-
PDF
- Split View
-
Views
-
Cite
Cite
Christophe Doddoli, Pascal Thomas, Xavier Thirion, Yves Serée, Roger Giudicelli, Pierre Fuentes, Postoperative complications in relation with induction therapy for lung cancer, European Journal of Cardio-Thoracic Surgery, Volume 20, Issue 2, August 2001, Pages 385–390, https://doi.org/10.1016/S1010-7940(01)00764-3
Close - Share Icon Share
Abstract
Objectives: The purpose of this study was to evaluate the risk of lung cancer surgery following induction chemotherapy and/or radiotherapy. Methods: This retrospective study included 69 patients treated from January 1990 to January 1998 for a primary lung cancer in whom surgery had been performed after induction treatment. Surgery had not been considered initially for the following reasons: N2 disease (IIIA, n = 25); temporary functional impairment (two stages IB and two stages IIIA (N2), n = 4); and doubtful resectability (stage IIIB (T4), n = 40). The medical regimen resulted in combined radio-chemotherapy in 43 patients who received two to four cycles of chemotherapy (average 2.9±0.8 cycles) and 43±8 Gy (range 20–60 Gy), or chemotherapy alone in 26 patients (3±0.7 cycles). Results: Exploratory thoracotomy was performed in four patients (6%). The in-hospital mortality was 9% (n = 6) from respiratory origin in all cases. There were four re-operations (6%): three for bronchial fistula and one for bleeding. Thirty-five patients (51%) required blood transfusion (4.5±3.8 cell packs). The incidence of early and delayed bronchial fistula after pneumonectomy was 15%. Thirteen patients had a postoperative pneumonia (19%). Conclusions: Surgery for lung cancer after induction chemotherapy and/or radiotherapy is associated with an increased risk. If the mortality seems ‘acceptable’, the morbidity rate, however, is high.
1 Introduction
Surgery remains the best treatment in non-small cell lung carcinoma (NSCLC) every time a complete resection of the tumor and the mediastinal nodes can be achieved. However, results of such a surgery for stage III are poor, especially for N2 disease patients [1]; the large majority (85%) of the patients succumb to their disease in the 2 years (85%) which follow the surgery. This is explained essentially because complete resection is often not possible and non-detectable distant metastasis are frequently present at the time of the diagnosis. Many trials have been done [2–4] using a combination of radiotherapy, chemotherapy, and surgery. The current efforts relate essentially to the evaluation of induction therapy [5–7]. The purpose of such therapy was to reduce the volume of the tumor and of the nodes, allowing a resection in a better condition, and to treat the distant undetectable metastasis in order to improve survival. The results of these studies are discussed. Studies of phase II [8,9] are limited by methodological problems such as the heterogeneity of the studied populations, or the rigor of the clinical staging. Three studies of phase III published to date [6,7,10] involve the same limits and comprise moreover a very reduced population of about 30 patients per group. Very schematically, one can illustrate the effectiveness of such therapy by the following rates: complete response 10%, partial response 50%, stability 30%, progression of the disease 10% [11,12]. Even if the correlation between response to the induction therapy and survival is not perfect, it seems that improvement of survival can be observed if there is a response to the oncological treatment. However, this long-term benefit is modest and could be completely cancelled out by the increase of the early postoperative risks associated with these combined treatments including surgery. Few data on the immediate postoperative risks are available; certain studies suggest an appreciable increase in the postoperative morbidity [13,14]. Thus, the purpose of our study was to evaluate the risk of lung cancer surgery following induction chemotherapy and/or radiotherapy.
