-
PDF
- Split View
-
Views
-
Cite
Cite
Regina Bökenkamp, Nico A. Blom, Daniel De Wolf, Kristine Francois, Jaap Ottenkamp, Mark G. Hazekamp, Intraoperative stenting of pulmonary arteries, European Journal of Cardio-Thoracic Surgery, Volume 27, Issue 4, April 2005, Pages 544–547, https://doi.org/10.1016/j.ejcts.2005.01.013
Close - Share Icon Share
Abstract
Objective: The surgical treatment of pulmonary artery stenoses in small children with complex cardiac lesions can be technically difficult. A hybrid-approach combining corrective surgery and intraoperative stent placement may be complementary in these patients. Methods: Descriptive study in 11 small children (age: one week to 12 years, median of 12 months, weight: 2.5–20kg) after previous cardiac surgery. Intraoperative stenting of pulmonary arteries was performed involving paediatric cardiologist and cardiac surgeon. Stenting was combined with repair of pulmonary atresia (n=2), right ventricular outflow and pulmonary artery reconstruction (n=3), unifocalisation of pulmonary arteries (n=1), revision of distal anastomosis of RV-PA-conduit after truncus repair (n=1), revision of distal anastomosis of RV-PA-conduit after AVSD/Fallot repair (n=1), aortic arch patch reconstruction after anatomical correction for transposition of the great arteries (n=1), bidirectional cavopulmonary anastomosis after Norwood I operation for hypoplastic left heart syndrome, (n=1) retrieval of a dislodged stent from the left pulmonary artery (n=1). In seven patients stenting was planned electively while in four patients it took place on an emergency base. Results: No complications occurred during stent implantation. One patient died three weeks postoperatively from diffuse bleeding due to a coagulation disorder. Ten patients left hospital after the surgical intervention with concomitant stent implantation. Stent re-dilatation was necessary in 4 patients 2–24 months postoperatively. After a median follow-up of 15 months ranging from 3 weeks to 7.5 years all stents were patent as diagnosed by angiography in 6 patients and by colour-Doppler in all other patients. One year after stent placement one stent was removed and another surgically opened during re-operation for conduit replacement in the smallest patient from this series. There was one late death during operative right ventricular outflow-tract reconstruction after initial stent placement. Conclusions: With intraoperative stent placement surgically difficult patch augmentation of small and fragile pulmonary vessels during repair of complex cardiac lesions can be avoided. Stents recruit pulmonary vessels and keep them open and amenable to future percutaneous or surgical interventions.
1 Introduction
Stenting of stenosed pulmonary arteries has become an accepted catheter intervention in older children [1]. In infants and small children, stent placement may interfere in a negative way with pulmonary artery growth and is therefore, relatively contraindicated [2]. However, also in small infants a subset of patients may benefit from a hybrid-approach combining corrective surgery and intraoperative stent placement [1,3]. This approach was used by us to rescue stenosed pulmonary arteries in 11 small children, either during elective repair of cardiac defects or on an emergency base in the direct postoperative phase.
2 Patients and methods
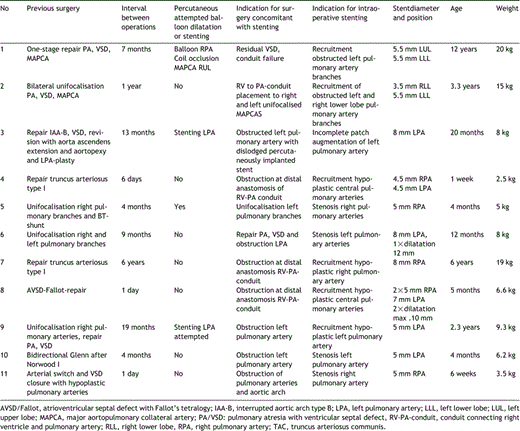
In recent years it has become our policy to perform intraoperative stenting in two clinical situations: (1) Elective stenting in patients requiring concomitant cardiac surgery and/or have surgically un-accessible stenoses in lobar pulmonary branches. (2) Urgent/rescue stenting in patients direct postoperatively or following failure of percutaneous stenting. Our experience includes 11 consecutive patients with severe pulmonary artery stenoses after previous surgery (Table 1). Their age ranged from one week to 12 years with a median of 12 months. The median weight was 8kg (range 2.1–20kg). The anatomical diagnoses of our patients included pulmonary atresia with VSD +/− MAPCA's (5), truncus arteriosus (2), aortic arch interruption with VSD (1), AVSD/ Fallot (1), TGA + VSD with hypoplastic right PA (1), hypoplastic left heart syndrome (1). Intraoperative stent placement was performed electively in 7 patients (9 months +/− 24.3 SD) after previous surgery. In 4 patients stenting was performed on an urgent base (1, 1 and 6 days after corrective surgery, 1 day after attempted percutaneous stenting) Intraoperative stent placement was combined with repair of pulmonary atresia (2), right ventricular outflow and pulmonary artery reconstruction (3), unifocalisation of pulmonary arteries (1), revision of truncus repair (1), revision of AVSD/Fallot repair (1), retrieval of a dislodged stent from the left pulmonary artery (1), aortic arch patch reconstruction after anatomical correction for transposition of the great arteries (n=1), bidirectional cavopulmonary anastomosis after Norwood I operation for hypoplastic left heart syndrome (n=1). In all but one patient preoperative angiographic data were available to select the exact stent dimensions. Intraoperative stenting was performed under direct vision during cardiopulmonary bypass. According to surgeons choice stenting was done with an arrested heart and short periods of low flow or circulatory arrest. In most of the cases a balloon-catheter carrying the stent was positioned over the wire, using standard exchange wires. In one case (no. 5) fluoroscopy was used during stent implantation on cardiopulmonary bypass. Care was taken to avoid obstruction of the more distal branches. In some cases the position of the stent was secured with surgical sutures. Inflation and dilatation of the stents was carried out by the paediatric cardiologist according to manufacturers instructions. In total 16 stents were implanted in the right or left pulmonary artery or lobar branches. The median stent diameter was 5.0mm at implantation ranging from 3.5to 8mm with a median of 5mm. Different types of stents were used. If possible the Palmaz P 188R (Johnson and Johnson) or in the more recent cases PalmazR Genesis, Cordis, Miami Lakes, FL, USA, were implanted in the left or right pulmonary artery. In case of extreme hypoplasia of the central pulmonary arteries or in the more peripheral position coronary artery stents (from Medtronic AVE, Minneapolis, MN, USA, Cordis, Miami Lakes, FL, USA) were used. In all patients antiplatelet treatment was started at the day after the operation with acetylic salicylic acid 2–5mg/kg/day.
