-
PDF
- Split View
-
Views
-
Cite
Cite
Richard D. Page, Aung Y. Oo, Glen N. Russell, Stephen H. Pennefather, Intravenous hydration versus naso-jejunal enteral feeding after esophagectomy: a randomised study, European Journal of Cardio-Thoracic Surgery, Volume 22, Issue 5, November 2002, Pages 666–672, https://doi.org/10.1016/S1010-7940(02)00489-X
Close - Share Icon Share
Abstract
Objective: Patients undergoing esophagectomy are typically nutritionally depleted and cannot establish oral feeding for up to a week after surgery. We have investigated the routine use of enteral feeding via a naso-jejunal tube. Methods: Forty consecutive patients undergoing a transthoracic esophagectomy for cancer were randomised to receive enteral feeding or intravenous crystalloid fluids after surgery. Nutritional indices were obtained prior to surgery and on the 7th post-operative day. Results: There were no post-operative deaths. Non-fatal complications occurred in 10 patients, without difference in morbidity between the two groups. Lean body mass did not change in either group over the study period. No differences in any other parameters were identified between the two groups. Conclusion: Enteral feeding via a naso-jejunal tube is safe and well tolerated after esophagectomy. It is a simple method of providing nutritional support prior to the re-introduction of oral feeding. However it provides no measurable benefit over intravenous hydration only for patients undergoing routine esophagectomy.
1 Introduction
Patients presenting with esophageal cancer frequently have nutritional difficulties due to the obstructive nature of their tumour in addition to the general catabolic effects of their malignancy. Significant weight loss is common and in patients who undergo surgery to resect the tumour, there is often concern that the patient's poor pre-operative nutritional status may increase the risk of post-operative complications. The fact that no oral intake is allowed until the esophageal anastomosis has healed sufficiently (i.e. for up to a week after surgery) makes the situation even more worrying. These concerns coupled with the catabolic response of the body to major surgery makes post-operative nutritional support logical in the early post-operative period for all patients undergoing esophageal resection [1–3].
Historically it has been our practice to avoid enteral or parenteral feeding, mainly for the sake of simplicity and early post-operative mobilisation. Our patients receive intravenous crystalloid infusions alone, usually via a peripheral vein. Oral water is commenced after 4 days and the patients’ intake is gradually increased toward unrestricted swallowing of semisolids by day 7. Using this regime we have achieved consistently acceptable results [4]. We have tended to avoid routine parenteral feeding via a central line, and have been concerned that a formal surgically created feeding jejunostomy was an unnecessary addition to the operation, given its potential for specific complications [5].
Nevertheless with the renewed interest in enteral feeding over recent years we felt it appropriate to investigate its place in the routine management of our patients undergoing esophageal resection. We therefore compared our standard technique of hydration only with crystalloid via a peripheral vein, with post-operative nutritional support using enteral feeding via a naso-jejunal feeding tube. Our aim was to detect: (1) whether this feeding technique was feasible and safe after esophagectomy; and (2) whether post-operative nutritional support led to any measurable benefit in our patients’ recovery after surgery.
2 Material and methods
The study was approved by the local hospital ethics committee. Informed consent was obtained from 46 consecutive patients undergoing esophageal resection for carcinoma under the care of one surgeon (RDP) over a 12-month period. The patients were referred from a population base of approximately 750 000 and were selected for surgery on the basis of an absence of metastatic or locally unresectable disease and adequate general performance status. Nutritional status per se was not a factor used in patient selection. Pre-operative weight loss and nutritional status were assessed, the latter by the bioimpedence method to give data on lean body mass, body fat and body water contents [6,7]. Serological and haematological indices were documented.
2.1 Operative technique
Patients were premedicated with oral diazepam and a thoracic epidural catheter was inserted at the T4–T7 level. All patients received a bolus of 10 μg/ml fentanyl in 0.1% bupivicaine. Anaesthesia was then induced with propofol and maintained with isofluarane in oxygen. Neuromuscular blockade was achieved with atracurium. A left-sided double lumen endobronchial tube was positioned with a fibre-optic bronchoscope to allow single lung ventilation. An infusion of fentanyl/bupivicaine was commenced into the epidural catheter, maintained throughout the subsequent surgery, and continued during convalescence at a rate of 2–10 ml/h until oral analgesia could be substituted on the 7th day after surgery. Antibiotic prophylaxis consisted of cefotaxime 1 g twice daily for 48 h.
