-
PDF
- Split View
-
Views
-
Cite
Cite
Vladimir A. Porhanov, Igor S. Poliakov, Andrew P. Selvaschuk, Anatoly I. Grechishkin, Sergei D. Sitnik, Igor F. Nikolaev, Jury P. Efimtsev, Leonid G. Marchenko, Indications and results of sleeve carinal resection, European Journal of Cardio-Thoracic Surgery, Volume 22, Issue 5, November 2002, Pages 685–694, https://doi.org/10.1016/S1010-7940(02)00523-7
Close - Share Icon Share
Abstract
Objective: Carinal resection is the most complicated procedure in tracheobronchial surgery. The main aspects of the technique are still debated at the present time. We present our experience of 231 carinal resections with analysis of operative techniques, complications and long-term survival. Methods: Since 1979 we have performed 231 carinal resections. Indications for surgery included lung cancer in 151 cases (65.4%), non-bronchogenic carcinoma in 45 (19.4%), main bronchus fistula with short stump in 25 (10.8%), stenosis of tuberculous and nonspecific etiology in nine (4%), and trauma in one case (0.4%). We have performed 156 right-sided resections (67.5%) and 75 left-sided (32.5%). In 162 cases carinal pneumonectomy was undertaken, carinal resection following pneumonectomy was performed in 28 cases, isolated resection of bronchial bifurcation was performed in 25 cases, and in 15 cases we combined lobectomy and resection of bifurcation. The length of resection extended from one to nine tracheal rings. The operative approach was lateral thoracotomy in 102 cases (44.2%), and sternotomy in 129 (55.8%). Results: Thirty-seven patients died postoperatively (16%). Complications were observed in 82 patients (35.4%), dominated by anastomotic problems which occurred in 58 cases (25.1%). The most frequent causes of death were respiratory distress syndrome and anastomotic dehiscence (P<0.05). Mortality and the incidence of complications were significantly correlated to length of resection, laryngeal nerves injury, and mode of intraoperative ventilation. Conclusions: The feasibility of carinal resection is limited by the patient's functional status and extension of tumor growth. Thorough selection of patients may improve immediate and long-term results.
1 Introduction
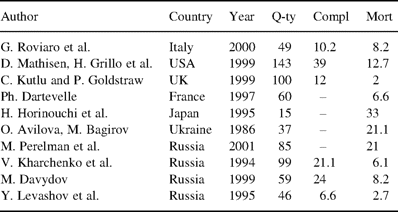
One of the most challenging operations in thoracic surgery is sleeve carinal resection, which involves dissection of the trachea and main bronchi, resection of the carina, and construction of an anastomosis between the trachea and bronchi. Indications for such operations occur rarely and in most cases they are because of tumor involvement of bronchial bifurcation, tracheobronchial angle or lower part of the trachea. Besides, limited recurrence of cancer in the main bronchus stump may result in carinal resection [1]. Rather seldom, proximal stenosis of the bronchi, benign tumors and main bronchial stump fistulae may require this procedure [2]. In the majority of cases, carinal pneumonectomy is undertaken. Less frequently, various types of resections may be performed with more than 15 modes of bronchial reconstruction [3,4]. The surgical technique, oncological sense, and the role of systematic lymph node dissection remain current topics of debate [5–7]. Published series of patients demonstrate that when following adequate principles, mortality and morbidity rates of such resections may become acceptable [1,2,7–16] (Table 1) . Accordingly, the absence of an alternative to surgery makes sleeve carinal resection an operation of choice and gives the patient a chance to be cured, or at least offers palliation and improved quality of life. The aim of this study was to review the experience of our center and to analyze both operative risk and long-term outcome.
2 Materials and methods
2.1 Patients
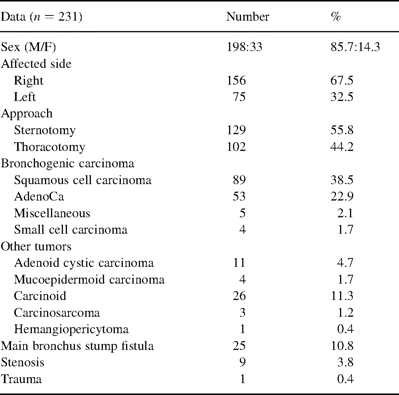
From January 1979 to April 2001, two medical institutions of the Krasnodar region (Regional Anti-Tuberculosis Clinic and Regional Thoracic Surgery Center) performed 231 sleeve carinal resections and 13 reoperations for anastomotic complications (Table 2) . These accounted for less than 1% of all pulmonary resections performed during the same period. There were 198 male patients (85.7%). The mean age of the patients was 59 years (range 19–71 years). Indications for surgery included 151 cases with bronchogenic carcinoma (65.4%); three of them had undergone pneumonectomy and had developed a limited recurrence within the bronchial stump. Histological examination revealed squamous cell carcinoma in 89 (38.5%), adenocarcinoma in 53 (22.9%), adenosquamous carcinoma in five (2.1%) and small cell cancer in four (1.7%) cases. Other types of malignancies were observed in 45 patients (19.4%): adenoid cystic carcinoma was found in 11 (4.7%), mucoepidermoid carcinoma in four (1.7%), carcinoid in 26 (11.3%), carcinosarcoma in three (1.2%), and hemangiopericytoma in one (0.4%). We performed carinal resection for bronchial fistula after pneumonectomy in 25 patients (10.8%), due to short stump main bronchus fistula or tracheal wall defect. In nine cases (3.8%), the operation was indicated because of scar tissue stenosis of the main bronchus and granulation tissue spreading over the carina and trachea. A final patient (0.4%) was admitted to the thoracic center following a car accident with full rupture of the right and left main bronchus.
