-
PDF
- Split View
-
Views
-
Cite
Cite
D. Dougenis, V. Patrinou, K.S. Filos, E. Theodori, K. Vagianos, A. Maniati, Blood use in lung resection for carcinoma: perioperative elective anaemia does not compromise the early outcome, European Journal of Cardio-Thoracic Surgery, Volume 20, Issue 2, August 2001, Pages 372–377, https://doi.org/10.1016/S1010-7940(01)00792-8
Close - Share Icon Share
Abstract
Objective: Blood transfusion may adversely affect the prognosis following surgery for non-small cell lung carcinoma (NSCLC). Conventionally by most thoracic surgeons, a perioperative haemoglobin (Hb) less than 10 g/dl has been considered a transfusion trigger. In this prospective trial we have (a) evaluated the overall blood transfusion requirements and factors associated with an increased need for transfusion and (b) in a subsequent subset of patients, tested the hypothesis that elective anaemia after major lung resection may be safely tolerated in the early postoperative period. Methods: A total of 198 (M/F 179/10, mean age 61.2, range 32–85 years) patients suffering from NSCLC were submitted to pneumonectomy n = 89 bilobectomy n = 19 and lobectomy n = 90 A rather strict protocol was used as a transfusion strategy. The transfusion requirements were analyzed and seven parameters (gender, age≫65, preoperative Hb≪11.5 g/dl, chest wall resection, history of previous thoracotomy, pneumonectomy and total blood loss) were statistically evaluated by univariate and logistic regression analysis. Subsequently, according to the perioperative Hb level during the first 48 h, patients were divided into group A (n = 49, Hb = 8.5-10) and group B n = 149, Hb > 10) with a view to estimate the risks of elective perioperative anaemia. Groups were comparable in terms of age, sex, type of operation performed, preoperative Hb, creatinine level, FEV1, arterial blood gases and history of heart disease. Results: The overall transfusion rate was 16%. Univariate analysis revealed that preoperative Hb≪11.5 g/dl (P < 0.01) and total blood loss P < 0.0001 were associated with increased need for transfusion, but only the total blood loss was identified as an independent variable in multivariate analysis. Statistical analysis between groups A and B showed no significant difference regarding postoperative morbidity and mortality: atelectasis (3 vs. 6), chest infection (2 vs. 9), sputum retention requiring bronchoscopy (5 vs. 12), admission to intensive care unit (5 vs. 7), ARDS (0 vs. 3), postoperative hospital stay (7.7±2.6 vs. 9.1±3.8 days) and deaths (1 vs. 3). Conclusions: The use of a strict transfusion strategy could help in reducing overall blood transfusion. Furthermore, a perioperative Hb of 8.5–10 g/dl could be considered safe in elective lung resections for carcinoma.
1 Introduction
The knowledge that blood transfusion carries a risk of HIV infection [1] and the increasing evidences that perioperative blood transfusion for cancer surgery in general have an adverse effect on disease-free and overall survival [2], forced a reevaluation of the indications for perioperative transfusion policy. The purpose of transfusion is to reduce the risks associated with anaemia. Until recently, it was generally accepted that a haemoglobin (Hb) level of 10 g/dl and a haematocrit (Hct) of 30% represent the lowest transfusion trigger for anaesthesia and surgery [3]. For certain elective operations in orthopaedics and general surgery, this transfusion trigger has recently shifted to lower levels.
Although some clinical studies have examined the relationship between blood transfusion and long-term outcome in patients undergoing curative resections for lung carcinomas with controversial results [4–9], there has been very few clinical data evaluating the blood transfusion trigger in patients subjected to major non-cardiac thoracic surgery. The purpose of this trial was to evaluate the efficacy of a certain transfusion protocol in eliminating unnecessary transfusions of red blood cells (RBCs). In the first part of our study, we evaluated the overall blood requirements and factors potentially associated with increased need for transfusion among the entire patient cohort. Subsequently, in a subset of patients, we have tested the hypothesis that elective anaemia after major lung resection, may be safely tolerated in the early postoperative period.