All resections were carried out via a left thoraco-abdominal sixth space incision. The tumour was resected en-bloc with adjacent tissue including areas of lymphatic drainage from the celiac axis and its branches to the level of esophageal transection. The latter was located to ensure removal of at least 10 cm of uninvolved oesophagus proximal to the tumour, and resulted in six patients requiring a cervical anastomosis (subsequently excluded from further study). The remaining 40 patients underwent an intrathoracic esophagogastric anastomosis using an anastomotic stapling device; in the majority of patients this was at the apex of the chest with the gastric tube being routed transpleurally lateral to the aortic arch. A gastric drainage procedure was not performed routinely but a pyloromyotomy was deemed necessary in seven patients because of evidence of pyloric scarring and narrowing.
After completion of the esophago-gastric anastomosis a double lumen naso-jejunal feeding tube (Entrafeed 2, Medicina, Cookham, Berkshire, UK – Fig. 1) was inserted by the anaesthetist and retrieved via the anterior distal gastrotomy which had been created to insert the anastomotic stapler. In all cases the distal limb (feeding) port of the tube was positioned in the proximal jejunum, and the proximal (aspiration) port positioned in the body of the stomach. The tube was secured with surgical tape to the patient's nose. After completion of surgery all patients were extubated immediately and nursed in a high dependency area. The position of the feeding tube was checked on plain chest and abdominal radiographs (Fig. 2) . The proximal port was left on free drainage to a bag and aspirated every 4 h to decompress the gastric tube. During surgery and over the following 24 h intravenous crystalloid was given for hydration and additional colloid or blood transfused depending on volume status.
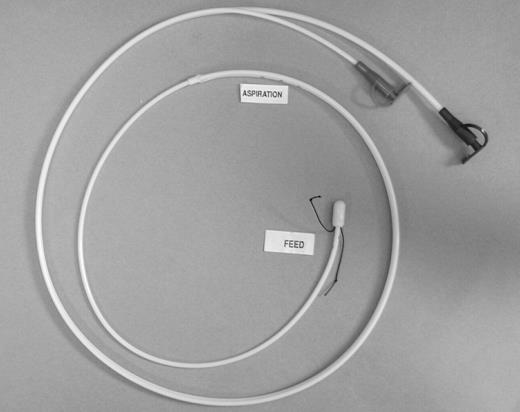
‘Entrafeed 2’ double lumen naso-jejunal feeding/aspiration tube. It measures 110 cm in length, the feeding lumen having an internal diameter of 1.5 mm. The aspiration port has multiple side holes and is of larger calibre. There is a silk suture at the tip for grasping with endoscopic forceps, should it be necessary to insert the tube with a flexible gastroscope.
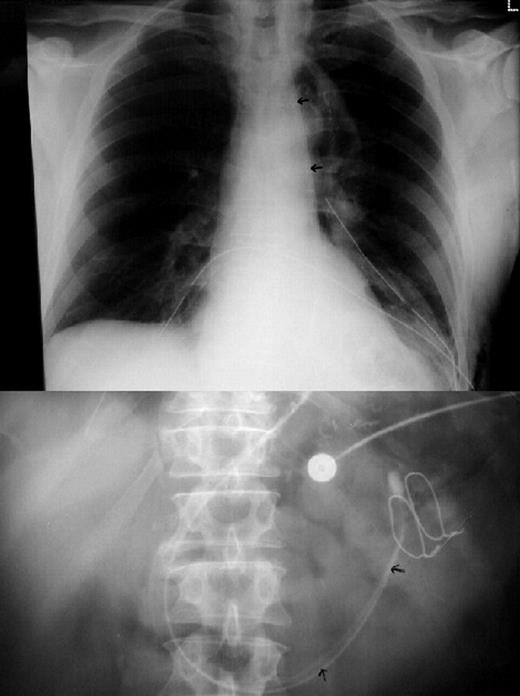
Plain chest (top); and abdominal (bottom) radiographs taken in the immediate postoperative period, to confirm accurate positioning of the feeding tube. The aspiration lumen and feeding lumen are both visible (arrowed) in the gastric tube and proximal small bowel.
2.2 Randomisation and feeding protocols
On the morning following surgery patients were randomly assigned to either control or feeding groups using sealed envelopes.
2.2.1 Control group
Hydration was maintained with intravenous crystalloid via a peripheral vein, using a combination of 5% dextrose, 0.9% saline and potassium supplements as indicated by daily serum electrolyte measurements. The naso-jejunal tube was maintained until aspirates from the proximal (gastric tube) port were minimal at which time the tube was removed, usually on day 2 after surgery. The distal (jejunal) port was not used. Oral intake was forbidden until day 4 after surgery when water was introduced at a rate of 30 ml/h for 24 h, rising to an unrestricted intake of water by the start of day 6. Intravenous crystalloid was reduced at a rate governed by the patient's oral intake, and discontinued on day 6. Nutritional oral intake was introduced in largely liquid form on day 7, and further increments of semi-solid and solid diet introduced depending on patient tolerance, aiming for a free and unrestricted diet by day 10.