2.2 Preoperative work-up
Selection for carinal pneumonectomy required adequate functional tests, predicting a postoperative FEV-1 of at least 1000 ml. Multiple views of chest X-ray, computer tomography (linear tomography before 1989), and rigid or fiber bronchoscopy were performed routinely in all patients. Morphology suggestive of lymph node involvement was taken into consideration, but suspected N2 involvement was not a contraindication to surgery. At first rigid bronchoscopy and then fiber bronchoscopy were standard procedures for patients. Mediastinoscopy was required only when we found peribronchial tumor invasion spreading over the trachea, when extracapsular tumor invasion from lower paratracheal and subcarinal lymph nodes was suspected, or when there was a tracheal compression above the anticipated resection line.
2.3 Anesthesia
Anesthesia was performed with intravenous drugs; inhalation medications were avoided. Early extubation in the ICU was carried out whenever possible. Ninety-eight (42%) patients were intubated with a thin single-lumen tube (No. 7-8), which was inserted in the contralateral main bronchus and left there during construction of the anastomosis. In the remaining patients, the operation was started with a double-lumen intubation. As soon as the feasibility of resection was ascertained, we resected the bifurcation and removed the specimen, and we performed across-the-field ventilation through a sterile intubation tube inserted into the distal part of the contralateral bronchus. In 91 (39%) cases we applied a flexible thin tube No. 5-6, which was sutured to the bronchial edge by an atraumatic ligature to avoid its displacement. In 42 cases (18%) high-jet ventilation was applied. The catheter was inserted through the wound as well as through the trachea. In the case of difficult exposure of the opposite side of the anastomosis due to the intubation tube (in three cases), we made an incision 1.5–2 cm lower than the resection edge of the main bronchus to insert distally either a thin intubation tube or a high-jet ventilation catheter. In all utilizations of the shunt-respiration system, orotracheal intubation was kept, but the tube with deflated cuff was pulled back to the level of the vocal cords. We thus avoided repeated intubation, with overbending of the neck and increased traction on the anastomosis.
2.4 Surgical approach and exposure of bifurcation
In order to approach the carina we applied either routine lateral thoracotomy, or sternotomy, or combined sterno-thoracotomy or thoracotomy-sternal accesses.
Many authors usually use right-sided thoracotomy. Access to lower and middle aspects of the trachea is not easy through a left thoracotomy due to the overhanging aortic arch and supraaortic vessels. These difficulties were overcome with two artifacts: mobilization of the aortic arch, and mobilization of the trachea with immediate preoperative mediastinoscopy. Moreover, pericardium was transected both from right and left sides before the resection. For malignant lesions right and left systematic lymph node dissection was a routine procedure.
During sternotomy access for malignant disease, routine lymph node dissection was performed bilaterally [17]. However, in the case of left carinal pneumonectomy performed through a sternotomy, access to the posterior part of the hilum was complicated because of the overhanging heart, and additional pressure on it resulted in undesirable effects – arrhythmia and lowered arterial pressure. To avoid these effects and to guarantee a full lymph node dissection, we used left-sided video assisted thoracoscopic surgery (VATS) prior to sternotomy, to transect inferior and sometimes superior pulmonary veins, and to resect hilar, paraesophageal and subcarinal lymph nodes [18]. Thus, we excluded tumor invasion of pleura, while facilitating further removal of lung via sternotomy access.
During sternotomy, regardless of the side of pulmonary resection, the main bronchus and trachea were exposed in the aorta-caval space. After removal of the specimen, the anastomosis was sutured in the same space. The suture line was covered with a pedicled flap of mediastinal and thymic fat, with a part of pericardium or with omentum. In all cases, before completing the anastomosis, the neck was strongly flexed. The chin was tagged to the anterior chest wall by one or two heavy sutures, left in place for 7–8 days. In the case of pneumonectomy, we attempted to seal open pleural space hermetically and take in mediastinal pleura. In that case a drainage tube behind the sternum was connected to the low-pressure aspiration (2–4 cm H2O). The intrapleural drain was put on a water seal. In other cases the number of drains and aspiration regimens depended on the operative volume.
