-
PDF
- Split View
-
Views
-
Cite
Cite
Jacek Gawrychowski, Krzysztof Bruliński, Eugeniusz Malinowski, Bolesław Papla, Prognosis and survival after radical resection of primary adenosquamous lung carcinoma, European Journal of Cardio-Thoracic Surgery, Volume 27, Issue 4, April 2005, Pages 686–692, https://doi.org/10.1016/j.ejcts.2004.12.030
Close - Share Icon Share
Abstract
Objective: In order to evaluate the follow-up study of surgical treatment for primary adenosquamous lung carcinoma (ASC) we specified prognostic criteria, also in comparison with primary adenocarcinoma (AC). Methods: The study group consisted of 96 patients discharged between 1990 and 1999 after radical surgical treatment for ASC—80 (83%) men and 16 (17%) women aged 34–73, mean 56 years. Consequently, we evaluated 252 patients operated during the same time period for primary AC. Results: Apart from grading, we did not find any significant differences between both ASC and AC groups of patients. Among the 96 patients operated radically for ASC median overall survival (OS) was 20 months. The cumulative postoperative survival rates at 5 and 10 years were 25.4 and 19.2%, respectively. By comparison, median OS for 252 patients with AC, discharged after surgical treatment in the same period, was 28.5 months and the cumulative postoperative survival rates at 5 and 10 years were 42.5 and 39.1%, respectively (P=0.006). At pathologic stages IA, the cumulative survival rate at 5 years was 63.3% for patients with ASC as compared with 72.1% for patients with AC (P=0.330). However, out of IB stage patients treated surgically for ASC 31.8% survived 5 years in comparison with 56.3% operated for AC (P=0.017). Study of survival rates did not differ significantly between ASC and AC patients at stage IIA (P=0.824) and stage IIB (P=0.217), respectively. Univariate analysis revealed that six factors of tumor size, T status, N status, as well visceral pleura involvement, tumor localization (central vs. peripheral) and tumor structure were significantly associated with the survival rate according to these variables. Multivariate analysis using Cox's proportional hazards model indicated that T factor, nodal involvement and one of the tumor components predominating were significant factors associated with the postoperative survival of patients with ASC. Conclusions: Our findings indicate that in patients after radical operation for ASC, predominance for one of the histopathological components (adenous or squamous) within primary tumor is attended by worst prognosis. Our study confirmed also that the prognosis of ASC of the lung was poorer than that of primary AC. Lack of generally accepted diagnostic criteria and unclear prognosis, even in the pathologic stage I suggest that there is a need for prospective studies in this respect.
1 Introduction
Adenosquamous cancers (ASC) are detected in 0.4–4.2% of patients treated for primary lung cancers [1–6]. Clinicopathological characteristics of ASC are still not clear. Some authors indicate that the properties of cancers are more similar to those of adenocarcinomas rather than squamous cancers [7]. Others believe that biological properties of ASC and behavior of both components of ASC are similar in most cases [8,9]. However, as ASC are rare conditions, the data are diversified, controversial and often conflicting. It is little wonder that a consensus about prognosing late results of treatment for ASC cannot be easily reached.
Basing on light microscopic examinations, McDowell demonstrated that many of squamous cancers (SQLC) and primary adenocarcinomas (AC) have obvious tendency to dichotomous differentiation in adenous or squamous direction. He believes that at least 46% of all lung cancers have in fact the character of adeno-squamous tumors [10].
Heterogenic nature of ASC makes it often impossible to determine their pathological characteristics. Consequently, proper selection of the patients in order to develop a suitable monitored surgical treatment plan is very difficult if not precluded. Therefore, there is a need for more precise criteria, both diagnostic and prognostic, for the purpose of limiting the discrepancies between expected and actual clinical course and prognosis in patients operated for ASC [7,9].