Neither enteral nor parenteral nutritional substances were given. Contrast swallows were not requested routinely during the post-operative period.
2.2.2 Feeding group
Standard isocaloric enteral feed (1048 kcal and 40 g protein per litre) was infused into the distal (jejunal) port of the naso-jejunal feeding tube. Energy and fluid requirements were calculated according to individual patient needs taking into account total body weight and bioelectrical impedance parameters. Infusion of feed commenced at 25 ml/h and increased by 25 ml/h every 4 h until the calculated target volume was reached (35 ml/kg body weight/day – e.g. for a 70 kg patient=2000–2500 kcal and 80–85 g of protein per day). Supplementation of the feed with water was carried out as indicated to ensure adequate patient hydration. Intravenous crystalloids were reduced proportionally as the enteral feeding was increased and discontinued once the target rate of enteral feeding was achieved. Aspiration from the proximal (gastric) port was continued every 4 h, to ensure the absence of reflux of feed into the interposed stomach, and to avoid gastric distension. Oral intake was established as in the control group, and enteral feeding discontinued when a free oral fluid intake had been achieved, usually by the end of day 6. The naso-jejunal tube was then removed.
The position of the naso-jejunal tube was monitored every 4 h at the patient's bedside, by measuring the length of tube lying outside the patient. Its position was checked radiographicly every day, or at anytime when there was a suspicion that it had become dislodged. If the distal feeding port became accidentally withdrawn into the interposed stomach, enteral feeding was abandoned, and the tube removed. The patient was then treated as per the control protocol.
In both feeding and control groups haematological, serological and nutritional variables as obtained prior to surgery were measured once again on day 7 after surgery.
2.3 Data analysis
All data within each group of patients were pooled and expressed as mean±standard deviation (SD). Values for pre-operative data (day 0) were compared with those available on day 7 within each group, using the paired Student's t-test. Days 0 and 7 values were then compared between groups using the unpaired t-test. Finally the change in value for each parameter (i.e. day 7 value minus day 0 value) was calculated for each patient, and the resulting values pooled as mean±SD. These values were then compared between the control and feeding groups, again using the unpaired t-test, to more sensitively identify a difference between the two treatment protocols. A P value of less than 0.05 was considered significant.
3 Results
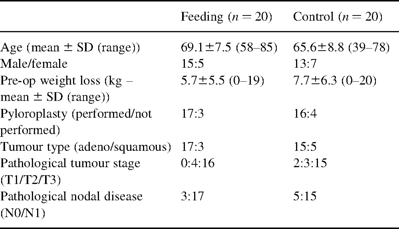
Six patients who required a cervical esophagogastric anastomosis because of the proximal position of their tumour, and who therefore could not have the naso-jejunal tube inserted under vision were deemed ineligible for the study immediately after surgery. The remaining forty patients were randomised as described. No patients in the study period were inoperable. There were no differences in demographic, operative or oncological variables between the feeding and control groups as shown in Table 1 . Pre-operative weight loss varied from 0 to 19 kg, and was of a similar degree between the two groups.
All patients fed enterally achieved their target feeding volume (35 ml/kg body weight/day) by the end of day 1, (i.e. on the day following surgery). The required rate of feed infusion was maintained without problems over the ensuing week. This resulted in patients receiving 2232±79 kcal/day, and 83.2±5.6 g of protein/day over the period of enteral feeding. The group of patients receiving crystalloid solutions only necessarily received a negligible calorific intake and no protein.
Enteral feeding was discontinued prematurely in seven out of the 20 patients because of accidental dislodgement of the naso-jejunal feeding tube, occurring most frequently on the 5th post-operative day (Table 2) . Overall feeding time was 5.3±1.0 days (range=3–7 days). In one patient who was experiencing problems with sputum retention we deliberately removed the tube on day 3 after surgery, because of our concern over his inability to expectorate effectively in the presence of the tube. Although pulmonary aspiration was of theoretical concern in this patient, the volume of fluid obtained from the stomach via the proximal port of the feeding tube was minimal in this and all the other patients. At no time did the enteral feed appear in the aspirated material. Other than the above, no specific problems were identified as being directly due to enteral feeding and the tube was reasonably well tolerated by the majority of patients.