2.5 Technical aspects of anastomosis
Following tracheal and bronchial mobilizations we fixed stay sutures proximally and distally to the line of transection. The anterior semi-circumference of airways was transected first. Thus, the posterior wall was exposed, which reduced the risk of injuring the neighboring structures of the bronchus. An elastic probe (15 mm in diameter) was inserted into the esophagus for accurate identification. At first, angle-sutures were placed on either side of the anastomosis at the membranous-cartilaginous angles. The membranous part of the anastomosis was sutured. After knotting the stitches and removing the cross-field ventilation system, ventilation was carried out with the trocar, which was temporally pushed beyond the suture line. The tube was pulled back into the trachea after hermetic sealing of the anastomosis.
In most cases ‘end-to-end’ anastomosis was applied. If the diameters of the trachea and bronchus did not correspond to each other, two technical variants were used. At first, the principle of the ‘telescope’ was used – the cartilaginous part of the main bronchus was inserted into the lumen of the trachea up to 1–2 mm (in 11 patients), and the membranous part was sutured end-to-end. In nine patients we performed so-called ‘rotating’ anastomosis [19]. A technical peculiarity was that the membranous parts of the trachea and main bronchus were rotated on each other by 30–60°. This facilitated the possibility of pulling the caliber of either part to be anastomosed, to avoid narrowing of the anastomotic lumen and to decrease tension along the suture line.
We performed three main types of carinal resections. In the majority of cases carinal pneumonectomy (or a similar procedure if a patient had a main bronchus stump fistula or tumor recurrence in the main bronchus stump) was performed (Fig. 1b,c) . The second type included isolated carinal resection, right and left main bronchi being anastomosed with the trachea as a ‘neo-carina’. The third type was a ‘complex’ anastomosis following sleeve carinal resection: an end-to-end anastomosis was performed between the trachea and one of the main bronchi, and the second bronchus was reimplanted ‘end-to-side’ above or below the tracheobronchial anastomosis. Both absorbable and unabsorbable atraumatic threads of 3-4/0 were used for sutures. A continuous suture was used for the membranous part, and separate knot sutures were used for the cartilaginous semi-circle of anastomosis. In 39 (16.8%) patients we used a continuous suture with a single polypropylene thread (‘parachute technique’) [20]. While creating reconstructions as a ‘double barrel gun’ after carinal transection, the left main bronchus was intubated, medial parts of bronchi were sutured and then the ‘double barrel gun’ was sutured with the trachea. In the case of reimplantation anastomosis was formed as an ‘end-to-side’ by means of separate knot sutures, when the main tracheobronchial anastomosis was sealed hermetically. The fenestration of the trachea or bronchus was created in a longitudinal direction on the cartilage, which prevented retraction of the lumen after anastomosis. Owing to the overhanging aortic arch, reimplantation of the left bronchus was carried out below the anastomotic line; after right-sided resection with preservation of the right upper lobe, the bronchus of the upper lobe was reimplanted above the anastomotic line into the lateral wall of the trachea (Fig. 1a).
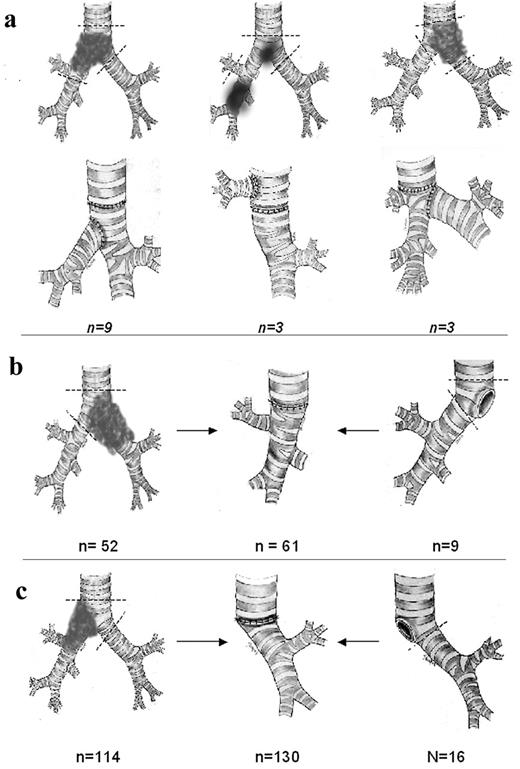
Different types of carinal resections: (a) the ‘complex reconstruction’ type of resections includes carina resection, preservation of intact lung, and formation of second anastomosis ‘end-to-side’; (b,c) sleeve resection of bifurcation with pneumonectomy or following pneumonectomy (for primary tumor or recurrence, main bronchus short stump fistula).
2.6 Analysis
Material was analyzed on the basis of case records, dispensary records of patients, personal correspondence and contact with patients. Statistical software was used to analyze the received information (SPSS v. 10.0.5 (C) for Windows 9X.NT/2000, Microsoft©). Results with P<0.05 were considered to be significant.
