-
PDF
- Split View
-
Views
-
Cite
Cite
Fabrice Barlesi, Christophe Doddoli, Céline Gimenez, Bruno Chetaille, Roger Giudicelli, Pierre Fuentes, Jean-Pierre Kleisbauer, Pascal Thomas, Bronchioloalveolar carcinoma: myths and realities in the surgical management, European Journal of Cardio-Thoracic Surgery, Volume 24, Issue 1, July 2003, Pages 159–164, https://doi.org/10.1016/S1010-7940(03)00190-8
Close - Share Icon Share
Abstract
Bronchioloalveolar carcinoma (BAC) of the lung is a subtype of adenocarcinoma with pure bronchoalveolar growth pattern and no evidence of stromal, vascular or pleural invasion (1999 WHO criteria), that seems to increase in incidence actually. BAC has its proper clinical spectrum, occurring more frequently in women and in younger patients. BAC also seems to be less dependent on tobacco exposure. Furthermore, original feature of this type of lung cancer is its intrapulmonary spreading and being infrequently systemic. Thus, surgical resection appears to have a pivotal role. This review of the literature attempted to assess whether or not patients with BAC should be treated according to the same oncological principles as those recommended for other non-small cell lung cancers, i.e. performance of anatomical resection combined with lymphadenectomy, and development of multimodality therapeutic strategies. Unilateral multinodular or pneumonic forms are best removed by lobectomy, or pneumonectomy when appropriate, combined with lymphadenectomy. Segmentectomy or wedge resection is a valuable option for the treatment of solitary lung nodules with pure pathological BAC patterns, provided specific conditions based upon computed tomography scan findings are present. The place of multimodality strategies is still unexplored. Treatment of bilateral BAC is challenging. Incomplete resection may be performed to palliate a severe intrapulmonary shunting. However, one hope of cure is provided by lung transplantation, even though disappointing results with disease recurrence on the grafts have been reported. The lack of large studies including only pure BAC gives a place for future biological and clinical research on this cancer.
1 Introduction
Bronchioloalveolar carcinoma (BAC) of the lung is one of the subtypes of adenocarcinoma [1] with an actually rising incidence [2]. According to the 1999 WHO criteria, pure BAC is defined as an adenocarcinoma with pure bronchoalveolar growth pattern and no evidence of stromal, vascular or pleural invasion. In the event of an invasive component, tumor is classified as an adenocarcinoma with BAC component. The percentage of the BAC component is, however, highly variable and its prognostic importance is actually discussed [3]. BAC has a broad spectrum of presentation with some specificity. Indeed, BAC occurred more frequently in women and in younger patients compared to other lung cancers [4] and BAC seems to be less dependent on tobacco exposure [5]. Furthermore, the original feature of this type of lung cancer is the intrapulmonary dissemination of the disease due to bronchial or lymphatic spread, leading to a high frequency of local or regional evolution and a rare metastatic dissemination. In this context, surgical resection particularly appears as a suitable approach in the treatment of BAC.
Regarding usual strategy in lung cancer management, the two goals of surgery are achievement of a complete resection and preservation of functional capacity. Lobectomy remains the rule and extension to bilobectomy or pneumonectomy has to be restricted to achieve complete resection.
Does surgical approach of BAC differ from other non-small cell lung cancer (NSCLC) surgery? To answer this question, we made a systematic review of the recent literature concerning surgical approach of BAC.
2 Material and methods
A computerized Internet PubMed search of English language medical literature was conducted using the words or key phrases: bronchioloalveolar lung carcinoma, thoracic surgery, surgical treatment, lobectomy, pneumonectomy, clinical trial and prognostic factors. Regarding rapid evolution of radiology, pathological classification and surgery, search was limited to the past 10 years. Articles exclusively focusing on radiological, pathological or biological aspects of BAC as well as case reports were excluded from analysis. Articles cited in retrieved publications and studying a large number of patients were also considered.
3 Results
Sixty-two papers met defined criteria. Nineteen addressed question of surgical approach of BAC whereas ten dealt with other types of NSCLC. Radiological, pathological and biological aspects of BAC were developed in five, five and nine papers, respectively. Furthermore, eight case reports and six other publications were excluded from analysis.
3.1 Surgery in a curative intent
3.1.1 Importance of completeness of resection
Whatever the type or the extension of BAC, achieving a complete resection is a necessity. Indeed, opportunity for long survival or cure without complete resection is quite null [6–10] and completeness of resection remains a major prognostic factor as shown in Table 1 .
