-
PDF
- Split View
-
Views
-
Cite
Cite
Alfred Maier, H. Pinter, F. Tomaselli, O. Sankin, S. Gabor, B. Ratzenhofer-Komenda, F.M. Smolle-Jüttner, Retrosternal pedicled jejunum interposition: an alternative for reconstruction after total esophago-gastrectomy, European Journal of Cardio-Thoracic Surgery, Volume 22, Issue 5, November 2002, Pages 661–665, https://doi.org/10.1016/S1010-7940(02)00522-5
Close - Share Icon Share
Abstract
Objective: If the colon cannot be used for reconstruction after total esophago-gastrectomy, alternatives have to be sought. Methods: From 1990 to 2001, retrosternal jejunum loop interposition was performed in 35 patients (male/female: 29:6; mean age 59.2, range 16–83 years) due to cancer in 32 cases and to esophageal perforation in three cases. In all patients reconstruction with stomach or colon, respectively, was impossible on behalf of the underlying histology, of previous resection of the stomach and impedient colonic diseases. A jejunal segment with abundant arcades was chosen, preserving a strong, distal arteriovenous mesenteric pedicle, while 2–3 proximal mesenteric vessels were severed. The loop was pulled up retrosternally, establishing a cervical end-to-side esophagojejunostomy. A Roux-en-Y anastomosis was done in a convenient position. Results: Two patients died perioperatively due to mediastinitis and consecutive multiorgan failure (one Boerhaave's syndrome, one suicidal ingestion of acid). In one case the oral part of the loop developed venous congestion and was replaced by a free jejunal transplant. The perioperative course of 32 patients was uneventful. Seventeen patients are up and well (1–8.5 years). Sixteen patients died of metastatic disease. The functional results are satisfactory. In about 50% of patients recurrent dilatations became necessary due to anastomotic scars. However, none of the patients complained about severe dysphagia. Conclusion: In cases of impedient colonic diseases, the pedicled, retrosternal jejunal loop with cervical anastomosis is an alternative for reconstruction after total esophagogastrectomy.
1 Introduction
Patients in whom total esophagogastrectomy is indicated, are relatively rare [1,2]. Sigillocellular carcinoma of the esophagogastric junction extending into the distal esophagus may show a long range submucosal spread. In these cases an adequate distance of the resection line to the margins of the submucosal tumour can sometimes not be provided by an intrathoracic procedure and resection at the cervical level is necessary. In rare cases, squamous-cell carcinoma of the distal esophagus extends down to the stomach, with lymph nodes invading the cardia and the lesser curvature. Under such circumstances especially in presence of a small stomach and/or prior resection treatment of the stomach, esophageal replacement by a gastric tube is not possible.
Reconstruction of the upper gastrointestinal continuity after total esophagogastrectomy is technically demanding, and associated with a high morbidity and functional swallowing disorders. Throughout the last century, various substitutes for reconstruction of the esophagogastric passage have been created. Segments of skin, the colon and the small bowel have been brought up to the cervical esophagus, using a subcutaneous, retrosternal, intrathoracic or posterior mediastinal pathway. There is still no agreement as to which is the ‘best’ operation [3].
The most often used substitute conduit for reconstruction of the upper gastrointestinal tract after total esophagogastrectomy is the iso- or anisoperistaltic colon interposition [4–7]. However, if for different reasons, i.e. intrinsic colonic diseases, the colon cannot be used, alternative techniques have to be sought.
The aim of this retrospective analysis of patients records was to evaluate the use of a pedicled retrosternal jejunum interposition with cervical esophago-jejunostomy and Roux-en-Y anastomosis, after total esophagogastrectomy. Technical considerations, perioperative complications, functional results, quality of life, and survival were considered as main objectives.
2 Patients and methods
The medical records of 35 patients (29 male and six female; mean age 59 years, range 16–83 years) who underwent total esophagogastrectomy and reconstruction by a retrosternal pedicled jejunum interposition with Roux-en-Y anastomosis were reviewed to determine demographic data, diagnosis, functional results and perioperative complications. Besides oncologically necessary investigations such as physical examination, chest roentgenograms, computed tomography (CT) scan of the thorax and the abdomen, barium swallow, endoscopy, and bone scan in carcinoma patients, follow-up investigations included body weight, presence or absence of dysphagia, nutritional habits, intolerance of certain types of food, borborygmi, singultus, breaking of wind or diarrhea which might give evidence of the functional aspects of the interposition of a jejunal loop. The follow-up intervals range from 1 to 8.5 years (median: 3.2 years).
Routine staging of carcinoma of the gastroesophageal junction and/or the esophagus was done by endoscopy and biopsy, endosonography, esophagogram, CT scan of the thorax and abdomen, and bone scan. In case of questionable resectability staging laparoscopy was performed. Functional assessment included ECG, spiroergometry and cardiac ultrasound. All patients were operated on by two senior surgeons between January 1990 and December 2001.