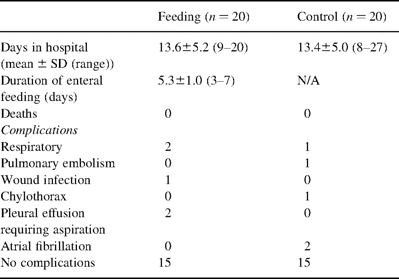
No patients in the study died in hospital. Post-operative morbidity and hospitalisation times are shown in Table 2. Septic complications requiring antibiotic therapy consisted of one wound infection, and three patients developing pneumonia. None of these patients required ventilatory support. No patients required surgical re-exploration for any reason. All anastomoses and gastric suture lines healed without problems. No differences were identified between feeding and control protocols.
Nutritional, haematological and serological parameters prior to surgery and on the 7th post-operative day are shown in detail in Table 3 . Total body weight, body mass index, lean body mass, body water content and body fat did not vary significantly over the study period in either the feeding or control groups. As would be expected haemoglobin concentrations fell significantly in both groups. Blood usage was similar in the two groups of patients with a mean±SD of 1.4±0.4 units being transfused per patient (range=0–4 units). Leukocyte counts and C-reactive protein levels rose in both groups, in keeping with the usual systemic inflammatory response to surgical trauma. The latter along with haemodilution presumably accounted also for the significant fall in serum protein, albumin and transferrin levels in the two groups. The changes in serum electrolyte, creatinine and urea concentrations seen in both groups were small.
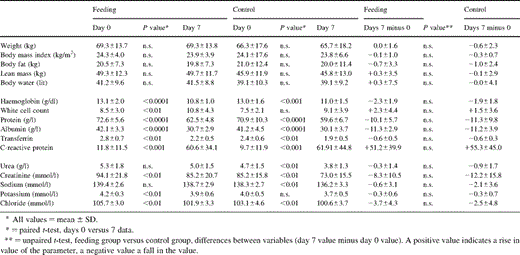
Nutritional, haematological and serological parameters prior to surgery and on the 7th post-operative day
No differences were detected when comparing the groups, with respect to both the pre-operative baseline data and the data on day 7. When analysing the differences in the above variables over the study period (i.e. the day 0 or pre-operative value minus value on the 7th post-operative day) the degree of variation was very similar when comparing the feeding and control groups.
4 Discussion
There is good evidence available from studies of patients involved in major trauma and undergoing major surgery that enteral feeding can reduce the incidence and harmful sequelae of major complications [8,9]. Immune competence may be enhanced and wound healing optimised. Keeping the gut functional by artificial enteral feeding is thought to normalise the resident intestinal bacteria population, and allow the intestinal mucosa to maintain its integrity [10,11]. The reduction in septic complications that results may be directly due to a reduction in translocation of bacteria from the gut into the circulation. It would seem logical that enteral feeding after esophageal resection would therefore be highly beneficial, in what is almost always a nutritionally challenged group of patients.
We were impressed by the simplicity and feasibility of enteral feeding via the naso-jejunal method in our patients. The tube was generally well tolerated, and functioned effectively, allowing all patients to receive their target caloric and protein intake. We had some concerns that this technique of enteral feeding would cause feed material to reflux into the transposed stomach from the small bowel, cause distension of the stomach, and thereby jeopardise the gastrotomy suture lines and esophageal anastomosis. Aspiration of feed into the lungs was another concern [12]. In the feeding group of patients these potential adverse effects of enteral feeding were not observed and minimal aspirates only were obtained from the proximal (aspiration) port of the feeding tube.
Although we feel the technique is safe and effective in providing nutritional support, the data obtained from this small study shows no measurable benefit to support the routine use of enteral feeding after esophageal resection. Patients who received intravenous crystalloids underwent very similar metabolic changes to those fed enterally when analysing the parameters measured in the study (plasma proteins, serum electrolytes, and haematological variables). More detailed nutritional analysis failed to reveal any real changes at all over the first post-operative week in any of the patients. Although no patients died in the study period, morbidity was unaffected by the decision to feed or not in this group of patients.
A valid criticism of studies that show no differences when trying to compare two techniques may be that insufficient patients have been recruited. However the fact that not even a trend to a benefit with enteral feeding was observed in our 40 patients (in the various nutritional and metabolic end-points presented in Table 3) suggests that enteral feeding is unlikely to confer a major and significant benefit for post-esophagectomy patients, compared with intra-venous hydration only.
