-
PDF
- Split View
-
Views
-
Cite
Cite
Tommaso Claudio Mineo, Vincenzo Ambrogi, Vincenzo Corsaro, Mario Roselli, Postoperative adjuvant therapy for stage IB non-small-cell lung cancer, European Journal of Cardio-Thoracic Surgery, Volume 20, Issue 2, August 2001, Pages 378–384, https://doi.org/10.1016/S1010-7940(01)00779-5
Close - Share Icon Share
Abstract
Objective: Although surgical resection alone is considered adequate treatment in stage IB non-small-cell lung cancer (NSCLC), long-term survival is not satisfactory and the recurrence rate is quite high. The validity of postoperative chemotherapy at stage IB in terms of disease-free and overall survival was assessed in a randomised trial. Methods: The trial was designed as a randomised, two-group study with postoperative adjuvant chemotherapy versus surgery alone as control group. All patients had stage IB disease (pT2N0) assessed after a radical surgical procedure. Chemotherapy consisted of treatment with cisplatin (100 mg/m2 on day 1) and etoposide (120 mg/m2 on days 1–3) for a total of six cycles. Results: Between January 1988 and December 1994, 66 patients were included in the study. Thirty-three belonged to the adjuvant chemotherapy group and 33 to the control group. Groups were homogeneous for conventional risk factors. There was no clinical significant morbidity associated to chemotherapy. Patients were followed for a minimum period of 5 years. The rates of locoregional recurrence and distant metastases were 18 and 30%, respectively, in the adjuvant chemotherapy group and 24 and 43%, respectively, in the control group. The 5-year disease-free survival rates were 59% in the adjuvant group and 30% in the control group P = 0.02. The difference in the Kaplan–Meier survival between the groups was significant as assessed using the log-rank test P = 0.04. Conclusions: Our results suggest that adjuvant chemotherapy may reduce recurrences and prolong overall survival in patients at stage IB NSCLC deemed radically operated. Despite being difficult to accept, the use of adjuvant chemotherapy might have better long-term results.
1 Introduction
Radical surgical resection is considered adequate treatment for providing survival benefits for early non-small-cell lung cancer (NSCLC), especially for those patients without lymph nodal involvement [1]. Nevertheless, long-term survival is not satisfactory and the recurrence rate is quite high even in the case of radical surgery [2–4]. This is probably due to micrometastases present at the time of primitive surgery [5–7] but not detectable by conventional diagnostic methods and not eradicable by surgery. It is well known that distant metastasis disease is the dominant site of recurrence in such patients and this observation has served as the basis for trials of postoperative systemic therapy. A recent meta-analysis of all randomised trials from January 1965 to December 1991 showed that the absolute risk of death was reduced by 3% at 2 years and 5% at 5 years for patients who were treated with postoperative cisplatin-containing regimens compared to patients who were treated with surgery alone [8]. On these bases, some institutions [9,10], in spite of drug toxicity, proposed the introduction of adjuvant chemotherapy even after presumed radical surgery at stage I. Nevertheless, according to another multicentric study [11], there was no significant effect on survival; hence the toxicity induced by chemotherapy appeared to radically operated patients, especially for those at stage I, to be too high cost to pay.
However, because of the frequent observation of recurrent disease in radically operated patients at stage I, we focused our attention on the risk–benefit of chemotherapy in clinical stage I NSCLC patients who underwent radical operation. With this purpose in mind, we developed a randomised trial including a limited but homogenous subset of NSCLC patients, staged pT2N0 (IB). The aim of our study was to assess the validity of postoperative chemotherapy in preventing recurrences and in prolonging survival.
2 Patients and methods
2.1 Patient eligibility criteria
The eligibility criteria for inclusion in the study included the following: (1) completely resected NSCLC patients in pathological stage T2N0; (2) patients aged less than 75 years with a preoperative performance status (Karnofsky index) equal to or greater than 90%; (3) rapid recovery from surgery to allow randomisation and beginning of adjuvant chemotherapy within 30 days; (4) postoperative forced expiratory volume in 1 s (FEV1) greater than 1 l; (5) no history of cancer or radiotherapy or chemotherapy within the last 5 years prior to surgery; and (6) no other organ (liver, kidney or heart) dysfunction limiting the study protocol – no elevation of bilirubin or creatinine level greater than 25% normal values, no recent myocardial infarction, or congestive heart impairment or history of ventricular arrhythmia requiring medication.