2 Material and methods
This is a retrospective study of 96 patients operated radically between 1990 and 1999 for primary ASC. They were 4.5% of 2132 patients operated radically at that time for various kinds of primary malignant lung tumors. Consequently, we evaluated 252 patients operated during the same time period for primary adenocarcinoma (AC) and carried out comparison with ASC group. Patients were identified basing on operation protocols and histopathological findings regarding primary tumors, removed lymph nodes and operation margins, in accordance with the proposal made in 1997 by American Joint Committee on Cancer (AJCC) and International Union on Cancer (UICC) [11]. The map and terminology relative to pulmonary or mediastinal groups of lymph nodes were in accordance with the criteria accepted in 1986 by UICC [12] and Naruke classification of 1978 [13].
Diagnostic management consisted of routine chest radiology, p-a and lateral projections, computer tomography of the chest and upper abdomen, abdomen USG and bronchofiberoscopy. If chest CT revealed an increase in mediastinal lymph nodes (≫1cm), mediastinoscopy was done (if the alterations in lymph nodes were located in aortic-pulmonary window, videothoracoscopy was chosen). In such a situation the surgery was only performed in the cases where histological assessment of mediastinal lymph node tissue was negative.
Radical resection meant a management (lobectomy or pneumonectomy) enabling to remove all macroscopic lesions (including lymph nodes) with resection margins free of any malignant cells by microscopic examination.
We considered systematic lymph node sampling as a routine procedure in all patients in whom lobectomy or pneumonectomy was performed. During left thoracotomy we routinely removed aortopulmonary region and subcarinal lymph nodes (5–10 as well as 4). When right thoracotomy was performed, clearing of the right paratracheal and subcarinal lymphatic tissue was done (2–4 and 7–10).
Upon their discharge from hospital, the patients were seen at least four times a year, i.e. every 3 months, within the first 5 years, and every half a year thereafter. Physical examinations, basic routine X-ray examinations and abdominal USG were performed. Computed tomography of the chest (twice a year), and if necessary abdomen or head was added. In order to confirm the dates of deaths, if any, the data were verified at Regional Station of Public Records Department for Telecommunication and Computer Science, Ministry of Home Affairs and Administration in Katowice, and at Medical Center of Analyses and Statistics, Silesian Center of Public Health in Katowice.
Local recurrence was defined as tumor relapse within the same lung or bronchial stump. Regional recurrence meant tumor relapse within mediastinal lymph nodes in spite of their dissection during primary operation, or metastases in mediastinal lymph nodes on the contra-lateral side and/or supraclavicular region. Remote metastases (or dissemination) were tumor lesions in other organs (outside the lung under treatment) or remote lymph nodes.
2.1 Histopathological analysis
Morphological diagnosis of both primary lung ASC and AC were established basing on the criteria accepted in 1999 by World Health Organization and International Association for the Study of Lung Cancer (WHO/IASLC) [14].
We defined the cancers as adenosquamous type if elements of both squamous cancer and adenocarcinoma could be seen by light microscope in not less than 10% percentage. However, if the percentage of one of the tumor components was smaller than 10%, such cancer was defined in accordance with predominating texture and the remark ‘…with elements…’ was added. Such patients were not included in the study. If each of the components was present in 40–60%, such situation was considered as balance.
Histological slides were assessed using archive material obtained from paraffin-embedded tumor specimens, including the widest cross-sections, coming from patients operated for 96 ASC and 252 AC. Paraffin blocks were cut on a microtome into sections about 5μm thick, then stained with hematoxylin and eosin. All histological slides were verified separately by two pathomorphologists. We used microscopic examination to find out both histologic structure and malignancy grade (G) of the tumor as well as the type of the adenocarcinoma which was one of the tumor components. Histologic malignancy grades were determined in accordance with traditional criteria described by Broders [15].
