-
PDF
- Split View
-
Views
-
Cite
Cite
Gianluca Guggino, Christophe Doddoli, Fabrice Barlesi, Pablo Acri, Bruno Chetaille, Pascal Thomas, Roger Giudicelli, Pierre Fuentes, Completion pneumonectomy in cancer patients: experience with 55 cases, European Journal of Cardio-Thoracic Surgery, Volume 25, Issue 3, March 2004, Pages 449–455, https://doi.org/10.1016/j.ejcts.2003.12.002
Close - Share Icon Share
Abstract
Objective: Analysis of a single institution experience with completion pneumonectomy. Methods: From 1989 to 2002, 55 consecutive cancer patients received completion pneumonectomy (mean age 62 years; 25–79). Indications were bronchogenic carcinoma in 38 patients (4 first cancers, 8 recurrent cancers, 26 second cancers), lung metastases in three (one each from breast cancer, colorectal neoplasm and lung cancer), lung sarcoma in one, and miscellaneous non-malignant conditions in 13 patients having been surgically treated for a non-small cell lung cancer previously (bronchopleural fistula in 4, radionecrosis in 3, aspergilloma in 2, pachypleura in 1, massive hemoptysis in 1 and pneumonia in 2). Before completion pneumonectomy, 50 patients had had a lobectomy, three a bilobectomy, and two lesser resections. The mean interval between the two procedures was 51 months for the whole group (1–469), 60 months for lung cancer (12–469), 43 months for pulmonary metastases (21–59) and 29 months for non-malignant disorders (1–126). Results: There were 35 right (64%) and 20 left (36%) resections. The surgical approaches were a posterolateral thoracotomy in 50 cases (91%) and a lateral thoracotomy in five cases (9%). Intrapericardial route was used in 49 patients (89%). Five patients had an extended resection (2 chest wall, 1 diaphragm, 1 subclavian artery and 1 superior vena cava). Operative mortality was 16.4% (n=9): 11.9% for malignant disease (n=5) and 30.8% for benign disease (n=4). Operative mortality was 20% for right completion pneumonectomies (n=7) and 10% for left-sided procedures (n=2). Twenty-three patients (42%) experienced non-fatal major complications. Actuarial 3- and 5-year survival rates from the time of completion pneumonectomy were 48.4 and 35.2% for the entire group. Three- and five-year survival for patients with bronchogenic carcinoma were 56.9 and 43.4%, respectively. Conclusions: These results suggest that completion pneumonectomy in the setting of lung malignancies can be done with an operative risk similar to the one reported for standard pneumonectomy. In contrast, in cancer patients, completion pneumonectomy for inflammatory disorders is a very high-risk procedure.
1 Introduction
Completion pneumonectomy is defined as the operative procedure in which the remainder of the lung is removed, after one or more ipsilateral resections of lung parenchyma. The indications for completion pneumonectomy are rather rare and include both benign and malignant diseases [1]. Completion pneumonectomy for benign conditions may also be required in cancer patients when chronic suppuration develops in a destroyed lung as a consequence of radiotherapy, or for complications of lung sparing cancer operations. Indications for malignancies include primary bronchogenic cancer occurring after lobectomy for benign disease, invaded bronchial margins after lobectomy, local recurrence, metachronous bronchogenic cancer, and relapsing metastases after primary cancer treatment.
The purpose of this study was to evaluate our experience with 55 consecutive completion pneumonectomies performed in cancer patients, with the aim of identifying factors that influence morbidity, immediate results and long-term survival.
2 Materials and methods
From 1989 to 2002, 696 consecutive patients received pneumonectomy at a single academic institution, among whom 55 had completion pneumonectomies (8%).
2.1 Definitions and inclusion criteria
The primary operation was defined as the first ipsilateral operation in which lung tissue was removed. Hospital mortality included all intraoperative and postoperative deaths during hospitalization or within 30 days after operation for patients who were discharged earlier. Tumor histology was classified according to the 2000 version of the Word Health Organization histologic typing of lung tumors. All lung cancers were staged with the 1997 UICC/AJCC TNM classification system.
Recurrent lung cancer and second primary lung cancer were discriminated using the following criteria: a second primary tumor was of different histology or if histology was the same, the disease-free interval between cancers was at least 2 years or the second cancer was in the different lobe but with no cancer in common lymphatic or extrapulmonary metastasis at the time of diagnosis.