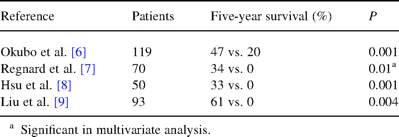
Influence of completeness of resection on survival in surgically treated BAC
3.1.2 Type of resection for multinodular or pneumonic forms of BAC: pneumonectomy, lobectomy or less?
Specificity of BAC in terms of multifocal pulmonary recurrence through alveolar spread leads to a maximal parenchymal saving policy. In a retrospective study published by Liu et al. [9], survival was better for lobectomy (median survival, 314 weeks) than for pneumonectomy (median survival, 119 weeks, P=0.017). These results are consistent with those reported by Regnard et al. in a second retrospective study [7] with 5-year survival of 45 and 8% for lobectomy and pneumonectomy, respectively. Okubo et al. reported in a third retrospective study [6] a significant difference in 5-year survival between segmentectomy (70%) and wedge resection (20%, P<0.01). However, extension of resection never remained significant in multivariate analysis maybe because surgical procedure is closely linked to tumor node metastases (TNM) stage.
3.1.3 Type of resection for nodular forms of BAC: could wedge resection be performed?
Despite results reported by Okubo et al. and discussed above, interest of limited resection in BAC should not be denied systematically. Indeed, several teams reported interesting results using wedge resection for BAC. Breathnach et al. studied 138 patients with stage I disease including 33 BAC and reported statistically not different 5-year disease free survival in patients resected by lobectomy or wedge resection (83 vs. 66%, P=0.6) [5] as Harpole et al. did in a study of 205 patients [11]. In a study concerning 17 patients with focal BAC showing pure ground-glass attenuation on computed tomography (CT), Watanabe et al. [12] performed wedge resection on 14 patients without postoperative mortality and a follow-up of 32 months with no cancer death or relapse. Yamato et al. published recently [13] the early results of a phase II prospective study of limited resection for 42 patients with BAC. They performed wedge resection in 34 patients and segmentectomy in two patients. Procedure was converted in lobectomy regarding per-operative findings in six patients. All patients were alive at 30 months without sign of recurrence. Radiologists are of great importance in the description of tumor especially regarding presence of ground-glass opacities on CT scan which seems to be predictive of BAC nature of the lesion [14,15]. It should be noted that positron emission tomography scan is probably less effective than CT scan in this setting [16]. To summarize, limited resection could be an acceptable alternative to lobectomy for histologically confirmed BAC with specific characteristics as type A and B adenocarcinomas described by Noguchi et al. [17]. However, wedge resection has to be avoided for other cases of BAC as recently demonstrated in a study of 100 patients with NSCLC 1 cm or less including 19 BAC [18] (5-year survival of 27% for wedge resection vs. 71% for lobectomy) and for all other types of lung cancer as previously demonstrated by several authors [6,19].
3.1.4 Should mediastinal lymph node dissection be undertaken in nodular forms of BAC?
Infrequency of mediastinal lymph node involvement in peripheral small-sized BAC and especially its absence in Noguchi's type A and B evokes the interest in mediastinal lymph node dissection. With this objective, Watanabe et al. performed a retrospective study of 225 patients with peripheral small-sized (2 cm or less) lung cancer [20]. Among 170 adenocarcinoma patients, 67.6% did not show lymph node involvement, especially adenocarcinoma ≤1 cm and Noguchi's classification type A and B. Then, they concluded that mediastinal nodal dissection in patients with BAC ≤2 cm without foci of active fibroblastic proliferation in histology would be unnecessary. This point of view is not shared by Miller et al. [18] who studied 100 patients with NSCLC ≤1 cm (including 19 BAC) and showing 18% of recurrence (local, distant and both). For this reason, they proposed systematic mediastinal lymph node dissection when medically possible even in NSCLC ≤1 cm.
3.1.5 Factors influencing survival in resected patients
Clinical and radiological presentation of disease seem to play a role on survival (Table 2 ) but independence of these factors is not constantly reported.
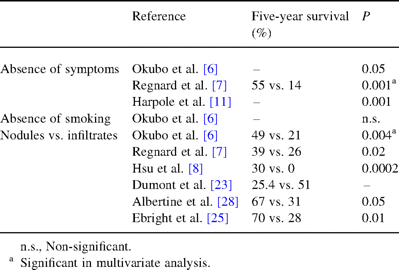
Clinical factors favorably influencing survival in surgically treated BAC
Role of completeness of resection was discussed above. TNM classification or lymph node dissemination exert same influence as for other type of NSCLC (see Table 3 ) [10,11,21–25].