2.2 Study design
The trial was designed as a prospective randomised, two-group study with postoperative adjuvant chemotherapy versus surgery alone as a control group. The study project was submitted and approved by the ethical committee of the University. Operative procedure included postero-lateral thoracotomy, pleural cavity exploration, dissection of incidental filmy adhesion of the tumour, routine mediastinal lymph node dissection according to the map of Naruke et al. [12], venous vessel preparation and ligation before the arteries and mechanical bronchial suture. All firm adhesions of the tumour with the parietal pleura were considered neoplastic and these patients were not considered for the test. All specimens were collected and sent for histological review. Once proven to be pT2N0 samples, with margin of resection free of tumour invasion and absence of viable regional or distant metastatic disease as required, we requested fully informed consent from each patient eligible for the study. The enrolled patients were randomly assigned to the two groups by a computer procedure that notified the staff of the patient's group only at the time of randomisation.
Chemotherapy regimen consisted of six courses of cisplatin (CDDP) (100 mg/m2) given on day 1 and etoposide (VP16) (120 mg/m2) administered on days 1–3. Treatment was repeated every 4 weeks. CDDP was infused over 1 h after intravenous hyperhydratation with 2000 ml saline plus potassium chloride. VP16 was administrated as a 30-min intravenous infusion diluted with 250 ml of saline.
Dose reduction and delay in therapy were performed according to haematologic and non-haematologic toxicity, with the exclusion of alopecia, assessed at the beginning of each cycle. Toxicity was scored according to the WHO toxicity scale graded from 0 to 4.
Chemotherapy was administrated at 75% of the planned dose in the event of grade 2 haematologic toxicity and at 50% in the presence of grade 2 neurotoxicity or a creatinine clearance rate of less than 60 ml/min. Dose reduction was handled the same way for both agents. Doses were withheld if myelotoxicity or neurotoxicity exceeded grade 2 or if creatinine clearance dropped below 40 ml/min. The persistence of grade 2 toxicity for a period longer than 14 days implied the interruption of treatment.
For comparison of the two groups, 5-year disease-free and overall survival rates were evaluated at the end. Recurrence rates and causes of death were compared for the two groups. The incidence and degree of severity of adverse effects of adjuvant chemotherapy were evaluated.
2.3 Clinical and pathological staging
Preoperative staging always included a complete history and physical examination, a complete blood cell count with differential, serum biochemistry, urine analysis, plain chest roentgenogram, respiratory functional tests, total-body computed tomography (CT) scan and fibrobronchoscopy. A bone scan was required only when symptoms were present or the alkaline phosphatase level was 50% greater than normal limits. Tumour marker (carcinoembryonic antigen) evaluation and electrocardiograms were obtained at baseline. Echocardiography and cardiopulmonary stress tests were performed when necessary. Hepatic, renal and cardiac impairment were researched where possible up to a maximum period of 6 months preoperatively. Mediastinoscopy was performed only for those who were found to have enlarged lymph nodes by CT scan.
We evaluated generic (i.e. age and sex) as well as specific preoperative risk factors. Among these, we considered a neutrophil count greater than or equal to 5400 cells/mm3, a lactic dehydrogenase (LDH) level greater than or equal to 149 U/l, a Karnofsky index less than 100%, a weight loss greater than or equal to 10% within the last 6 months before operation and concomitant extrapulmonary diseases. Tumour histology (squamous versus non-squamous) and tumour localisation (central versus peripheral according to fibrobronchoscopy visualisation proved by biopsy) were also considered. Operative factors were type of resection (lobectomy versus pneumonectomy) and surgery-related morbidity.
Functional assessment (i.e. electrocardiogram, clearance creatinine, urea nitrogen, bilirubin and liver enzymes) was repeated before each chemotherapic cycle. Follow-up was repeated with the same time schedule for both groups and included a clinical visit, blood biochemical, serum tumour marker assay and chest X-ray, to be performed every 3 months for the first 2 years and thereafter twice per year. A total-body CT scan was done twice per year for the first 2 years and then yearly.
2.4 Statistical analysis
Univariate analysis was conducted between factors capable of conditioning intergroup differences. Continuous variables were tested by the Mann–Whitney non-parametric test and dependence between categoric variables was analyzed by the chi-square test or Fisher's exact test when appropriate. All tests were two-tailed with a significant level of 0.05.