3 Results
No complications occurred during stent implantation. Individual courses are summarized in Fig. 1. Ten patients left hospital after the surgical intervention with concomitant stent implantation. Patient 11 died three weeks postoperatively from diffuse bleeding due to a coagulation disorder. A dramatic improvement of right ventricular function, facilitating weaning from inotropic support and artificial ventilation within few days after stent placement was observed in the 3 patients (4, 8,11) stented on an emergency basis (Fig. 2). After a median follow-up time of 15 months ranging from 3 weeks to 7.5 years all stents were patent as diagnosed by angiography in 6 patients (Figs. 3 and 4) and by colour-Doppler in all the other patients. In four patients the stents were re-dilated. Patient 4, the smallest patient from our series, underwent re-dilatation of the stents and re-operation. More than one year after stenting he was re-operated because of fibrotic obstruction at the anastomosis between conduit and pulmonary artery bifurcation. One stent was removed and the other stent was opened surgically to allow for wider conduit implantation. The only late death occurred in patient 6 during operative right ventricular outflow-tract reconstruction unrelated to earlier stent placement. In patient 1 the xenograft used for RVOT reconstruction had to be explanted because of graft-failure and was replaced by a homograft.
4 Discussion
Percutaneous stenting of stenosed pulmonary arteries has become the therapy of choice and a less invasive alternative to patch angioplasty in older children. [4,5,6,7] In small children limited vascular access, the need of relatively large sheaths and the potential impairment of further vessel growth by stents are points of concern when stenting of pulmonary artery stenoses is considered [2]. However, for a highly selected group of patients reported in this paper, we suggest a hybrid approach combining corrective surgery and intraoperative stent placement.
The development of interventional techniques avoiding long sheaths for stent implantation [8] and the possibility of transhepatic and translumbar approach for patients with iliofemoral obstruction [9,10] has made interventional stent implantation feasible for smaller patients. All patients described in the present paper, needed concomitant surgery or suffered from haemodynamic instability directly after cardiac surgery. Therefore, the combination of redo surgery and intraoperative stent placement was indicated in these patients. With intraoperative stent placement surgically difficult patch augmentation of small and fragile vessels is avoided. As fluoroscopic control is usually not available in the operating room, some concern about accurate stent positioning may arise. However, no stent embolisations, pulmonary vessel damage at the distal end of the stent, or obstruction of contra lateral pulmonary branches occurred in our patients. Nevertheless combined cardiac catheter lab/surgery suites designed to meet the requirements for paediatric applications are desirable. These new developments will facilitate the introduction of other hybrid procedures in which fluoroscopy cannot be avoided.
Re-dilatation of stents because of incomplete dilatation of the distal end of the stents was necessary in one patient (no. 8) two months postoperatively. Re-dilatation of stents during long-term follow-up was performed in four patients. In patient 8 we were able to increase the stent diameter from 5 and 7mm to 10mm 24 months after intraoperative stenting. With direct operative stent placement iliofemoral vessels were left untouched and remained patent for future percutaneous procedures. Early postoperative catheter-interventions although possible [11], could be avoided with all procedure related risks. Intraoperative stenting in some highly selected small infants remains sometimes the only option to reopen severely stenotic vessels [3,12–15]. Advances in stent-technology have led to smaller stents (PalmazR Genesis, Cordis, Miami Lakes, FL, USA) with the option for later percutaneous dilatation of the stented vessel. These newly developed stents, which were not available when the first implantations in this study took place, may help to prevent re-operations due to patient's growth. Nowadays we would prefer to use this type of stents in small infants.
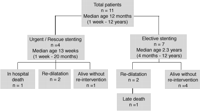
Flowchart illustrating the treatment and clinical course of our patients.
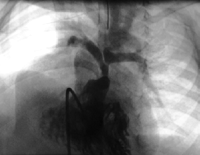
Preoperative right ventricular angiogram of patient. Hypoplastic central pulmonary arteries.
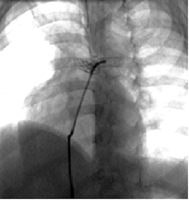
Position of the stents in the pulmonary arteries 2 months after implantation in the same patient.
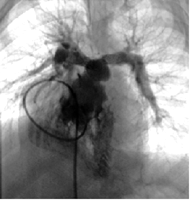
Angiogram of the stented pulmonary arteries 2 months after stent implantation in the same patient.
Presented at the joint 18th Annual Meeting of the European Association for Cardio-thoracic Surgery and the 12th Annual Meeting of the European Society of Thoracic Surgeons, Leipzig, Germany, September 12–15, 2004.