Primary lung cancer was the indication for completion pneumonectomy if lung cancer had not been the indication for the primary operation or if completion pneumonectomy was performed during the same hospitalization as the primary operation because of a positive resection margin. The criteria for completion pneumonectomy with pulmonary metastases included reliable control of the primary tumor, absence of other sites of distant metastasis, no contralateral pulmonary metastases and reasonable disease-free interval between the most recent metastasectomy and the completion pneumonectomy.
2.2 Patients
There were 39 male and 16 female patients. Mean age at the time of primary operation was 57 years (14–74). Mean age at the time of completion pneumonectomy was 62 years (25–79). Preoperative evaluation included assessment of the respiratory, cardiac and renal functions. The patients were considered eligible for completion pneumonectomy if they had a predicted post-operative forced expiratory volume in 1 s (FEV1) of more than 1 l/s, estimated with spirometry exam and lung perfusion scan.
In the group with malignant disease all patients have been re-staged by total body computed tomographic scan, technetium bone scan and abdominal ultrasound. Mediastinoscopy was performed when computed tomographic scan findings showed mediastinal node involvement with a mean diameter wider than 1 cm. Preoperative bronchoscopic examination was performed for all patients.
The initial lung resection had been performed for malignant disease in 52 patients (95%), and benign lung disease in three (5%). The indications for primary operations were primary bronchogenic cancer in 46 patients (21 squamous cell carcinoma, 17 adenocarcinoma, 4 undifferentiated carcinoma, 2 bronchioloalveolar carcinoma, 2 adenosquamous carcinoma), lung metastasis in four (metastases of colorectal carcinoma, breast carcinoma, renal cell carcinoma, and endometrial tumor) and other malignancies in two (hemangiopericytoma and Hodgkin's lymphoma). Benign lung diseases included pulmonary tuberculosis in two cases and emphysema in one.
The 55 first surgical procedures consisted of 15 right upper lobectomies, 14 left upper lobectomies, 9 right lower lobectomies, 5 left lower lobectomies, 3 bilobectomies, 1 middle lobectomy, 1 lingulectomy, 2 sleeve lobectomies, 2 right upper lobectomies with wedge resection, 1 right lower lobectomy with wedge resection, 1 right lower lobectomy with segmentectomy, and 1 wedge with chest wall resection.
The postoperative staging of the 46 lung cancer was stage I in 33 (10 T1 N0 M0 and 23 T2 N0 M0), stage II in 4 (1 T1 N1 M0 and 3 T2 N1 M0), stage IIIA in 2 (1 T3 N1 M0 and 1 T2 N2 M0), stage IIIB in 4 (2 T4 N0 M0 and 2 T4 N1 M0) and stage IV in 3 (1 T1 N0 M1, 1 T2 N0 M1 and 1 T4 N0 M1).
Twenty-one patients had postoperative adjuvant therapy, among whom seven had received radiation therapy, five chemotherapy and nine both treatments.
2.3 Statistics
Numbers were expressed as mean and range in brackets. The χ2-test was only used if all expected frequencies were ≥5. Otherwise, Fischer's exact test was used. The Kaplan–Meier method was used to calculate the expected survival rates after completion pneumonectomy and the data included operative mortality. Statistical significance was calculated with the log-rank test and a P-value of the 0.05 or less was considered significant. Survival analysis was performed for the entire group of patients, for second primary cancer, metachronous cancer, metastasis and recurrence, for stage, and for interval from first resection to completion pneumonectomy for completely resected patients.
Follow-up was complete for all patients and was obtained from the patient physician, family, or from city hall registries.
3 Results
The indications for completion pneumonectomy were a malignant disease in 42 patients (76%) and miscellaneous non-malignant conditions in 13 (24%). In the 42 patients who underwent completion pneumonectomy for malignant diseases, pathological examination showed a primary bronchogenic carcinoma in 38 patients (91%), lung metastases in 3 cases (7%) and a hemangiopericytoma in 1 case (2%). In the group with primary bronchogenic carcinoma, there were a squamous cell carcinoma in 16 cases (42.5%), an adenocarcinoma in 16 cases (42.5%), an undifferentiated carcinoma in 2 cases (5%), a bronchioloalveolar carcinoma in 2 cases (5%) and an adenosquamous carcinoma in 2 cases (5%). In the group with lung metastases, the primary tumor was a colorectal carcinoma in one case (33%), a lung cancer in one case (33%) and a breast cancer in one case (33%).