According to the cognitive nature of the study for the sample size we decided to present the results for the first 50 patients randomised after a 5-year period from the operation. Overall survival and disease-free survival curves were estimated using the Kaplan–Meier method [13] and the significance test was based on the log-rank test as given by Mantel [14]. The survival time was counted from the day of surgery. Any new neoplastic lesion, second primaries included, was considered a recurrence. All causes of death were treated as final events. Major factors capable of influencing recurrence or death were entered into a regression Cox's proportional hazards model [15] for multivariate analysis.
3 Results
3.1 Study population
The study was activated in January 1988. The total number of patients considered for randomisation was 149, 69 of whom were judged ineligible because they did not meet the spirometric entry criteria n = 27 or had clinically significant comorbidities or delayed recovery from surgery n = 42. Five were excluded because they showed metastasis within 30 days after surgery. Seven patients refused randomisation.
Furthermore, two patients who were randomised for the study presented major protocol violations at further review and were ruled out from the study. Mean time between surgery and randomisation was 21±3.9 days (range 14–28). Afterwards, chemotherapy was medially started after 2.7 days.
All randomised patients were included in the survival analysis, regardless of whether their planned treatment was completed or not. Nevertheless, they were included for the final analysis, to avoid a selection bias, thus improving the survival of the adjuvant group.
Until December 1994 the study accrued a total of 66 evaluable patients. Thirty-three belonged to the adjuvant chemotherapy group and 33 to the control group. Descriptive analysis of the study population is summarised in Table 1 . No significant differences were noted in the distribution of preoperative, operative and postoperative risk factors between the two groups in the study (Table 1). Except for those who died earlier, all patients were followed up for a period of at least 5 years.
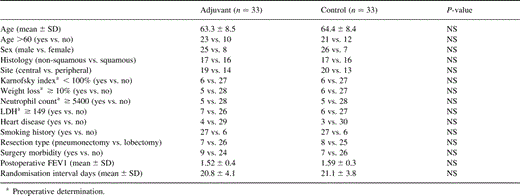
3.2 Treatment compliance
Treatment compliance was evaluated for each patient. The chemotherapy regimen was well tolerated, even though nausea and vomiting were the major treatment-related toxicity. These side effects were difficult to treat with the antiemetic drugs in use at the time of the study.
Twenty-five patients (75.7%) were able to receive the six planned courses of the treatment regimen, 20 (80%) of them as per schedule. Eight patients did not complete the planned chemotherapy: two refused further treatment after three cycles and another three patients after four cycles.
The last three patients developed rapid disease progression (mediastinal and cerebral metastases) after the completion of only two courses of chemotherapy. They suspended the planned therapy and were considered for supportive care only.
3.3 Recurrence and survival
Recurrence was detected in 16 patients in the adjuvant group and in 23 in the control group. Recurrence patterns are shown in Table 2 . In 35 cases, imaging devices showed evidence of recurrence and, in 31 of these patients, this evidence was confirmed by histology. In the remaining four patients, the recurrence was suspected only on a clinical basis. Median disease-free time and time till recurrence showed a statistically significant difference, using the log-rank test, between the two groups studied.
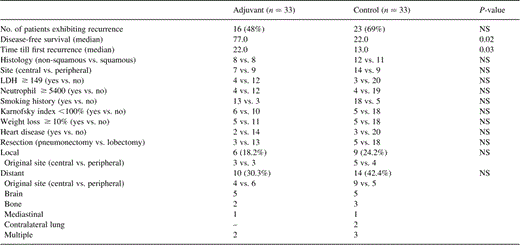
The rates of locoregional recurrence and distant metastases were 18 (6/33) and 30% (10/33) in the adjuvant chemotherapy group and 24 (9/33) and 42% (14/33) in the control group, respectively. There was no significant difference in local versus distant recurrences stratified for histology or for tumour site (Table 2). Three patients developed recurrence during the treatment period: one in the brain and the other two in the mediastinum.
Fig. 1 shows the disease-free survival curve for the two groups. The 5-year disease-free survival rates were 59% in the adjuvant group and 30% in the control group P = 0.02. None of the other risk factors evaluated by the log-rank test influenced the probability of recurrence in a significant manner. Using Cox's model, the absence of chemotherapy was reported to be only mildly significant (Wald test P < 0.05, odd ratio=2.73).