An almost identical study protocol, applied to patients undergoing non-esophageal intestinal resection was published recently [13]. Although the authors felt that enteral feeding was beneficial when compared to intravenous crystalloid alone, nutritional data did not support this view. There was a lower incidence of relatively minor complications such as nausea, diarrhoea, and abdominal distension in the patients fed enterally. An earlier study of similar design, again in patients undergoing non-esophageal intestinal resection failed to show any nutritional benefit with enteral feeding [14]. Although some of our patients did experience diarrhoea at various times during their hospital stay, this was not troublesome, did not delay their recovery and did not require specific treatment.
Our ability to re-establish adequate oral feeding in all the patients in the study within only a week after surgery has presumably negated any beneficial effect early enteral feeding might have given to the subjects. Although complications did occur, none were serious enough to delay oral intake, and in particular none of the patients had problems with healing of gastric suture lines or the esophago-gastric anastomosis. There was no observable difference between the two groups in the ability to re-establish oral feeding in the days leading to discharge from hospital. It might have been assumed that those patients fed enterally would have intestines which would thereby be ‘prepared’ to more easily accept the re-introduction of oral feeding, leading to some benefits between day 7 (when most of our observations were made) and discharge from hospital. However this potential benefit was not in evidence, and the hospitalisation times of the two groups were almost identical (Table 2). Although it would have been interesting to obtain further nutritional data at a later stage in convalescence for example at day 14 after surgery, this would have meant keeping many patients in hospital longer than otherwise indicated. We felt this was not justified, especially as the day 7 data showed no trend at all to indicate a benefit from enteral feeding.
In other series of patients undergoing esophagectomy early enteral feeding has been given in the post-operative period via a surgically constructed jejunostomy. In addition to its use in hospital, we are aware of some surgeons who routinely discharge patients home before adequate oral feeding has been established (e.g. within a week of surgery), and who use out-patient enteral feeding supplementation via a surgical jejunostomy for several weeks. This has the potential nutritional advantage of allowing the patient a longer period of time to adjust the difficulty with post-prandial fullness that always results to some degree after esophagectomy. In addition it is self-evident that if there are problems with the healing of internal suture lines (e.g. anastomotic leakage or dehiscence) then a previously constructed formal jejunostomy has the advantage of providing a means of nutritional support in the context of a sick and septic patient. In this situation enteral feeding is likely to be much more beneficial than the patient recovering from an esophagectomy without these specific complications. However although many authors attest to the safety of a surgical jejunostomy as well as efficacy in terms of delivery of nutrients [1–3,15–18], others have reported a less favourable experience [5,14,19]. One series [5] looking at 143 patients was particularly worrying in this regard. Complications included peritonitis, aspiration pneumonia, necrotising fasciitis at the site of catheter insertion, catheter dislodgement, occlusion and leakage. The overall complication rate was 55% with ten patients suffering serious or fatal problems, including five cases of fatal small bowel necrosis. The newer technique of needle jejunostomy may be safer than the traditional tunnel method [20] but can still cause major problems. In a series of 100 patients undergoing major abdominal surgery described by De Gottardi three patients required re-operation because of jejunostomy-related complications [21]. As we could detect no measurable benefit for our patients undergoing esophageal resection who received enteral feeding, we do not feel the routine use of a surgically-constructed feeding jejunostomy is justified, given its potential for serious procedure-related morbidity.
The naso-jejunal method has proved to be safe in both our study and that of Carr's [13]. Apart from dislodgement or patient discomfort it would seem to be free from specific problems. We continue to use it in those patients who we feel have a high risk of complications after esophagectomy, e.g. those with severe weight loss, cachexia, etc. For patients who for some reason are unable to establish oral feeding within a week after surgery, the tube (if not already in place) can be inserted in the post-operative period. A flexible gastroscope, with a snare or biopsy forceps is used to hold the silk thread adjacent to the distal port of the tube, and manipulate it into the jejunum. General anaesthesia facilitates the gentle introduction of the gastroscope and tube, to obviate trauma to internal suture lines. One other indication is for patients requiring specific drugs in the early post-operative period, which can only be administered via the enteral route (e.g. some anti-Parkinson and anti-angina medications). In these patients the tube is inserted under direct vision at the time of esophageal resection.
In summary, post-operative nutritional support via a naso-jejunal feeding tube is safe and effective after esophagectomy. However this small study shows no detectable objective benefit in terms of an impact on the rate of complications, ease of re-introduction of oral feeding, or a reduced hospital stay. For our routine practice we continue to support our patients with intravenous crystalloid only until they are swallowing normally. We have been impressed with the ease of use of the double lumen naso-jejunal feeding tube, and prefer it to a surgically constructed jejunostomy in those patients in whom we feel enteral feeding is appropriate.