2 Materials and methods
This is a retrospective study carried out from January 1990 to January 1998. We included all patients who underwent pulmonary resection for a bronchopulmonary cancer after induction therapy (n = 69). This series accounts for 5% of the total number of patients operated on for lung cancer during this same period. There were 60 men and nine women. The average age was 57±11 years. Thirty patients had a squamous cell carcinoma, 25 an adenocarcinoma and 14 an undifferentiated carcinoma. Radio-chemotherapy had been performed in 43 patients who had received from two to four cycles (mean 2.9±0.8 cycles), and 43±8 Gy (from 20 to 60 Gy). Twenty-six patients had had an exclusive chemotherapy (3±0.7 cures). All regimens of chemotherapy included cisplatin. Surgery had not been considered initially for the following reasons: N2 disease (IIIA, n = 25); temporary functional impairment (two stages IB and two stages IIIA (N2), n = 4); and doubtful resectability (stage IIIB (T4), n = 40). The mediastinal tumor extension and node involvement were mainly appreciated on computed tomodensitometric data. Seventeen patients (25%) had had a mediastinoscopy in the pre-therapeutic assessment. Among the 40 patients in whom the resectability of the tumor appeared to be uncertain, 18 of these (45%) had not previously seen a thoracic surgeon and were only evaluated by pneumologists. The analysis was related to the surgical indication, the type of intervention, and the results of the surgery in terms of postoperative morbimortality by assessing particularly the pulmonary infectious, hemorrhagic and bronchial fistula risk. Data are presented as the mean±standard deviation. The χ2-test was only used if all expected frequencies were greater than or equal to five. Otherwise, Fisher's exact test was used. The unpaired t-test was only used for approximately normally distributed data. Otherwise, the Mann–Whitney U-test was used. Operative mortality included all deaths occurring within 30 days after pulmonary resection or during the same hospital stay. Probability values of 0.05 or less were considered statistically significant.
3 Results
Four patients were operated on before the completion of the treatment because of a complication directly related to this medical therapy: one pneumothorax by tumoral necrosis and three recurrent pneumonitis. The new cardiorespiratory functional status of the four patients contraindicated initially permitted the surgical resection. For the 61 other patients, surgery was indicated in the absence of disease progression, after the neoadjuvant treatment. Among these 65 patients, no complete response as assessed clinically was observed but 35 patients had a partial or major response. The surgery was performed from 4 to 8 weeks after the completion of medical therapy, except for the four patients operated on in a relative emergency.
Open and closed thoracotomy was performed in four patients (6%). Complete resection (R0) was possible in 59 patients. Of the six remaining patients, four had a macroscopic residual tumor (R2) and two had a microscopic residual tumor found on specimen analysis (R1). There were 33 pneumonectomies, 23 lobectomies, one bilobectomy and eight lung sparing resections. Among the 65 patients who underwent a pulmonary resection, 42 (65%) had an extended resection and 23 (35%) had a standard resection. Extensive resections included the pericardium in 25 cases, the thoracic wall in 11 cases, the carena in three cases and the subclavian vessels in three cases.
Thirty-three pneumonectomies were performed, 22 on the right side and 11 on the left side. The bronchial stump was covered 19 times on the right side (90%) and twice on the left side (18%). The type of protection was a pleural flap in 16 cases (76%) or an intercostal pedicle flap in five cases (24%). Each resection included an extensive mediastinal lymph node dissection. A postoperative pathology study of the specimen and nodes showed a down-staging for 50% of the patients in comparison with the pre-oncological treatment; no tumor cells were found in 11 patients (16%).
3.1 Postoperative mortality
The overall in-hospital mortality rate was 9% (n = 6), 8 and 9% following lobectomy and pneumonectomy, respectively. The mortality rate for right pneumonectomy was 14%. There was no difference in mortality regarding the type of induction therapy (RTCT (9.3%) vs. CT (7.7%), P = 0.59, Fisher's test).
3.2 Non-fatal postoperative morbidity
Thirty-four patients had an uneventful postoperative course. Twenty-nine (42%) presented one or more non-fatal complications of very variable gravity. Major complications occurred among 12 patients (17%) and accounted for 41% of all complications. There were four re-operations: three for bronchial fistula, and one for hemothorax. There were six infectious pneumonia, one pulmonary embolism and one renal failure. Major complications were not correlated to the type of resection (lobectomy (8%) vs. pneumonectomy (21%), P = 0.17, Fisher's test) or the type of induction therapy (RTCT (12%) vs. CT (27%), P = 0.09, Fisher's test). Seventeen patients (25%) experienced at least a minor complication. There were five prolonged air leaks (16%), five bronchopulmonary obstructions including two atelectasis (16%) requiring bronchoscopy, four arrhythmias, four infectious pneumonia, two vocal cord paralyses and one allergy to heparin.