3 Results
3.1 Approval of resectability
Before an incision was made, it was not always possible to determine the necessity for carinal resection. In 55 (23%) cases resection was ‘forced’, i.e. its need was revealed during the operation. The carina or the lower part of the trachea were involved in 89 cases of ‘T4’ lung cancer (38.5%) (Table 3) . Thirty patients (12.9%) were found to have carinal invasion by lymph nodes (LN 7, LN 4). In 39 cases (16.8%), the visible edge of the tumor was located 1–7 mm from the carina (classified as T3). Of those, the distance to the carina was 7–5 mm in four (1.7%), 4–3 mm in 21 (9%), and less than 3 mm in 13 (5.6%) cases. In this group of patients we tried to perform carinal resection. Histology revealed tumor growth at the resection margin in 21 cases. In 18 patients we performed sleeve resection because it was impossible to close the planned wedge defect.
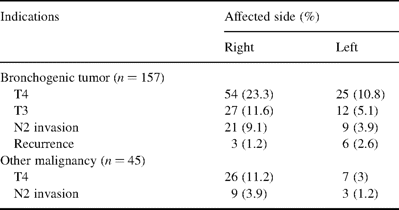
Indications for sleeve resection of bifurcation: malignant pathology
Among 45 patients with non-bronchogenic carcinoma, three had invasion of lymph nodes. Nine patients with carcinoid tumors had lymph node invasion and invasion into the carina and trachea. Another 33 patients (14.2%) were found to have tumor invasion of bifurcation. Eight out of nine scar stenoses (3.4%) had developed after tuberculous bronchitis, and had existed for 3–14 months. In one patient iatrogenic postintubation injury of the trachea and right main bronchus resulted in stenosis 3 weeks following upper right lobectomy. In all patients of this group, the lung on the operated side required its full resection because of irreversible changes. After a car accident one patient had ruptures of right and left main bronchi with massive hematoma along the line of rupture. This necessitated resection of the carina with formation of ‘neo-carina’ anastomosis.
Short stump fistula of the main bronchus was an indication for surgery in 25 patients (10.8%). The diameter of the fistula ranged from 10 to 30 mm. The operation was undertaken from 35 days to 28 months following pneumonectomy. Six of the patients had incidental tumor cells in the fistula.
3.2 Preoperative endoscopic management
Prior to the operation, we performed endoscopic ablation of obstructive tumor or granulation tissue from the trachea or main bronchus in 43 patients (18.6%). The obstacle was removed with the tip of the bronchoscope, forceps or electric loop. Among these patients, there were three with bronchogenic carcinoma (1.2% of the total), nine with scar stenoses (3.8% of the total), 11 with mucoepidermoid cancer (4.7% of the total), seven with adenocystic cancer (3% of the total), and 13 with carcinoid tumors (5.6% of the total). In all nine patients with scar stenosis of the carina, attempts to treat the stenosis by endoscopic means failed as re-stenosis developed. Preoperative irradiation was administered to 39 patients (19.3%). Four had small cell lung cancer, 26 had bronchogenic carcinoma, and four had adenocystic cancer. Furthermore, five of the nine patients with tumor recurrence in the bronchial stump were given induction radiotherapy. The dose of radiotherapy varied from 45 to 60 Gy. Neo-adjuvant chemotherapy was administered to two patients with adenocarcinoma.
All patients with tuberculosis had been given specific anti-tuberculous therapy for 3 months before the operation. None of them had a positive sputum test by the time of the operation. In 25 cases with bronchial fistula, initial VATS was performed in order to debride empyema, and we placed drainage tubes in all of them.
3.3 Surgery
Lateral thoracotomy was used in 102 patients (44.2%), left-sided in 29 (12.5%). There were 54 right carinal pneumonectomies (23.4%), and 26 left carinal pneumonectomies (11.25%). In seven cases the lung was preserved and the carina was resected with construction of an anastomosis of the double-barrel type. In 15 cases, various types of complex tracheo-bronchial reconstructions were performed.
Sternotomy was undertaken in 129 patients (55.8%), 46 of them having right-sided tumors (19.9%). Right carinal pneumonectomy was performed in 84 patients (36.6%), and left carinal pneumonectomy in 25 (10.8%). We formed a transsternal neo-carina anastomosis in 18 cases (7.7%). In all 25 patients with a main bronchus fistula (nine left-sided) and in three patients with tumor recurrence in the main bronchus stump (two left-sided), we performed resections via a sternotomy.
Combined access was applied in 26 cases (11.2%). Of those, 12 had undergone VATS surgery before left carinal resection was performed (5.1%). Eight patients required thoracotomy to be performed (3.5%): five on the left because of dense adhesion with the descending aorta, and three on the right side due to invasion of the esophagus. In six patients (2.6%), left thoracotomy was combined with sternotomy. It was necessary to mobilize the trachea and right lung in order to reduce the tension of the anastomosis line, and to remove enlarged right mediastinal lymph nodes.
Among patients with malignant disease, carinal resection was combined with superior vena cava reconstruction in two cases (0.8%), resection of the muscular wall of the esophagus in three cases (1.2%), resection of the chest wall in 12 cases (5.1%), wedge resection of the aorta in one case (0.43%) and resection of the atrium in 13 cases (5.6%).