2.2 Methods of statistical analysis
Continuous variables with normal distribution (Gaussian decomposition) were compared using parametric tests, i.e. Student's t-test for two trials and variance analysis for more trials. Non-parametric U Mann–Whitney test or Kruskal–Wallis rank variance analysis were used for trials showing other than normal distribution or homogenous variance. Interrelationship of nominal variables was verified using Parson's independence chi-square test and shi-square test basing on the highest reliability function (if small groups were analyzed). Precise Fisher's test was also used. Survival curves were determined by Kaplan–Meier method. Kaplan–Meier curves for two trials were compared using F.Cox's test. Chi-square increment statistics were used for more trials. Simultaneous effect of many factors on Kaplan–Meier curve was measured by Cox's multiple regression analysis using Cox's proportional risk model. The patients who died from other than cancer causes, or from unknown causes, were included in the group of censored observations.
3 Results
Among the group of 100 patients operated for ASC, 4 (death rate 4.0%) died during immediate postoperative period (within 30 days). Two (2.8%) patients died after lobectomy or bilobectomy, and 2 (7.1%) after pneumonectomy. Clinical and postmortem examinations revealed the following causes of death: hemorrhage during early postoperative period due to disseminated intravascular clotting syndrome (DIC) (1×), respiratory-circulatory failure (2×), single lung pneumonia (1×).
Finally, the study group consisted of 96 patients undergoing surgical treatment for primary adeno-squamous cancers. There were 80 (83.3%) men and 16 (16.7%) women aged 34–73, mean 56 years. Lobectomy was done in 70 (72.9%) operated patients, and pneumonectomy in 26 (27.1%). Twenty-three (24%) cases were in pathologic stage IA, 25 (26%) in stage IB, 8 (8.3%) in stage IIA, 15 (15.6%) in stage IIB, 22 (22.9%) in stage IIIA and 3 (3.1%) in stage IV. (Table 1). Apart from grading, we did not find any significant differences between both ASC and AC groups of patients.
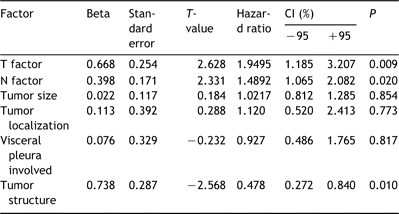
Among the 96 patients operated for ASC median overall survival (OS) was 20 months. The cumulative postoperative survival rates at 5 and 10 years were 25.4 and 19.2%, respectively. By comparison, median OS for 252 patients with AC, discharged after surgical treatment in the same period, was 28.5 months and the cumulative postoperative survival rates at 5 and 10 years were 42.5 and 39.1%, respectively (Fig. 1). A significant difference (P=0.006) between these two groups was seen.
The association of various prognostic factors with postoperative survival was examined using univariate analysis. As summarized in Table 2, six factors of tumor size, T status, N status, as well visceral pleura involvement, tumor localization (central vs. peripheral) and tumor structure were significantly associated with the survival rate according to these variables.
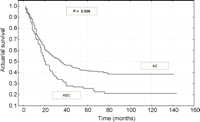
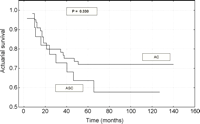
At pathologic stages IA, the cumulative survival rate at 5 years was 63.3% for patients with ASC compared with 72.1% for patients with AC (P=0.330) (Fig. 2). However, out of IB stage patients treated surgically for ASC 31.8% survived 5 years in comparison with 56.3% operated for AC (P=0.017) (Fig. 3). No significant differences between these two groups of patients were apparent for visceral pleura involvement and/or tumor size greater than 5cm (P=0.785). These factors were identified among IB stage patients operated for ASC and AC, in 13/25 (52%) and 43/78 (55.1%), respectively.
The cumulative postoperative survival rate for stage IIA ASC patients at 5 years was 26%. However, among those operated for stage IIB or IIIA no patient survived 5 years. In the group of 15 patients with stage IIB, 2 (14.3%) survived 3 years, and in the group of 22 patients with stage IIIA only 1 (4.5%) survived 3 years. Next, at pathologic stages IIA and IIB AC patients the cumulative survival rates at 5 years were 30 and 23.9%, respectively. None of stage IIIA patients with AC survived 5 years. This study of survival rates did not differ significantly between ASC and AC patients at stage IIA (P=0.824) and stage IIB (P=0.217), respectively.