3.3 Hemorrhagic risk
Thirty-five patients (51%) were transfused of on average 4.5±3.8 cell packs (Table 1) . There were significantly more transfusions in the group of patients who received an exclusive chemotherapy.
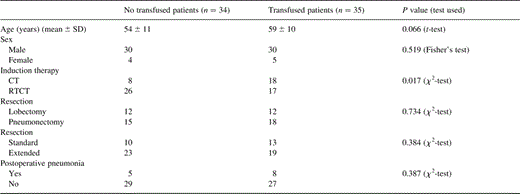
Characteristics of patients according to risk of blood transfusion
3.4 Pulmonary infectious risk
Thirteen cases of postoperative pneumonia (19%) were observed, nine severe (13%) including three having led to death, and four minor (6%). Among these pneumoniae, there were three cytomegalovirus pneumoniae, and one fungic pneumonia. Older patients had had a significantly higher incidence of postoperative pneumoniae (Table 2) .
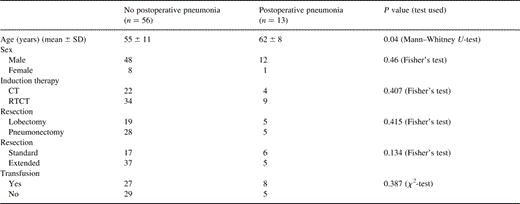
Characteristics of patients according to risk of postoperative pneumonia
3.5 Bronchial fistula
The characteristics of the five patients who developed a bronchial fistula are shown in Table 3 . This complication represented 15% of the patients who underwent a pneumonectomy. All these bronchial fistula occurred on the right side representing a 23% incidence following right pneumonectomy. Patient 1 died at month 10. Patient 5 died at day 40 from a hemorrhage due to the erosion of the superior vena cava related to iterative dressing changes. Patient 4 died at month 6. Fistulae were more common following radio-chemotherapy (19%) than chemotherapy alone (8%), but the difference was not significant (P = 0.39, Fisher's test).

Characteristics of patients who developed a postoperative bronchial fistula
4 Discussion
The overall rate of mortality observed in this series is 9%. This rate is ‘acceptable’ since in reports relating to induction therapy, postoperative mortality varies from 5.2 to 11% [2,3,12,14–16]. However, this rate is higher than what is currently observed after a surgery of first intention [17,18], suggesting an increase risk of preoperative oncological therapy. We observed a postoperative mortality of 8% after lobectomy. Mathisen et al. [3] and Rice et al. [12] also reported higher rates of 7.4 and 10%, respectively. A mortality lower than 2% is indeed commonly allowed under standard conditions. On the other hand, almost all the series report on an overmortality of pneumonectomy, ranging from 10 to 17.5% [2,12,14,15,19]. It was 9% in our experience. In a series of 13 patients, Fowler et al. [13] reported three deaths (43%) and warns against the induction therapy associating RT and CT. This excess of mortality following pneumonectomy is usually explained by the risks of bronchial fistula and pulmonary edema [2,13,15,19]. Macchiarini et al. [19] and Martini et al. [15] estimated that this edema was primarily related to the pulmonary toxicity of mitomycin. In our experience, only one patient received such chemotherapy. The pneumotoxic effect of the mitomycin is probably increased by a preoperative 100% O2 delivery and a combined RT exceeding 45 Gy [20,21]. Other factors are probably involved, such as the interruption of the lymphatic drainage of the remaining lung due to extensive lymph node dissection, explaining why these organic edemas are more frequently observed after right pneumonectomy, which is associated with a more complete radical lymph node resection than left pneumonectomy. Infectious factors, in particular of viral origin, can also be a cause [22]. In the study of Macchiarini et al. [19] the complications were more frequent after RTCT (42%) that after isolated CT (9%). In our series the operative mortality was not associated with the type of resection (lobectomy vs. pneumonectomy) or the type of induction therapy (RTCT vs. CT). Two series having used preoperative radiotherapy of 60 Gy report a prohibitory mortality after pneumonectomy, reaching 33% [8] and 43% [13].