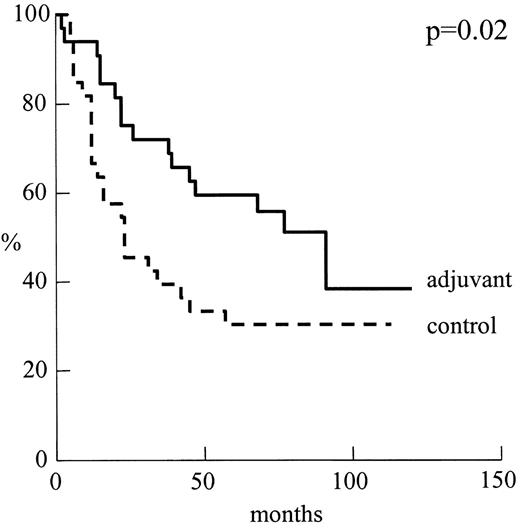
Disease-free curves for patients by treatment group: adjuvant n = 33 versus control n = 25.
Fourteen (42.4%) deaths occurred in the chemotherapy group and 21 (63.6%) in the control group (Table 3 ). With regard to the causes of death, the primary disease compared with other causes of death, including other diseases, accidents and unknown causes, accounted for 12 versus two (acute heart ischaemia) in the adjuvant group and 19 versus two (acute heart ischaemia and unknown) in the control group, respectively.
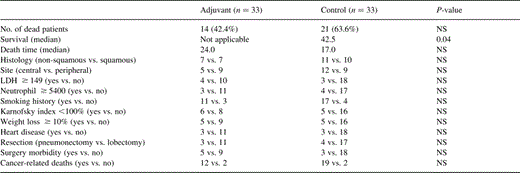
Fig. 2 shows the Kaplan–Meier survival curve. The 5-year survival rates were 63% in the adjuvant group and 45% in the control group, respectively. The difference of the Kaplan–Meier survival between the groups was significant when tested using the log-rank test P = 0.04. No other prognostic factor was found to be significant using the log-rank test. Using Cox's model, the absence of chemotherapy was found to be a significant negative prognosticator (P < 0.05, odd ratio=2.12).
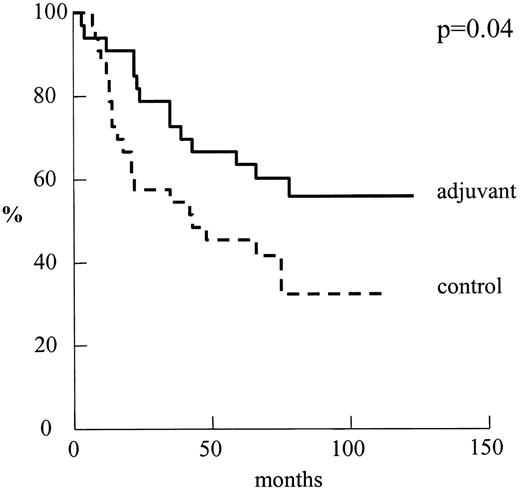
Overall survival for patients by treatment group: adjuvant n = 33 vs. control n = 33.
3.4 Adverse effects
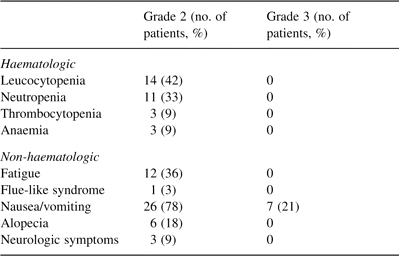
The treatment was generally well tolerated. There was no toxic death. Most adverse effects were manifested starting from the third course. Treatment-related toxicity is reported in Table 4 . Major discomforts were nausea and frequent vomiting (100%), fatigue (36%), alopecia (18%). With regard to haematologic toxicity, leucocytopenia was quite relevant (42% of the patients), especially considering that granulocyte-colony stimulating factors were not available at that time.
4 Discussion
Surgery with curative intent is the gold standard of treatment in early stages of NSCLC. However, depending on the stage at the time of surgery, the 5-year survival rate for patients with NSCLC who undergo complete surgical resection is only 40–69% [16–19]. Thus, despite radical surgical resection, the patients with early stage NSCLC have unsatisfactory 5-year survival when treated by surgery alone. This might be due to the fact that many of these patients have undetectable remaining viable tumour cells, either locally or systemically, which may cause future failure [5–7]. Furthermore, the treatment of recurrent disease after it has become apparent is rarely curative; on the contrary, it is highly invalidating [20].