The length of resection was defined by the length of the resected part of the trachea and bronchus. Resection up to 2 cm was performed in 31 cases (13.4%), to 4 cm in 112 (48.5%), to 6 cm in 69 (29.9%) and to more than 6 cm in 19 (8.2%) cases. The mean length of resection was 3.8 cm.
The anastomosis was wrapped with a pedicled pericardial flap in 112 patients (48.5%), remnants of thymus in 63 (27.3%), omentum in 21 (9.1%) and adjacent fat in five cases (2.1%). In 30 cases (12.9%) anastomosis was not protected.
3.4 Complications and mortality
We observed complications in 82 patients (35.6%) during the hospital stay (Table 4) . Intraoperatively, there were injuries to great vessels in four patients (1.7%): pulmonary artery in two patients, and superior vena cava in two patients. In one patient bleeding developed because the vessel clamp sprang off the atrium; a sudden blood loss of about 1000 ml was rapidly followed by hypotension and cardiac arrest, but normal hemodynamics were recovered after infusion therapy and defibrillation. Intraoperative blood loss among all other patients ranged from 200 to 800 ml, and required blood transfusion in one patient only. In 21 cases (9.1%) we observed cardiac arrhythmia intraoperatively.
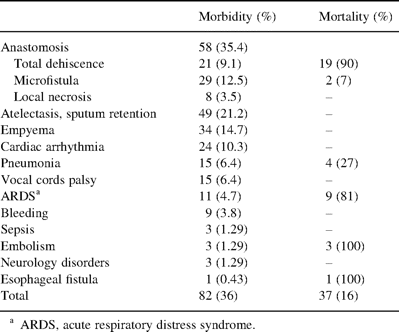
Morbidity and mortality rates following sleeve carinal resection (n=231)
Impaired anastomotic healing was observed in 58 cases (25.1%). There was a complete dehiscence in 21 patients (9.1%): 17 had undergone a carinal pneumonectomy (7.3%), three a neo-carina operation (1.3%), and one a complex reconstruction (0.43%). All cases of dehiscence were declared within 6 days after the operation. Another 29 patients (50%) were found to have a microfistula, measuring from 1 to 3 mm, disclosed by endoscopy between 3 and 14 days postoperatively. Local necrosis of anastomosis without visible disturbance of its integrity was observed in eight patients (13.7%).
Pleural empyema and local mediastinitis developed in 34 patients (41.6%), and five patients (6.1%) had sepsis. All patients were drained. Intrapleural bleeding and clotted hemothorax were found in nine patients (10.9%); re-thoracotomy was required in five cases (55%) and re-sternotomy was required in one case (11%). Sputum retention and lung atelectasis developed in 49 patients (59.7%). In addition to fibrobronchoscopy we performed microtracheostomy in 19 patients.
Cardiac arrhythmia occurred in 24 patients: the disorder appeared intraoperatively in 19 cases (79%), and 11 events (58%) developed during left pneumonectomy. Among three patients with resection extended to the esophageal muscular wall, one (33%) was found to have esophageal-pleural fistula on postoperative day 12. He died of associated sepsis.
There were no cases of intraoperative mortality. The overall hospital mortality rate was 16% (37 patients). Anastomotic complications were the most common cause of death and applied to 21 patients: 19 had complete dehiscence, and two had a microfistula which resulted in erosive bleeding. Pneumonia, sepsis, pulmonary and cerebral edema and ARDS resulted in death. The ARDS mortality rate was 81%. Pneumonia was associated with a 26% mortality rate (four of 15). Pulmonary artery embolism (in two) and superior vena cava (SVC) graft thrombosis (one) were associated with a 100% mortality rate.
3.5 Reoperations
We performed 13 reoperations (5.6%): 11 patients had a total dehiscence of anastomoses, and two had a microfistula. Operations were performed from 2 h to 3 days after diagnosis of the complication. Re-resection of the carina was associated with a high risk of failure and resulted in death in nine cases (70%). ARDS developed in two patients (15%) and caused their death. The overall mortality rate of such operations was 85%.
3.6 Long-term follow-up
Long-term follow-up was available in 161 (82%) patients and ranged from 2 months to 12 years. All patients with trauma and scar stenoses were followed up. The patient operated on for traumatic disruption has survived for 5 years without complications. Among the nine patients with scar stenoses, one patient (11%) was found to have granulation tissue growth along the anterior semi-circle of anastomosis, at the location of healed microfistula, 38 days after primary resection. The lumen of the anastomosis was constricted at 1/4. Granulation tissue was successfully removed by a laser device. Two patients of that group developed late recurrence of single lung tuberculosis. They died of non-controlled progressive tuberculosis within 2 years of the operation. Two patients were still alive 8 and 3 years after surgery. The other died in different terms because of unrelated causes.
Nine patients (3.8%) who underwent carinal resection due to main bronchus fistula required removal of granulation tissue along the anastomotic line; a temporary tracheal stent was left in place in two patients. One of these two patients recovered and has no restenosis after removal of the stent, whereas the second patient developed an arterio-bronchial fistula under the inserted stent and died of sudden hemoptysis.