The incidence of bronchial fistula in our series (15%) is considerable and represents 23% after right pneumonectomy. Sugarbaker et al. [11] observed three cases from a total of 17 pneumonectomies, i.e. 15%. Fowler et al. [13] and Deutsch et al. [8] also reported a high frequency of bronchial fistula when preoperative radiotherapy of 60 Gy had been used. All these rates are definitely higher than those observed after standard surgery which vary from 4 to 7% [17,18]. It seems that this excess of bronchial fistula is related to tissue damage due to RT.
It was not possible from a statistical point of view to identify predictive factors of bronchial fistula for reasons of sample size. Nevertheless, our descriptive data are eloquent. It should be stressed that all these bronchial fistula occurred in spite of bronchial stump reinforcement. This failure has two explanations: on one hand, radical lymph node resection performed routinely by our team that favors the devitalization of bronchial tissue, already deteriorated by the preoperative therapy, and on the other hand, the quality of the flap used to cover the bronchus (pleura or intercostal muscle). The intercostal muscle [23] flap may be deteriorated by RT because it is initially located in the RT field. The pleural flap conveys insufficient tissues to widely cover the bronchial stump. For all these reasons, we now favor a diaphragmatic flap [24].
The use of the serratus anterior muscle was also reported [23] but its transposition may lead to scapular winging; moreover, it is always in an irradiated field. Finally, we reserve the use of the omentum for an eventual postoperative empyema with or without fistula [14]. Indeed, requiring a laparotomy which raises the deterioration of the pulmonary function due to the thoracotomy, its use of first intention [23] seems a matter of debate.
Fifty percent of the patients were transfused. This hemorrhagic risk was already reported [2,13,16,19,25] in surgical series after induction therapy. This is of concern regarding the potential risks of infectious disease.
The incidence of pneumonia in this series was 19%. This rate is very high when compared to rates in the literature [2,8,11–14,16,19,25], which range from 0 to 9%. This difference is probably explained by the problem of the criteria used to define postoperative pneumonia. It appears in our work that an advanced age is a predictor of postoperative pneumonia. In our series four patients had a viral or fungic pneumonia, etiologies which are not common after ‘conventional’ surgery. This fact is probably in accordance with the immunosuppressive effect induced by the medical therapy. This specific morbidity had never been mentioned. The diagnosis of cytomegalovirus pneumonia remains difficult, and usually requires a histological diagnosis complemented by a possible positive viremy or positive cultures from bronchioloalveolar lavage. This histological confirmation can be obtained from a transbronchic biopsy, or from a surgical lung biopsy which only provides a sufficient quantity of tissue for multiple microbiological searches.
The rate of re-operation observed in this series (6%) was directly dependent on the hemorrhagic and early bronchial fistula risks. The risk of re-operation is seldom mentioned in work relating to the surgical resection of lung cancer after oncological treatment. Nevertheless, rates of 17% [9,19] and 33% [14] were reported.
Table 4 summarizes the data available in the literature dealing with postoperative deaths and complications after induction therapy.
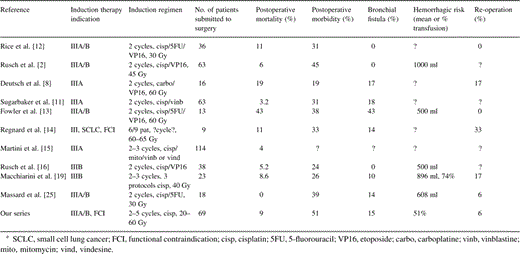
Data available in the literature dealing with postoperative deaths and complications after induction therapya
5 Conclusion
Surgery after induction therapy is associated with an increased risk as the consequence of (1) the high incidence of pneumonectomy in this subset of patients, and (2) the occurrence of ‘specific’ complications. This suggests favoring extended arterial and bronchial lobectomies whenever possible, and reinforcing the bronchial stump with a diaphragmatic flap when the pneumonectomy is unavoidable.
References
- radiation therapy
- hemorrhage
- chemotherapy regimen
- postoperative complications
- blood transfusion
- bronchial fistula
- hospital mortality
- neoadjuvant therapy
- pneumonectomy
- surgical procedures, operative
- thoracotomy
- morbidity
- mortality
- surgery specialty
- lung cancer
- radiochemotherapy
- functional impairment
- postoperative pneumonia
- chemotherapy, neoadjuvant