Of the 38 patients with a primary bronchogenic carcinoma, 26 had a metachronous lung cancer (68%), 6 had recurrent lung cancer (16%), 4 had a primary lung cancer (11%) and 2 had a positive bronchial margin at the first operation (5%).
Postoperative staging of the 38 lung cancer patients is shown in Table 1 . In the group of cancer patients with non-malignant conditions, the indication for completion pneumonectomy was related to the occurrence of a bronchopleural fistula after lobectomy/bilobectomy in four cases (31%). In eight patients, completion pneumonectomy was done because of a clinical suspicion of cancer recurrence. In those cases, pathology disclosed destroying radio necrosis in three cases (23%), aspergilloma in two (15%), cytomegalovirus pneumonia in two (15%), and fibrothorax in one (8%). The remaining patient experienced massive hemoptysis and received surgery as a salvage procedure. In this group of patients, nine had had adjuvant therapy after the first lung cancer operation.
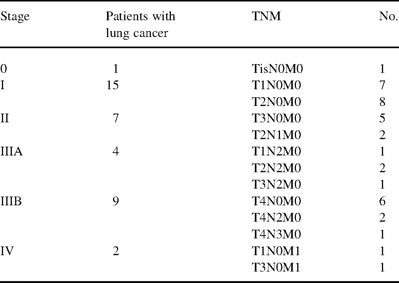
Postoperative staging of the lung cancer patients undergoing completion pneumonectomy
The mean interval between the first operation and completion pneumonectomy was 51 months (1–469) for the whole group, 60 months for lung cancer (12–469), and 29 months for benign disease (1–126). The mean interval was 47 months (12–208) for metachronous cancer, 12 months (1–24) for recurrent cancer, 245 months (16–469) for second primary lung cancer and 43 months (21–59) for pulmonary metastases.
Thirty-five completion pneumonectomies were performed on the right side (64%) and 20 on the left side (36%). Completion pneumonectomy was performed through a lateral thoracotomy in five patients (9%). All other operations were performed through a posterolateral thoracotomy (91%). The control of hilar components was achieved through an intrapericardial route in 49 patients (89%). Five patients had an extended resection (2 chest wall, 1 diaphragm, 1 subclavian artery and 1 superior vena cava).
The bronchial stump was hand-sutured in 13 patients (24%) with synthetic monofilament suture and closed with a mechanical stapler in 42 (76%). After closure of the proximal end, the bronchial stump was covered with viable tissue in 23 patients (42%), included pedicled mediastinal fat in 9 cases, pleural flap in 7, diaphragm flap in 5 and pedicled pericardium in 2. The pleural space was drained with a chest tube to balance the mediastinum, and removed 48–72 h after the operation.
Complete resection (R0) was achieved in 38 of the 42 patients with a malignant disease (90%). Four remaining patients had a microscopic residual tumor found at specimen analysis (R1).
Thirty-two patients (58.2%) had major complications (21 of the 38 lung cancer, 55.3%; 9 of the 13 benign disease, 69.2%; and 2 of the 3 metastases, 66.7%) and were fatal in nine. These complications are detailed in Table 2 . Prolonged mechanical ventilation of the remaining lung necessitated a postoperative tracheostomy in seven patients. Sixty-nine percent of the complications (24 of the 35) were observed after right completion pneumonectomy. Nine patients required rethoracotomy (bronchopleural fistula in seven and excessive postoperative bleeding with intrapleural clotting in two).
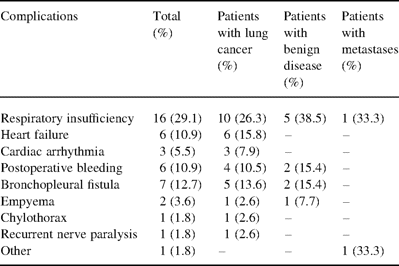
Seven bronchopleural fistulas (12.7%) were observed. Potentially involved clinical, technical and therapeutic variables are given in Table 3 . Three of the seven bronchopleural fistulas were delayed 29 months (3–45) after right completion pneumonectomy. All bronchopleural fistulas were treated with rethoracotomy and muscle flap or omental flap coverage, and thoracoplasty.