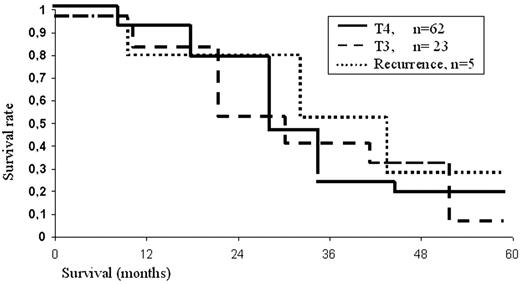
One hundred and one patients with bronchogenic carcinoma were followed up from 1.5 months to 8 years. The overall 5-year survival rate was 24.7% (from 0% to 32% in different groups). In cases with direct invasion only 19 (30.6%) of 62 patients reached 5-year survival, including those with N2 disease. The overall 5-year survival rate was 19%. Only one patient who had an isolated right lesion of tracheo-bronchial lymph nodes and had undergone carinal resection for carinal and tracheal invasion by mediastinal lymph nodes (out of a total of 16 patients) survived more than 5 years. In the subgroup with preoperatively diagnosed T3, four of 23 patients (17.3%) survived 5 years, and actuarial 5-year survival was 7% (Fig. 2) . Such low survival of these 23 patients is explained by the fact that 61% were N2. In all groups of patients mediastinal lymph node involvement was a deleterious factor. There were significant differences in survival rates between N0–1 and N2 patients, with respective 5-year survival rates of 32% versus 7.5%. Another deleterious factor was direct invasion of lymph nodes into the carina or trachea, since 5-year survival in such patients was only 2.6% (Fig. 3) .
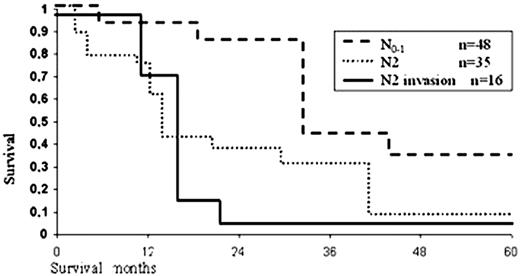
Survival rate for bronchogenic carcinoma related to N-status (Kaplan–Meier estimated).
For five patients with tumor recurrence in the stump, overall 5-year survival was 24%, but among the survivors none had involved mediastinal lymph nodes.
Twenty-six patients with non-primary bronchogenic cancer have been followed-up over 9 years. Unfavorable prognosis included the presence of carcinosarcoma, as two patients having undergone carinal resection died during the next 2 years of tumor recurrence. Five-year survival was observed in 16 cases (61%); of those, 12 patients (63%) had carcinoid tumors, and four patients (80%) had adenocystic cancer. Estimated actuarial survival was 66% and 75%, respectively. None of them revealed metastatic spread in mediastinal lymph nodes or invasion of surrounding structures.
For malignant pathology, chemotherapy was administered in three patients (2.4%) with adenocarcinoma and in two patients (1.6%) with small cell cancer. Radiotherapy of doses varying from 45 to 55 Gy was applied postoperatively in 21 (16.5%) patients.
Long-term complications of surgery included intrabronchial granulomas and empyema. Granuloma along the suture line was observed in 17 patients. In all cases this complication was cured by rigid bronchoscopy. Chronic pleural empyema developed in 15 patients, and was associated with fistula in 12 patients. Empyema was healed after a period of 23–79 days and fistula was relieved independently.
4 Discussion
Sleeve carinal pneumonectomy is a rather rare procedure, combined with a high rate of postoperative morbidity and mortality [9,21,22]. We have analyzed our own experience of 231 carinal resections performed for various diseases, including 13 reoperations. Operations had been performed over a time-span of 22 years. There were about ten operations per year in general, and these represented about 1.5% of all open operations. Malignant tumor was an indication to surgery in the majority of cases (88%).
The population of our region is about 5.2 million people. Annually about 3000 people are diagnosed with lung cancer. Of them more than 60% have advanced stages of lung cancer. Tumor invasion of the carina usually precludes surgery. The issue of patient selection has not yet been solved. To a definite extent it deals with the difficulties of preoperative assessment of resectability. Computer tomography, videobronchoscopes with high resolution, and a wider application of mediastinoscopy enables a more thorough selection of patients for operation [1–3]. In our experience, the number of ‘unplanned’ carinal resections decreased from 39% in the first decade to 23% in the second one.
The anesthesiological technique should be directed towards development of adequate ventilation and earlier extubation of the patient. Having applied various modes of ventilation we revealed that distal ventilation was the most suitable device which was safe for the patient and convenient for the surgeon. We have started applying jet ventilation if other methods are not suitable. However, we should note a more frequent development of ARDS: eight of 11 cases while applying jet ventilation (P<0.05).
The left-sided resection is related to a higher rate of complications [2]. In our experience we have not found reliable data regarding this fact. However, left-sided operations have their own peculiarities of performance. Thoracotomy access is supposed to allow for a tracheal resection up to 2–3 cm. To perform more extended tracheal resection, mobilization of aorta should be undertaken. This manipulation increases operative trauma and hence the complications rate although this access is convenient to form complex anastomoses. In our experience we prefer sternotomy for the left-sided resections routinely.