Twenty-eight patients had adenocarcinoma extending the proximal stomach and distal esophagus. In three cases squamous cell carcinoma of the esophagus in presence of a previous Billroth II resection due to ulcer disease was found. One patient had an esophageal stomal sarcoma also extending into the proximal stomach. Eight patients had TNM stage I, 14 stage II, and 10 stage III carcinoma. In three cases the underlying disease was perforation of the esophagus due to non-malignant causes (Boerhaave's syndrome n=1; acid ingestion n=2).
Reconstruction with gastric pull-up or colon interposition was impossible because of previous resections of the stomach (n=6), and/or tumour extending into the proximal stomach (n=27), and/or impedient colonic diseases such as diverticulitis/diverticulosis (n=23) or a history of colon resection (n=6).
2.1 Surgical technique
The patient was brought into a supine position with the head turned to the right. The entire abdomen, chest, and neck were prepared and draped as a single sterile field. After having confirmed the resectability, the mobilization of the cervical esophagus over a left cervical incision and the dissection of the abdominal esophagus were done simultaneously by two teams.
In presence of a tumour at the esophago-gastric junction the esophagus was dissected transhiatally, with direct view to its distal third, enabling lymph node dissection in this area. The middle- and proximal third were in part bluntly dissected. In presence of lymph nodes showing tumour infiltration during intraoperative frozen section histology, or in case of a T3 tumour inaccessible by the transhiatal approach, the approach was changed to an anterolateral thoracotomy enabling further dissection of the esophagus and its tributary nodes under direct view. After severing the esophagus at a suitable level, its proximal part was pulled out through the cervical incision. Gastrectomy including meticulous lymph node dissection in the left gastric area, at the porta hepatis and around the celiac axis was done.
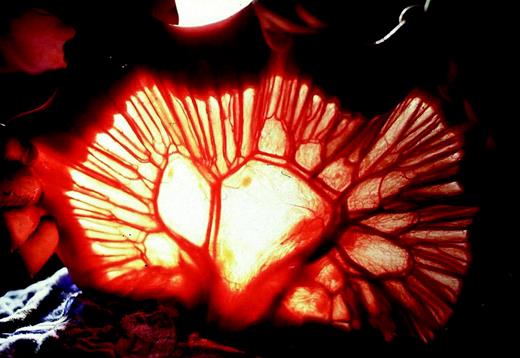
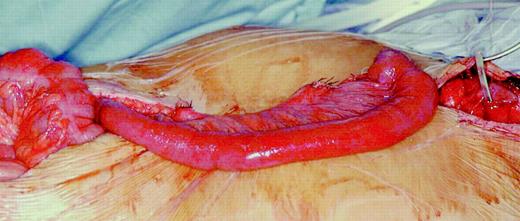
For reconstruction a jejunal segment with abundant arcades was selected. In order to ensure a meticulous dissection of the jejunum, transillumination of the mesenterium was used (Fig. 1) . Fifteen to 20 cm of jejunum distal to the duodenojejunal fold were preserved. From this point the necessary length of jejunum and mesenterium, respectively, were measured, using a tape. At the distal end of the segment planned for interposition the strongest arterio-venous mesenteric pedicle was identified. By tentative dissection and clamping of 3–4 mesenteric vessels proximal to this pedicle for at least 10 min the presence of sufficient collaterals over the arcades was verified. If pulsations in the collateral vessels remained strong and the bowel retained a healthy pink color, the clamped vessels were ligated and severed close to their mesenteric origin. Subsequently, the mesentery was dissected up to the very collateral arcades, enabling a further gain in loop length. Care was taken to preserve the first mesenteric vessel distal to the duodenojejunal fold, ensuring vascular supply to the proximal rest of the jejunum. The jejunum was severed at a position determined by the most arboral branch of the first mesenteric vessel distal to the duodenojejunal fold by using the GIA device (Ethicon Endosurgery Inc., Cincinnati, OH, USA). By this technique, a sufficient length of loop was ensured as documented by tentative presternal positioning (Fig. 2) .
The loop was guided into a retrocolic position; in two cases, however, an exceedingly short mesocolon transversum necessitated the antecolic way. After blunt dissection of the retrosternal space the jejunum was pulled up establishing a cervical end-to-side anastomosis at the level of the thyroid gland in a one-layer manual suturing technique. Care was taken to create a large opening behind the manubrium sterni which would give enough room for the jejunal loop, preventing venous congestion.
A nasojejunal feeding/enteric decompression catheter (Dobbhoff catheter, Sherwood, Davis and Geck, Gosport, UK) was placed in a convenient position and the cervical wound was loosely closed, allowing direct view to the anastomosis for the first postoperative days. The reconstruction was completed by an abdominal Roux-en-Y anastomosis in convenient position.