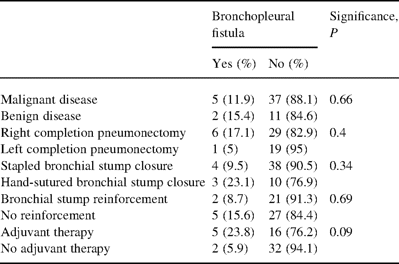
Risk factors for the development of bronchopleural fistula after completion pneumonectomy
Twenty-one patients had received an adjuvant treatment after the first operation (38%), of whom 15 had major complications (71.4%): 3 of the 5 patients treated with chemotherapy alone (60%), 5 of the 7 treated with radiotherapy (71.4%) and 7 of the 9 treated with both therapies (78%; P=0.38). Five bronchopleural fistulas have been observed in those patients who had received adjuvant treatment after the first surgery and two in those who had not (23.8 vs. 5.9%; P=0.09).
Seventeen patients (30%) were transfused of an average of two cell packs (2–4) after completion pneumonectomy, for malignant diseases in 13 (31%) and benign diseases in 4 (31%).
Overall operative mortality was 16.4%—11.9% for malignant diseases, 13.2% for lung cancer and 30.8% for benign disease (P=0.19). Operative mortality was 28.6% in patients who had received adjuvant therapy after the first operation and 8.8% in patients who had not (P=0.07). Operative mortality was 20% following right completion pneumonectomies and 10% following left procedures (P=0.46). Causes of death were multiple organ failure in five patients and heart failure in four.
Actuarial 3- and 5-years survival rates from the time of completion pneumonectomy were 48.4 and 35.2% for the entire group. Three- and 5-year survival for malignant diseases were 57.8 and 44%, 3-year survival for patients with benign disease was 17.9% (P<0.05; Fig. 1) . Three- and 5-year survival were 56.9 and 43.4% for patients with lung cancer: 50 and 50% for patients with primary lung cancer, 55.7 and 44.6% for patients with a metachronous cancer, and 58.3 and 29.2% for patients with recurrent lung cancer (P=0.81; Fig. 2) . Three- and 5-year survival for patients with stage I lung cancer was 78.8 and 67.5%, 33.3% in stage II, 60% in stage IIIA, 51.9 and 34.6% in stage IIIB, and 0% in stage IV (P=0.1).

Survival for malignant lung disease and benign disease after completion pneumonectomies using the Kaplan–Meier method.
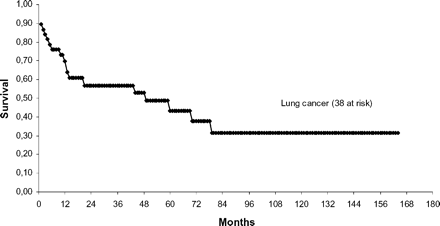
Survival for lung cancer after completion pneumonectomies using the Kaplan–Meier method.
In patients operated for a recurrent or metachronous tumor after the initial lung cancer resection, the 3- and 5-year survival were 55 and 35.4% when the delay between both events was <2 years, while they were 62.4 and 54.6% when the delay exceeded 2 years (P=0.1).
4 Discussion
Completion pneumonectomy is a surgical procedure which has become a relatively frequent therapeutic option in the fields of thoracic surgery. The indications for completion pneumonectomy for malignant diseases have augmented with the increased incidence of lung cancer, longer survival of patients with resected early-stage lung tumors, and increased request for repeat pulmonary metastases resection. However, extensive experience with this challenging operation remains rare, and limited to high-volume institutions.
The procedure is likely to carry a higher risk of operative mortality and morbidity than does standard pneumonectomy. The first report detailing the results of completion pneumonectomy have been that of McGovern and colleagues from the Mayo Clinic in 1988, who witnessed of an overall operative mortality rate of 12.4% [2]. Since then, various authors and institutions reported operative mortality rates ranging from 0 to 15.2% for malignant diseases, and from 0 to 35.3% for benign disease, balancing an actuarial 5-year survival from 18.3 to 44.5% (Table 4) . Our operative mortality rate for completion pneumonectomy was 11.9% for malignant diseases and 30.8% in non-malignant conditions, in a surgical cohort in which the 5-year survival rate was 35.2%, thus in the range of those previously published data.