Right thoracotomy is a more wide-spread and convenient access to the tracheobronchial bifurcation [1]. However, in the majority of patients carinal resection is performed for malignant pathology; therefore, a systematic removal and analysis of mediastinal lymph nodes is required to adequately stage the patient and to predict a prognosis of survival [6,23]. Our retrospective analysis demonstrates that mediastinal lymph node involvement (N2) is an unfavorable factor for survival, especially if there is invasion of the tumor from the lymph nodes into the carina or bronchi (Fig. 2). Nevertheless, operation is indicated under the conditions of systematic mediastinal lymph node dissection (unilateral or bilateral). It was recorded that all survivors with N2 disease had undergone systematic lymph node dissection. There is no doubt that sternotomy is the most suitable access for complete removal of lymph nodes and mediastinal fat. Besides, other advantages of this access are lower pain and lower harm to respiratory mechanics, which are of great importance for patients with reduced pulmonary function [6]. We consider that contraindications to sternotomy in the event of posterior mediastinal invasion or tumor localization in the aorto-pulmonary window are relative. Combined sterno-thoracotomy access [24] or VATS [1] for hilar mobilization and lymph node dissection may be a compromise. Besides, sternotomy enables us to perform total mobilization of both trachea and lung and to avoid laryngeal mobilization (it has never been used in our series).
Extension of resection influences complication and mortality rates, especially those related to the anastomosis. We consider resection of 2–4 cm to be safe [2,5]. In our series, the mean length of resection was 5.3 cm among patients who died postoperatively versus 3.4 cm in the group of survivors (P<0.01). It is possible that besides the tension on the suture line, devascularization of the area, which occurs during extensive resections, may lead to difficulties relating to bronchial trophicity.
We have not found any differences in complications and mortality rates when analyzing types of sutures (an interrupted suture, a blanket suture, and a combined suture) and types of anastomosis (simple membrane-to-membrane, cartilage-to-cartilage, ‘telescoping’ type, and ‘rotating’ type). We prefer a blanket prolene suture, which is placed in monofilament, as it is simple, rapid and convenient in performance. In the case of limited leakage after completion of the anastomosis, it is possible to place one to two interrupted sutures. In two cases anastomosis was created by means of a microsurgical technique, separately stitching mucous membrane and muscle carcass [25]. This is a rather complicated technique and the absence of advantages compared to other methods resulted in it being rejected in common practice.
The choice of adequate material to cover the anastomosis is an important component of surgery. An omentum flap is the best choice although its application increases operative traumatism. It is especially helpful for reoperations and management of complications. No vascularized flap was used in cases when tension along the anastomotic line was slight [1]. Development of total dehiscence of anastomosis was not correlated with the covering of the anastomotic line. However, in the case of a microfistula appearing during the first 6–8 days postoperatively, the presence of a muscle flap was meaningful – the microfistula healed without any further surgery. On the other hand, two patients developed rigid fibrous cuirass of a flap around the anastomosis, which led to incurable anastomotic constriction.
Malignant disease is a certain risk factor, as the majority of complications occurring in such patients resulted in death: 91.8% were lung cancer patients versus 8.2% with other diseases. There are some possible explanations for this fact. In patients with benign diseases, the main purpose was to perform economic resection with the least possible extent. At the same time, the objective of achieving clean margins of resection in cancer patients influenced the extension of resection and increased the complications rate. The volume of parenchymal resection was also one of the significant factors heralding postoperative complications (P<0.05). Operations preserving both lungs (‘neo-carina’, ‘complex’ types) were characterized by a rather low rate of morbidity and mortality (Table 5) . Almost 94.5% of patients dying postoperatively underwent sleeve pneumonectomy, with an overall mortality rate of 18.3%. Pneumonia of a single lung also resulted in death in 90% of patients versus 5.2% following conservative operations. Preservation even of a part of the lung is likely to provide an improved ‘functional stability’ when complications arise.

Morbidities caused by anastomosis and total mortality rate: various types of carinal resections
Repeated operations were authentically correlated with a high mortality rate (84%). Purulent process, ischemia of anastomotic edges, and increased tension of a new anastomosis because of a more extended resection were the main causes of failure of repeated surgery. Despite the fact that the remade anastomosis was wrapped with an omental flap, there was not enough time for its revascularization to develop. A repeated dehiscence developed and a patient died of erosive bleeding and sepsis. Among four survivors, three patients (75%) developed malacia of anastomosis with further uncontrollable stenosis, and those patients finally died within 38 days to 7 months postoperatively.
We conclude that carinal sleeve resection with various modes of reconstruction is an elective option for advanced lung cancer, and may be safely performed for other pulmonary pathologies. The surgical approach to the carina and suture types, and planned reconstruction are defined by the experience and skills of the surgeon. Proper selection of patients and an ideal operative technique aiming to release tension along the anastomosis favor a successful outcome. Systematic mediastinal lymph node dissection should be a routine procedure, especially in patients with invasion of mediastinal lymph nodes.