Patients with structural balance (40–60%) between the two components of the tumor had significantly better prognosis than those with one of the components predominating (60–90%) (Pos=0.013) (Fig. 4). Median OS for patients with tumors showing adenous texture predominance were 16 months whereas for those with squamous texture predominance were 15. On the other hand, median OS for patients with ASC structural balance were 36 months. In the group of patients with tumor structural balance the cumulative survival rates at 5 and 10 years were 49%. In the group of operated with adenous or squamous component predominance cumulative survival rates at 5 years were 14.3 and 7%, respectively. None of them survived 10 years. However, histopathologic type of the adenous component had no effect on the surgical treatment late results (P=0.313).
Table 3 shows the pattern of recurrent disease for all patients who died for ASC as well as AC. We did not find any significant differences.
Multivariate analysis using Cox's proportional hazards model indicated that T factor, nodal involvement and one of the tumor components predominating were significant factors associated with the postoperative survival of patients with ASC (Table 4).
4 Discussion
The 96 patients presented in this study are the largest so far group undergoing surgical treatment for ASC. Similar clinicopathologic characteristics of both ASC and AC groups of patients, enable to increase inareliable way our knowledge about this uncommon lung neoplasm.
The lack of generally accepted criteria makes prognosis controversial. In our study, ASC is defined as a primary tumor containing at least 10% of the minor texture. Japan Lung Cancer Society permits to diagnose ASC when either adenous or squamous component of the primary tumor covers at least 20% of the minor texture. Otherwise, the tumor is defined as having the major texture ‘with focuses of…’ [16]. According to WHO, adenosquamous cancers (ASC) consist of both squamous and adenous elements, but their proportion is not given [17].
The outcome of our surgically treated ASC patients was poorer than that of AC. These results confirmed the observations made by others, showing that late results of surgical treatment for ASC were worse than for other types of lung cancer [1–4,18–20]. Analysis of the literature indicates that among the patients operated for primary non-small cell lung cancers, those with ASC have the worst prognosis [1–4]. Naruke showed additionally that even among the patients operated for small cell cancers, 5-year survival rate was twice as high as after operation for ASC (36.7 vs. 18.9%) with TNM stage relatively the same. Moreover, Naruke,Nakagawa and Takamori reported that the worse results of surgical treatment for ASC, as compared to other non-small cell lung cancers, were observed in all patients regardless of clinicopathological stages [1,3,18]. These results do not agree with those presented by Hsia who found no statistically significant differences in 5-year survival rates between patients operated for ASC and those operated for AC or squamous lung cancer (SQLC) [5].
Our analysis of survival according to pathologic stage of ASC was not explicit. This analysis showed at pathologic stage IA similar outcome for both ASC and AC patients. On the other hand we have showed that, IB stage ASC patients showed poorer outcome than those with IB stage AC patients. Even after censoring cases involving patients with tumor greater than 5cm and/or visceral pleura infiltration the outcome of ASC patients was poorer than that of AC (P=0.032). In these groups, the cumulative survival rates at 5 years were 58.8 and 23.8%, respectively for ASC and AC cases. In this place we would like to stress, that our patients operated for ASC did not show significantly greater progression of pathologic primary tumor, N factor as well as pleural invasion in comparison with those treated surgically for AC.
We conclude on the basis of Kaplan–Meier curves and Cox's multivariate analysis that patients with a balance between the two ASC components have better prognosis than those with one of the components predominating. These results are different than those presented by Takamori[3], probably due to our definition of structural balance (each tissue component being present at the level of 40–60%) and larger number of patients undergoing statistical analysis. Takamori and Shimizu showed more frequent, but not statistically significant, metastases in regional lymph nodes of the lung and predominant adenous component within primary tumor [3,4].