In a further three cases who had been planned for jejunal interposition, the intervention could not be done due to anatomical considerations (too short mesenterium, poor collaterals). The crucial points in dissecting the pedicled jejunal loop are the preserving of a strong arteriovenous pedicle, and sufficient collateral vessels.
2.2 Postoperative management
Parenteral fluid substitution and early enteral feeding were started within the first 24 h postoperatively, while the retrosternal jejunal loop was decompressed over the second channel of the catheter. The cervical anastomosis was inspected every 6 h throughout the first 2 postoperative days. After fluoroscopy using a water-soluble contrast medium on day 6 the catheter was removed and oral feeding was initiated.
3 Results
The operation time ranged between 4 and 6 h depending on the necessity of an additional thoracotomy. In 32 out of 35 cases planned for jejunal interposition, no technical problems whatsoever occurred. In three cases, however, the mesenterium was fatty and short with sparse collaterals, making dissection of a sufficiently long pedicle extremely difficult. In one of these cases, the upper third of the jejunum developed intraoperative venous congestion due to an insufficient venous drainage. The congested part was resected and anastomosed to a free jejunal loop for which cervical vascular anastomoses (inferior thyroid artery, internal jugular vein) were provided [8]. Esophagojejunostomy was performed between the free jejunal graft and the cervical esophagus.
Two patients died perioperatively due to mediastinitis and consecutive multiorgan failure (one Boerhaave's syndrome and one acid ingestion). Four patients developed anastomotic dehiscence which healed uneventfully within 10 days. All of them had shown a livid hue of the jejunum at the anastomosis on the first or second postoperative day.
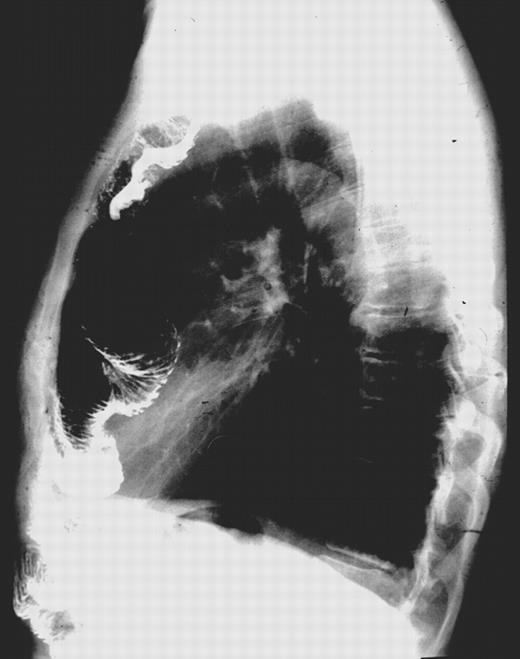
In the other patients the peri- and postoperative courses were uneventful. From the functional point of view, the procedure was very well tolerated. In 17 of 35 patients recurrent dilations of the esophagojejunostomy due to cicatriceal anastomotic stenosis were done on an outpatient basis. There was neither jejunoesophageal reflux nor belching. After an initial weight loss of 7–10%, the weight stabilized. With the exception of milk and other non-fermented dairy products which were not well tolerated by most patients, an almost normal diet was possible. Only one patient who had suffered from intermittent diarrhea due to an irritable colon preoperatively, experienced inconvenient breaking of wind and an increasing frequency of diarrhea after the operation. During follow-up, fluoroscopically controlled contrast swallows showed an undisturbed peristalsis within the jejunal loop during the first year, with slight deterioration of propulsive movements during the second. Thereafter no further changes in peristalsis were found (Fig. 3) .
In the 17 patients who are still alive the functional situation after the jejunal loop interposition was reported as good in 10 of 17 and moderate seven of 17. The main subjective problems were the necessity for the intake of many, small-portioned meals and an intolerance of unfermented dairy products, as well as retrosternal borborygmi. None of the patients complained about dysphagia or regurgitation.
Seventeen patients are up and well (1–8.5 years after operation) and 16 patients died of metastatic disease. None of the patients ever developed local recurrence with consecutive dysphagia.