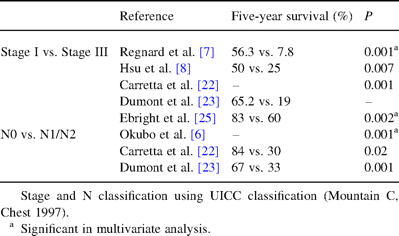
Surgical factors favorably influencing survival in surgically treated BAC
Pathological factors were at the center of prognostic evaluation in the past decades and several authors accorded an adverse prognostic influence to the mucinous histologic type [26,27]. Nevertheless, most recent studies, but one failed to retrieve this effect [28]. Prognosis influence of non-sclerosing type, presence or absence of scar, lymphocyte infiltration or vascular invasion are summarized in Table 4 . Percentage of BAC in the tumor seems to be controversial. Indeed, Carretta et al. [22] demonstrated a favorable influence on survival for each 10% increase of BAC in IA or IB patients (relative risk of 0.93 with 95% confidence interval of 0.08–0.98) while Okubo et al. [6] reported an adverse effect of 100% BAC tumors. Higashiyama et al. [3] did not show the same favorable effect of increased percent of BAC in adenocarcinoma in the entire population of its patients i.e. 206 (5-year survival of 100 vs. 60%, P<0.0001) than in stage I disease (100 vs. 95%, non-significant). These results assess the need for a strict histological definition of BAC patients included in studies and new analysis in this area. No influence of grade or mitosis is reported.
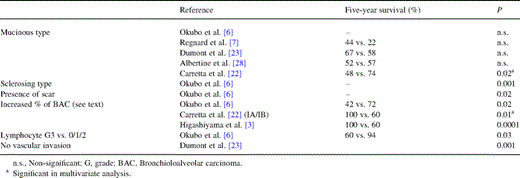
Pathological factors favorably influencing survival in surgically treated BAC
3.1.6 Combined treatments
No data are available concerning neoadjuvant or adjuvant chemotherapy or radiotherapy in the specific area of BAC. However, partial results could be extrapolated from clinical trials including variable proportion of BAC while BAC is an usual exclusion criterion from NSCLC clinical trials. In a trial including seven BAC (4.5%) patients, postoperative thoracic radiotherapy led to decrease in loco-regional recurrences, without the influence of histologic subtype [29]. Chemotherapy of BAC is also discussed because BAC is usually reported as chemoresistant tumor while efficacy of cisplatin in this subtype of adenocarcinomas is in fact comparable to that of chemotherapy in the treatment of metastatic adenocarcinomas of the lung [30]. Fujimoto et al. [31] even reported a better clinical response rate for BAC than for non-BAC stage IV patients, in a retrospective study of 53 cases without reaching survival impact, however. Trial interested in study of paclitaxel by 96-h infusion reported poor efficacy (12% of partial response and 40% of stable disease on 53 BAC patients with 1-year survival of 45%) [32]. At last, in the general setting of adenocarcinoma, Georgoulias et al. reported a significantly better efficacy for the combination of docetaxel and gemcitabine than for the platinum-based regimen [33]. Moreover, targeted therapy, especially epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 (Iressa®) recently available in NSCLC treatment was also tested in BAC patients and Janne et al. showed a promising activity of this molecule in recurrent BAC with 25% of partial response on 12 patients [34]. Some authors hypothesized a better efficacy of ZD1839 in adenocarcinomas and further research is warranted in this setting. Neoadjuvant or adjuvant strategy should also be tested in the context of BAC which despite its so-called rarity, represents approximately 5% of one of the leading causes of death worldwide.
3.2 Palliative surgery for BAC
Surgical management of patients presenting with bilateral BAC with unequal involvement of the lungs and major hypoxemia in relation to a severe intrapulmonary shunting [35] should not be denied systematically. Both symptoms and respiratory function could be quickly improved postoperatively with tolerable morbidity and comparable survey to the patients treated with chemotherapy. Palliative pneumonectomy or lobectomy (targeted on the most involved portion) should be discussed in a multidisciplinary manner but has to be considered exclusively for very highly selected patients [36].