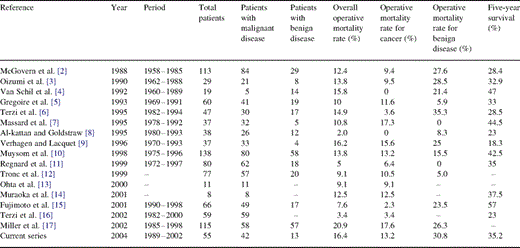
Results of operative mortality and survival after completion pneumonectomy in the literature
Data in the literature illustrating the operative risk of completion pneumonectomy for benign diseases show major discrepancies. Mc Govern et al. [2], Oizumi et al. [3], Verhagen et al. [9], Miller et al. [17] and Terzi et al. [6] reported a mortality rate of 27.6, 28.5, 25, 26.3 and 35.3%, respectively, while Grégoire et al. [5] as well as Massard et al. [7] reported a 5.9 and 0% mortality rates correspondingly. Our 30.8% rate is at the upper bound of the interval. The explanation links to the following: (1) all these patients, although presenting with non-malignant conditions, were cancer patients; (2) most indications were related to complications of their initial treatment; and (3) some operations were performed as emergent and even salvage procedures. Concerning bronchial fistula following lobectomy/bilobectomy, our experience suggests clearly that to complete the lung resection is probably the worst treatment, compared to alternative options such as muscle or omentum transposition combined with thoracoplasty when required.
Six patients of the present series underwent early completion pneumonectomy, within few weeks after the primary operation, because of the presence of invaded bronchial margins (n=2), or the occurrence of a bronchial fistula (n=4) following lesser resections. Although such cases have been included in other series customarily, the technique-related risk of the procedure is probably not comparable to late completion pneumonectomy since no adhesions are present, rethoracotomy is simple, and all structures have been already dissected. Conversely, late completion pneumonectomy is a technically demanding operation, requiring in almost all cases an extrapleural and intrapericardial dissection through a wide approach. This is illustrated indirectly by the proportion of patients requiring blood transfusions (30% in our experience). Unfortunately, this outcome measure is seldom reported in the literature [18].
Major complications occurred in 58.2% of our patients, among which cardio-pulmonary complications were the most common. This morbidity was observed mainly in patients operated for benign diseases, and after right-sided procedures. Indeed, 69% of major complications developed after right completion pneumonectomies, with an operative mortality rate of 20%, twice as high as that of left procedures. These features are commonly reported following standard pneumonectomy and there is no rationale to hope that it could be different in the setting of completion pneumonectomy.
Bronchopleural fistula was a fearing event. It occurred in nearly 13% of our patients, whereas rates reported in the literature range from 2.7 to 17.2% (Table 4). Bronchopleural fistula has been observed after right completion pneumonectomy in the vast majority of the cases, three times more frequently than for left-sided operations. We failed to identify any technical variable correlated with its occurrence, even if stapled bronchial closure and routine reinforcement seemed to be protective. We prefer the stapled technique for the bronchial closure because of its simplicity and safety. Although we observed a higher incidence of bronchopleural fistula in manually sutured bronchi than in stapled bronchi, the small number of sutured bronchi did not allow us to draw firm conclusions about the benefits of the stapled technique. Furthermore, the hand-fashioned technique was performed in almost all cases in those problematic cases where the suture needed to be performed very close to the carina, a well-known situation placing the patient at risk of stump leakage. Adjuvant therapy following the first lung resection was associated with a higher incidence of bronchopleural fistula (24 vs. 6%; P=0.09, Fischer's test), and a higher mortality (28 vs. 9%, P=0.07). These trends have been already emphasized previously [19,20].
In the present series we have observed a statistically significant difference in survival in cancer patients according to the clinical situation, malignant versus non-malignant, paradoxically detrimental to those patients operated on for non-malignant conditions. Respective survival curves showed that the difference was not due to the higher operative mortality exclusively, but continued along with time. Five-year overall survival rate for patients with bronchogenic carcinoma was 43.3%, with no dramatically different behaviors of patients operated for primary cancer, recurrent cancer, or metachronous cancer, thus reflecting a judicious patients selection. In contrast, in patients with metachronous or recurrent tumors, a disease-free interval <2 years seemed to influence adversely the overall outcome, as emphasized by Martini and Melamed [21].
To conclude, indications for completion pneumonectomy in cancer patients rely on various situations. The best ones probably include left-sided elective surgery for a metachronous cancer occurring >2 years after the treatment of an initial bronchogenic carcinoma, managed by surgery alone at that time. It remains, however, a technically demanding procedure, which carries an increased operative mortality and morbidity. Blood loss, bronchial fistula, and cardiopulmonary compromise dominate the spectrum of possible complications. Only those situations where a good prospect for long-term survival exist, justify the higher risk.