Interestingly, among our study tumors no adenous component was the texture of bronchio-alveolar cancer. We were not able to compare these observations with the literature. Neither could we find a confirmation that adenous component of ASC had no significant effect on late results of the surgical treatment.
Our findings indicate that in patients after radical operation for ASC predominance for one of the histopathological components (adenous or squamous) within primary tumor is attended by worst prognosis. Our study confirmed also that the prognosis of ASC of the lung was poorer than of primary AC. Lack of generally accepted diagnostic criteria and unclear prognosis, even in the pathologic stage I, suggest that there is a need for prospective studies in this respect.
Survival curves after resection of 96 patients with adenosquamous carcinoma and 252 with adenocarcinoma.
Survival curves after resection of patients with stage IA (T1N0M0) adenosquamous carcinoma and adenocarcinoma.
Survival curves after resection of patients with stage IB (T2N0M0) adenosquamous carcinoma and adenocarcinoma.

Survival curves after resection of patients with adenosquamous carcinoma depending on tumor structure (component predominance).
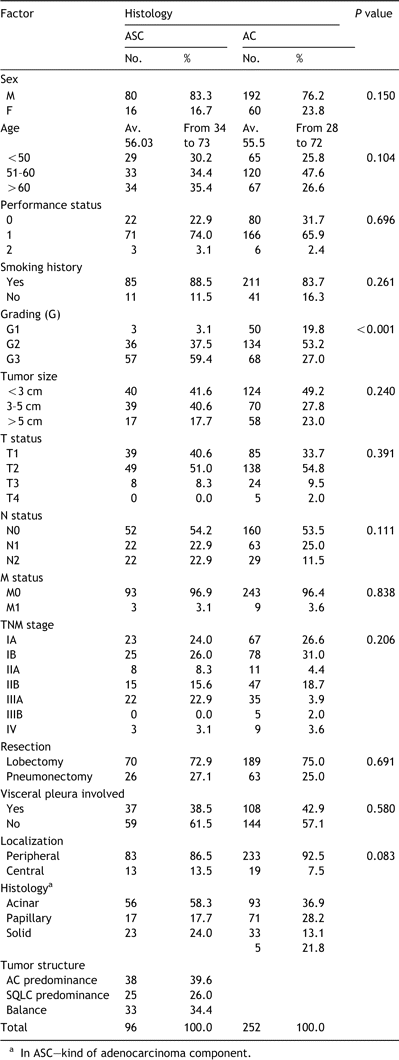
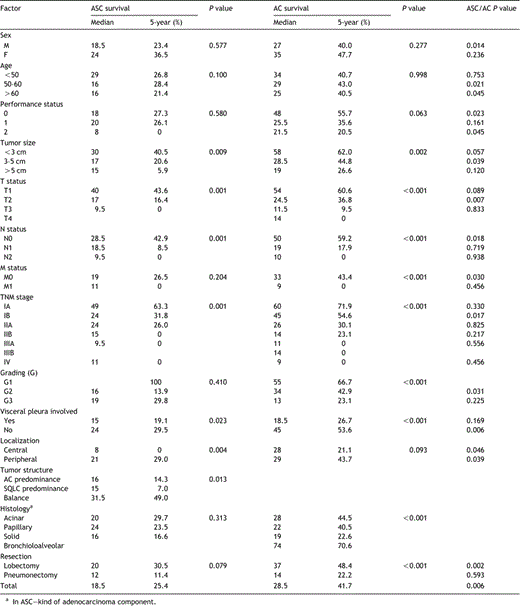
Univariate analysis of factors associated with postoperative survival of patients with ASC and AC
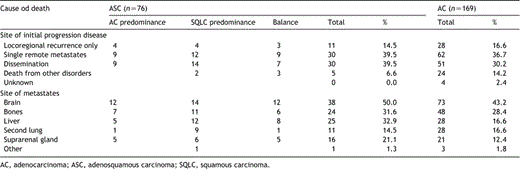
Cause of death and site of initial progression disease in the groups of ASC and AC patients