3.3 Transplantation
Lung cancer is usually considered as a contraindication to pulmonary transplantation. However, BAC is characterized by high incidence of intrapulmonary dissemination while lymph node and extrathoracic metastasis are rare. In this context, lung transplantation could be an option in the treatment of BAC and was proposed for selected patients. Four publications are reported in the literature including 20 patients [37–41]. These patients share known characteristics of patients with BAC with predominance of women (13/20), young age (median, 45 years; range 31–58 years) and absence of tobacco exposure in six cases (data non-available in nine cases). Ten patients firstly experienced a conventional surgery (lobectomy, four; double lobectomy, two; and pneumonectomy, four) with chemotherapy in five cases and radiotherapy in two cases (50 Gy in one case, data non-available in the second). Lung transplantation consisted of double-lung transplantation in 16 cases and single-lung transplantation in four cases. Patterns of relapses and survival are summarized in Table 5 . Eleven patients relapsed. Four underwent second transplantation and four others a surgical resection of involved lung tissue.
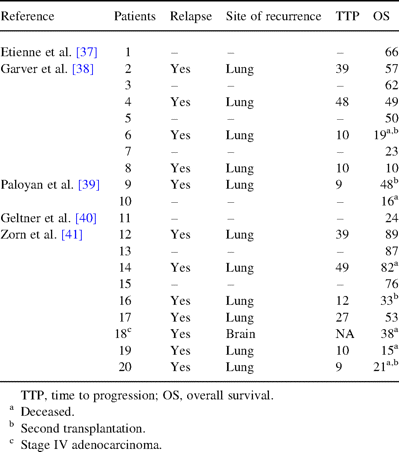
Patterns of relapse and survival in transplanted patients for BAC
However, study of original and recurrent tumors performed by Garver et al. [38] using polymerase chain reaction to amplify microsatellite DNA demonstrated that recurrent tumors in three patients originated from the recipients of the transplants. Even if the sanctuary site from which recurrent BAC arose within the donor lungs remains unclear (trachea, main bronchus, dormant cells in blood or bone marrow), hopes placed in lung transplantation are slightly disappointing.
Further examination of tumor genotype is needed for the definition of patients with favorable predictable outcome after lung transplantation.
4 Discussion
BAC presents specific clinical, radiological presentation and particular evolution as well. These characteristics offer some difference in the surgical management of BAC compared to other NSCLC. First, major risk of pulmonary relapse after surgery leads to a strict parenchymal sparing policy. In this aim, several studies are conducted to evaluate efficiency of limited resection and under specific conditions [14–17], wedge resection could be considered as a realistic alternative to lobectomy [12,13]. Although discussed by some teams [20], mediastinal lymph node dissection should probably be considered in the same way as for other type of NSCLC even for small-sized tumor [18]. Secondly, evolution almost always confined to the pulmonary parenchyma led to consider lung transplantation in selected patients [37–41]. Even though its results are disappointing (reported data are, however, heterogeneous), this opportunity should not be systematically denied regarding the patients who are alive without recurrence sometimes several years after transplantation. Furthermore, progress in our knowledge of genetics of BAC [42,43] will perhaps allow us for a better selection of candidates for transplantation for BAC. Finally, development of severe intrapulmonary shunting could lead to palliative pneumonectomy in selected patients [36].
Nevertheless, general management of BAC does not radically differ from other NSCLC and regarding major prognostic factors of surgically treated BAC as TNM, nodal involvement or completeness of resection, no difference appears compared to other NSCLC. One of the surprising conditions is certainly the lack of study on combined treatment. Only one datum available on adjuvant treatment is the results of study including a small proportion of BAC [29,44] while BAC is generally an exclusion criteria for trial on NSCLC.
This last point offers perspectives for international trials of a tumor certainly rare but representing 5% of one of the leading causes of death in western countries. Future will probably also permit progress in research of a genetic specificity and etiology [45] of BAC as well.
5 Conclusion
BAC presents specificity in its clinical, radiological and evolutionary or therapeutic aspects as well. Principal characteristic of surgical management of BAC is the need of parenchymal saving. Possibility in highly selected patients of lung transplantation in a context of lung cancer is undoubtedly a second particularity. However, numerous questions on the most suitable management of BAC are unresolved and a strict pathological definition of tumors considered as BAC in studies published is also needed now.
References
- lung transplantation
- computed tomography
- lung
- cancer
- adenocarcinoma
- bronchioloalveolar carcinoma
- non-small-cell lung carcinoma
- pulmonary coin lesion
- mastectomy, segmental
- pneumonectomy
- surgical procedures, operative
- tobacco
- tissue transplants
- world health organization
- palliative care
- pleura
- lymph node dissection
- lung cancer
- lobectomy
- wedge resection
- excision
- shunt intrapulmonary
- clinical research
- misconceptions