4 Discussion
Colon interposition is the standard procedure for reconstruction after total esophagogastrectomy [5–7]. However, it carries a number of problems: A minimum of three anastomoses is needed, including a colo-colic anastomosis which has an increased risk of anastomotic breakdown. Especially in anisoperistaltic colon loops, there is the problem of regurgitation with silent aspiration. Moreover, the bulky colon requires a lot of space at the thoracic inlet which often gives rise to external compression of the loop with consecutive problems in perfusion. Postoperatively, an unsightly bulge at the neck as created by the colon may disturb the patients. Redundant segments of the colon loop may give rise to stagnation of food which results in odorous breath. Late complications such as unexplained massive gastrointestinal hemorrhage or colon carcinoma have been reported [9,10]
This is why many surgeons took great interest in the development of alternative surgical techniques for esophageal and esophagogastric replacement [3]. The first successful bypass of the esophagus was performed by Roux in an 11-year-old boy who had an impermeable caustic stricture (1907) [11]. He anastomosed the distal end of an isolated segment of jejunum to the stomach and brought the proximal end up to the level of the clavicles, using a subcutaneous method. When feeding was done over a tube jejunostomy, he observed active peristalsis in the subcutaneous jejunum. Four years later, the esophagus and the jejunum were anastomosed successfully. The jejunum was favoured for esophageal substitution through the 1940s. By 1952, 12 patients had survived antethoracic jejunal reconstruction at the Boston Children's Hospital [12]. In 1944, Yudin reported the largest series in which antethoracic jejunum was used as a substitute for caustic burns of the esophagus [13]. Yet a number of investigators [4–7] found unsatisfactory results of pedicled jejunum interposition because of the crucial blood supply to the highest point of the jejunum and to the organ's susceptibility to gastric acid. Subsequently, the technique seems to have been abandoned. On the other hand, free jejunal autotransplants have been used recently, mainly for cervical esophageal and hypopharyngeal carcinomas [8]. The death rate owing to total necrosis of the transplant remains high [14,15]. Apart from the long duration of the operation due to its requirement for many anastomoses the functional results are far from satisfactory. Due to its complete denervation the free graft is subject to hypo- or a-peristalsis in due course [9,10].
We tried to reinstall the technique of the ‘high’ jejunal loop based upon the following considerations: on the one hand, peptic complications are obviously not to be expected, if the jejunal loop is used for reconstruction after total esophagogastrectomy rather than after esophagectomy; on the other, the potentially precarious vascular situation would be improved by using the retrosternal rather than the antesternal path.
Actually, we saw only few problems in perfusion of the loop. Intraoperatively, both a good arterialization and a sufficient venous drainage of the whole jejunal loop was found in all but one of our 35 patients. Postoperatively, an additional four patients developed a reduced perfusion at the highest point of the jejunum, resulting in anastomotic dehiscence which, however, healed spontaneously without further consequences.
We were able to document that from the technical point of view the dissection of a pedicled jejunal loop with sufficient length to extend up to the neck is possible in most patients, except those with a constitutionally short mesenterium and poorly developed mesenteric arcades. Up to this time a planned retrosternal jejunal loop interposition failed in only three cases. In order to ensure a viable loop, transillumination for localization of appropriate mesenteric vessels and their tentative clamping prior to severing are mandatory. Because of the superficial position of the cervical esophagus the manual suturing of the anastomosis did not present any problems. Due to the need for only two anastomoses the risk of postoperative intra-abdominal anastomotic complications is low. Accordingly, none of our patients showed any intra-abdominal problems. In case of cervical anastomotic breakdown, which we observed in four patients, the infection does not tend to create other than local problems.
Though we did not resect redundant bowel from the middle of the loop, there was no functional impairment: The enteral passage showed hardly any difference to routine intrathoracic esophagojejunostomy. After an initial need for anastomotic dilatation, oral intake did not cause problems in any of our patients [16]. In contrast to reconstruction with the stomach or with anisoperistaltic colon, there was no regurgitation at all. After an initial weight loss the nutritional status of our patients remained stable as long as there was no recurrence of tumour. These findings confirm the satisfactory results of former functional studies concerning absorption by retrosternal loops [16].
In conclusion, cervical esophagojejunostomy using a pedicled retrosternal jejunal segment with distal Roux-en-Y anastomosis seems to be a valid alternative method for esophagogastric replacement, if for one reason or another the colon is not viable. Further studies will be necessary to evaluate the limitations of anatomical feasibility and the functional long-term results.
Dr M. Ximenes (Brazilia, Brazil): Where was the stomach in your cases, was it removed or was it in place?
Dr Pinter: It was removed due to other previous resections.
Dr Ximenes: All or part of it?
Dr Pinter: All.
Dr G. Friedel (Gerlingen, Germany): What was the cause for the two patients with mediastinitis? Were there anastomotic complications or baffle complications?
Dr Pinter: They died due to mediastinitis, one due to acid ingestion and one spontaneous perforation of the esophagus in Boerhaave's syndrome.
References
- boerhaave's syndrome
- deglutition disorders
- gastrectomy
- roux-en-y anastomosis
- anastomosis, surgical
- colonic diseases
- dilatation, pathologic
- mediastinitis
- mesentery
- neoplasm metastasis
- reconstructive surgical procedures
- colon
- histology
- jejunum
- stomach
- transplantation
- multiple organ dysfunction syndrome
- ingestion
- esophagojejunostomy
- esophagogastrectomy
- pedicle