2 Materials and methods
We have evaluated 198 consecutive patients who underwent curative major lung resection for non-small cell lung carcinoma (NSCLC) in our hospital between 1994 and 1999. The mean age was 60.1 years, range 33–83. Patients with a history of acute myocardial infarction during the last 6 months, or recent coronary angioplasty for unstable angina, as well as those presented with severe haemoptysis necessitating urgent surgery, have been excluded from this study. None of our patients had undergone autologous blood predonation, or had acute normovolemic haemodilution or intraoperative cell salvage. Preoperative staging included for all chest-computed tomography (CT) and more recently spiral CT with contrast medium, brain and upper and lower abdomen CT, as well as bone radionucleotide scanning. For those anticipated to undergo pneumonectomy, a transthoracic echocardiography was also performed prior to surgery. Six patients had previously undergone resection of single brain metastasis and two were scheduled to receive similar surgery after resection of the primary tumour. Operations were performed via a lateral thoracotomy and the thoracic cavity was routinely drained. Double lumen endotracheal tube and thoracic epidural catheter analgesia were standard procedures. Frozen sections were routinely carried out for all suspicious mediastinal lymph nodes found during the procedure. Resection margins were also screened by frozen section in tumours close to the tracheal bifurcation. Although we intend not to operate on N2 disease, in the presence of a positive mediastinal lymph node, a full mediastinal dissection and clearance was performed. After extubation, patients remained in the recovery room under close monitoring until stabilized and have a chest radiography undertaken, and subsequently were transferred to the ward with hourly observation and recordings for the next approximately 16 h. Arterial blood gases including Hb were routinely taken at 3, 8 and 16 h after transfer to the ward, or as required. The arterial line was usually removed after 24 h and the central venous line, when inserted, after 48 h. Blood, only as RBCs, was transfused according to a certain protocol (Table 1 ), which was previously discussed and accepted by the blood transfusion department and also approved by our institutional ethics committee. Data were prospectively collected. The surgeon was always involved in making the decision for any RBC transfusion. Early reoperation was performed when indicated (Table 1). End points of this study were the lowest levels of Hb and Hct recorded during the perioperative period (within 48 h of surgery) and at discharge, the number of RBCs transfused, as well as the noted postoperative complications, hospital stay and mortality. Only patients receiving RBCs were included in the study; the administration of platelets (1/198), fresh frozen plasma (62/198) and cryoprecipitate (3/198) were not analyzed. None of the patients in this study had been given erythropoetin or other pharmacological agents to reduce transfusion requirements and blood loss, while fibrin sealing has only recently been adopted in our surgical practice. At the time of discharge, all patients had an acceptable performance status and were able to climb up a level of stairs.

Our protocol for blood transfusion representing the general transfusion policy followed in this studya
For all 198 patients of the study, seven variables (age≫65, gender, preoperative Hb, chest wall resection, history of previous thoracotomy, pneumonectomy, total blood loss) possibly related to increased need for blood transfusion were statistically evaluated by univariate analysis.
Subsequently, according to the perioperative level of Hb, patients were divided into two groups: group A consisted of 49 patients with Hb 8.5–10 g/dl and group B of 149 patients with Hb≫10 g/dl. Groups were analogous in terms of age, staging, tumour histology, surgical procedure performed, preoperative Hb, creatinine, forced expiratory volume/second and arterial blood gases (PO2 and PCO2). The clinicopathological characteristics of both groups are shown in Table 2 . The two groups were compared by means of postoperative morbidity, hospital stay and mortality.
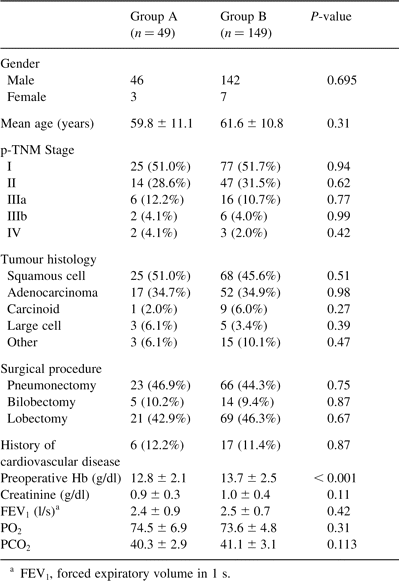
Clinicopathological characteristics of patients of group A (Hb 8.5–10 g/dl) and group B (Hb≫10 g/dl) of the study
Univariate statistics was performed using analysis of variance (ANOVA), unpaired t-test, chi-square or Fisher's exact test as appropriate. A P value ≪0.05 was considered significant. Numerical variables were expressed as mean ±standard deviation. Following univariate analysis, logistic regression analysis was used to determine which of the variables were independently associated with the transfusion of RBCs in the whole patient cohort, using a backward elimination procedure to isolate the significant variables. At each stage of the elimination procedure, the variable with the largest current P-value was eliminated until all remaining variables were or became statistically significant P < 0.05 The statistical package used was STATISTICA version 4.5F.