Among patients with resected stage I NSCLC, the most common pattern of failure following complete resection is distant metastatic disease. Matthews et al. [5] reported that 24% of patients who had died of tumour-related diseases within 30 days after complete resection of lung cancer were found to have distant metastasis. Feld et al. [2] reported a local recurrence rate of 11% and a distant recurrence rate of 30% after curative resection, in a group of 196 patients at the T2N0 stage. In another report, Pairolero et al. [21] found, in 158 patients at the T2N0 stage, a 6% local and a 23% distant recurrence rate.
Hence, with the aim of enhancing early systemic control of the disease in order to reduce the probability of recurrence, postoperative adjuvant chemotherapy was proposed as the treatment of choice to be administered to those patients radically treated with surgery [9–11]. The rationale for adjuvant chemotherapy was based on the concept that effectiveness of therapy is inversely related to the tumour burden.
Holmes [22] showed that 141 patients with completely resected stage II or III adenocarcinoma and large-cell NSCLC had a significant increment in disease-free survival in the adjuvant group. Niiranen et al. [9] demonstrated a significant improvement in overall survival (56 vs. 67%, P = 0.05) in 110 patients with operated T1–3 N0 by the addition of six courses of cyclophosphamide, adriamycin and cisplatin (termed CAP); unfortunately, in this test the distribution for type of resection (lobectomy versus pneumonectomy) was too penalising on the control group.
On the other hand, the Lung Cancer Study Group, in their trial 801 [11], designed as a two-group study (130 patients per group) for stage I patients, including both T2N0 (85%) and T1N1 (15%), revealed no advantage in recurrence-free or overall survival in the adjuvant chemotherapy group. Feld et al. [11] tried to explain the poor results by imbalances in prognostic factors between the two groups, unusual behaviour among non-squamous tumours and bad patient compliance to chemotherapy (four courses of CAP) enhanced by the inadequate antiemetic drugs available at that time.
Since then, in order to diminish the negative impact of incomplete chemotherapy treatment, more tolerable regimens have been introduced. In a recent Japanese test, completely resected NSCLC patients in pathological stage I or II were randomised for chemotherapy versus a control group [10]. The postoperative chemotherapy consisted of two courses of cisplatin, vindesine, mitomycin C (termed PVM) and daily oral tegafur, a fluorouracil derivate for 1 year. The overall and disease-free survival rate between the two groups was similar, 76.8 and 74.1 versus 71.1 and 69.8%. However, in terms of subgroups, the pT1N0 group patients revealed 5-year survival rates significantly greater for the adjuvant group. Another ongoing test (CALGB 9633) is gathering a group of 500 patients with T2N0 randomised surgery alone versus surgery plus chemotherapy with carboplatin and paclitaxel [23].
In our trial, we reported that the 5-year survival rate in patients who underwent adjuvant chemotherapy is significantly higher P = 0.04. Noticeably, the survival rate of our control group is lower than rates (52.7–65%) reported in the literature [16–18] for similar patients. However, data from Van Rens et al. [19] in a group of 797 patients showed a 3-year survival rate of 59% and a 5-year survival rate of 47%, a value not dissimilar to the rate of our control group. Like Van Rens et al. [19], we have defined the terminal event as death by any cause and this could be one of the reasons to be taken into account.
Our results suggest that adjuvant chemotherapy may reduce locoregional recurrences and distant metastases rates in patients at stage IB NSCLC deemed radically operated. This might be explained by the presence of tumour cells in circulation or hidden in lymphatic organs, which can be correlated to poorer prognosis. Indeed, evidence of micrometastases has been demonstrated already in lymph nodes judged negative to microscopic examination by using immunohistochemical staining for the p-53 protein [7]. Monoclonal antibodies to cytokeratin 18 allowed the detection of micrometastasis in the bone marrow [6].
A definitive answer could be given by evaluating the clinical impact of discovering tumour cells or their related protein in circulation. The use of reverse-transcriptase-polymerase chain reaction (RT-PRC) to analyze the blood of carcinoma patients for the detection of m-RNA expressed in tumours cells may aid in predicting which patients will have a favourable outcome following removal of the primary lesion versus those patients considered to be at high risk of relapse, who may require additional treatment such as chemotherapy.
This paper has been carried out within the Research Fellowship program entitled Dottorato di Ricerca in Oncologia Toracica appointed by the Tor Vergata University of Rome.




