcome or to increase the number of resected lymph nodes. Conversely, extended lymphadenectomy was performed to improve staging and to improve the outcome of patients with the strong will to increase the number of resected lymph nodes. So this is the difference between the number of resected lymph nodes.
Dr K. Moghissi (Goole, UK): I have a bit of a problem with your classification in that adenocarcinoma in particular can be of two or three varieties. One is that you have adenocarcinoma of the lower esophagus, at the cardia. I can understand that you do a lymphadenectomy on this one, and if you did the extended lymphadenectomy, you probably would not get much difference than with just the regional one, nonextended. In the second group is adenocarcinoma in Barrett's esophagus, which can go very, very high, and I did not think that you had made a differentiation between these two types of adenocarcinoma: one which is a localized one and one which extends. Added to these, there are skip lesions in adenocarcinoma, and I do not think that you mentioned that. Can you clarify that for me?
Dr D'Journo: It is difficult for me to explain that. Your classification is right, but in the compared two groups, there is the same rate of the two varieties of tumours.
Dr P. Thomas (Marseille, France): May I answer for Dr D'Journo? Because of historical reasons, in Marseille the majority of our patients presents with Barrett's esophagus. We have no type III or type IV tumours according to the Siewert classification, because these patients are usually referred to abdominal surgeons. The majority of our patients have adenocarcinoma of the esophagus located in the lower part of the esophagus in relation with a Barrett's esophagus in almost all cases.
Dr W. Klepetko (Vienna, Austria): I would like to also ask you about the use of preoperative induction chemotherapy. Was there a timely difference between the most recent cohort in terms that those patients might have had a higher percentage of preoperative chemotherapies versus earlier times?
Dr D'Journo: There is the same rate of neoadjuvant therapy in the two groups, about 53%.
Dr Klepetko: But was there any difference over the time?
Dr D'Journo: No, there is no difference.
Dr Klepetko: So you had the same protocol throughout the whole period of the investigation?
Dr D'Journo: Yes, the same protocol for all patients.
Dr M. Dusmet (London, United Kingdom): What surprised me in your presentation is that, for example, for lung resection it has been shown time and time again that systematic nodal dissection does not have a significant impact on morbidity, and yet here, doing a very similar lymph node dissection in the upper mediastinum, you have this tremendous impact on morbidity. Do you have any explanation for this?
Dr D'Journo: For the increasing morbidity?
Dr Dusmet: Yes.
Dr D'Journo: In the extended lymphadenectomy there is an increase in respiratory complications. Probably two explanations can be postulated. The first is related to the surgical injuries caused by the extensive lymphadenectomy into the mediastinum and in particular on the vascularization of the airway, especially for bronchial arteries. These injuries lead to the development of an inflammatory response into the airway. The second explanation is probably related to the interruption of the lymphatic backflow caused by the lymphatic dissection and leading to development of pulmonary oedema by the increasing of the pulmonary permeability and the increasing of the extra-vascular lung water.
Presented at the joint 18th Annual Meeting of the European Association for Cardio-thoracic Surgery and the 12th Annual Meeting of the European Society of Thoracic Surgeons, Leipzig, Germany, September 12–15, 2004.