Dr H. Toomes (Stuttgart,Germany): You have a very big experience with this kind of treatment, but I think the times are changing. Have you changed your policy in the last years, because we also in the earlier years performed many sleeve resections of the carina, but with the new multimodal treatments, chemoradiotherapy, we nearly don't have any more?
Dr Poliakov: Yes, but you have to know our circumstances, because our region is around 3.5 thousand cases per year of lung cancer, only 20% have to be resected, and multimodality treatment is usually impossible, only 5% of our patients got multimodality treatment. So surgery is the only one way to treat these kinds of patients. Maybe it is old, but it is true.
Dr M. Furrer (Chur,Switzerland): Resection of a T4 nonsmall cell carcinoma is usually considered to be justified only without mediastinal lymph node involvement. You showed us that you had only a 30% rate of mediastinoscopy. Could you comment on your preoperative work-up of these patients?
Dr Poliakov: It is a useful procedure, mediastinoscopy, but even if you perform mediastinoscopy, you can show about the lymph node involvement totally. You have to perform open surgery to obtain each lymph node. You have to see what happened in the pleural cavity in the mediastinum. It is the best way to obtain resectability.
Dr G. Stamatis (Essen,Germany): I have two questions. The first one, you have shown that in N2 disease the five-year survival is under 7%. Do you exclude now patients with N2 disease for resection? And the second question, you have shown a high incidence of anastomosis problem. Do you need tissue to cover the anastomosis?
Dr Poliakov: Yes, all patients who had N2 were included in the overall survival rate, with T3 and T4, of course. But there was only a small amount of N2 patients. So without N2, I showed N0N1 survival more than 40%, to the one question.
And the second one, usually we used a pedicle flap from pericardium or surrounding tissue as an omental flap, but in 40 cases we didn't use any covering for the anastomosis. It means when the length of resection less than around 2 cm or 3 cm.
Dr P. Macchiarini (Hannover,Germany): I think this is a wonderful work because it is so far as I know the largest experience worldwide on carinal resection, which is twice as much T1 presented last year by Mr Grillo's group in the Harvard Medical School. I would like to ask a question and make some comments.
The first one is that I had the distinct privilege to be trained by Philippe Dartevelle, who has a wonderful experience on this topic, and he told me, since the beginning, don't do an operation like this in patients with N2 disease except if you have a station 7, which makes sense in terms of long-term survival. Yet I see from your presentation that you have, depending on the T stage, T3, T4, an N2 status in between 30 and 66%, and unfortunately, well, you did 33% in the patients mediastinoscopy, but, as you stated, maybe incorrectly, only to open the pretracheal plane and not to dissect the mediastinum. Yet of these patients, only 20% underwent some form of radiation therapy or chemotherapy. Would you like to comment on that, please.
Dr Poliakov: It is our policy, we perform mediastinoscopy for these patients, but it is better to see what is happening in the pleural cavity and the mediastinum without mediastinoscopy. But the mediastinoscopy is the usual procedure for a left thoracotomy for the left-side resection, especially for the left thoracotomy.
Dr Macchiarini: The second question is, Mr Grillo showed us that if you exceed the 4 cm length of resection of the tracheobronchial tree, you have always a problem in terms of tension of the anastomosis, and yet you had 37% of patients that had more than 6 cm.
Dr Poliakov: Yes, it is true, but it is included in the mortality, because sometimes it is unexpected resection during the surgery. So it is a small number of patients with a resection more than 6 cm.
Dr P. Dartevelle (LePlessisRobinson,France): This is one of the biggest series ever to be presented. I have two questions for you. You had quite a lot of anastomotic problems as well as fistulas and empyemas. I wonder whether preoperative radiotherapy you think has contributed to that, and if you looked at those patients without radiotherapy, if the anastomotic problems were less?
And the second question concerns complex carinal resection, do you have facilities for extracorporeal circulation to be assisted when it is necessary?
Dr Poliakov: Related to the second question, we have no possibilities for extracorporeal circulation.
And for the first question, yes, there is no significance between the patient who was done with radiotherapy and for other patients; there are no differences, significant differences.
Dr D. Dougenis (Patras,Greece): It is a very nice series, but I don't agree with your indications because you cannot do an operation with a 50% mortality rate for N2 disease when you have no five-year survival rate, and I think that half of your indications were bad indications. In our series in my own hospital, the survival rate after 100 carinal pneumonectomies is 45% for selected patients, and the mortality rate is 6%, and it is probably because the patients are very well selected.
But I agree with you for the technique. I think that even for wide pneumonectomy, for wide carinal pneumonectomy, perhaps the median sternotomy is the best approach, and for left carinal pneumonectomy, it is true that the median sternotomy is the best approach to do a radical resection, and it is not very difficult if you mobilize totally the aortic arch of the aorta.
Dr Poliakov: Thank you very much, but it is our policy, it is more convenient for us to perform this by sternotomy because we did not need to mobilize the aortic arch in these cases. It is our manner.