3 Results
3.1 Blood transfusion requirements
Overall, a total of 32 out of the 198 patients (16%) were transfused with 67 units RBCs, eight during the operation, 14 in the recovery room and 10 during the perioperative period. Reoperation was required in seven (3.5%), because of postoperative bleeding (four), or air leak (three). Total blood loss was equally distributed among the various surgical procedures (Fig. 1 ). The highest blood requirements (19.8%) were noticed in the subset of patients undergoing pneumonectomy (Table 3 and Fig. 1). A preoperative Hb≪11.5 g/dl as well as the total blood loss were the single variables statistically associated with increased need for blood transfusion identified in the univariate analysis (Table 4 ). However, logistic regression analysis revealed the total blood loss as the only independent variable predisposing to transfusion P = 0.00001 odds ratio 0.984, 95% confidence limits 0.977–0.991, area under ROC = 0.9972.
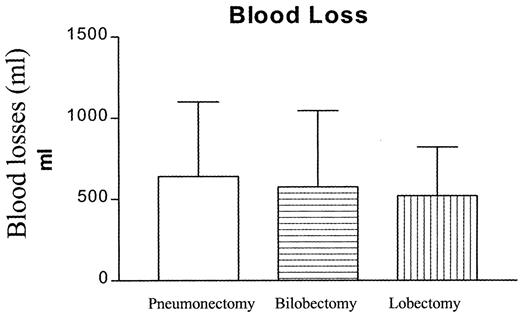
Total blood loss distribution according to the specific surgical procedure for the entire patients cohort of the study.
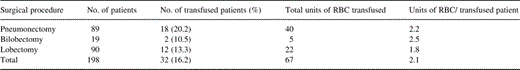
Perioperative blood transfusion according to the procedure performed for the entire patient population in the study P = 0.14

Univariate analysis among factors associated with perioperative blood transfusion for the entire patient cohort
3.2 Comparison between the groups
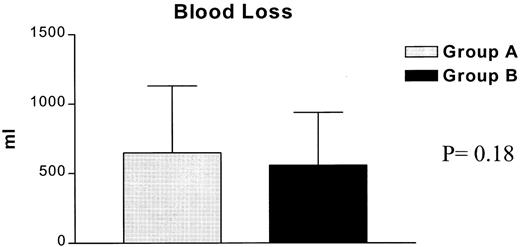
In the subsequent comparison between the two study groups to evaluate the potential effect of perioperative anaemia in the clinical outcome, we were not able to detect any statistical difference regarding complications, hospital stay and mortality (Table 5 ). Mortality was not different between groups, with one death occurring in group A and three in group B. The causes of death were respiratory failure and adult respiratory distress syndrome in three patients and major cerebral infarction in one. Only one of these patients had been transfused. Blood losses (Fig. 2 ) as well as blood transfusions (Fig. 3 ) were equally distributed between the groups. Postoperative complications were equally distributed in both the groups. A total of 52 complications occurred in 41 patients and the overall morbidity was 20.6% (Table 5). The two most common were sputum retention requiring suction bronchoscopy and/or mini-tracheostomy, and atrial fibrillation in 5/12 and 4/12 patients, respectively. Finally, 12 patients (6%), five in group A and seven in group B, required transfer to the intensive care unit because of postoperative respiratory failure requiring mechanical ventilation (11), or development of acute ischemic syndrome (1). Mean Hb at the time of discharge was 11.6 g/dl for group A and 12.9 g/dl for group B. Over an approximately 5 days further hospitalisation period, a statistically significant increase between the perioperative and discharge Hb P < 0.0001 was noted for both groups without any further transfusion (Fig. 4 ).
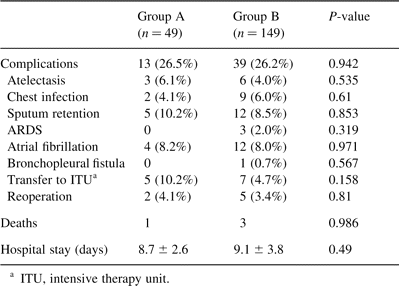
Comparison of complications, mortality and hospital stay between the two groups of the study
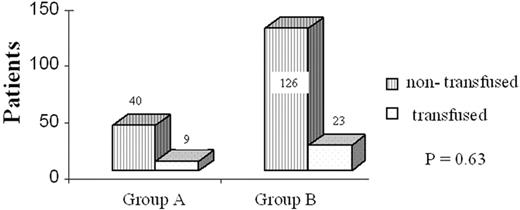
Histogram depicting the blood transfusion requirements for both groups of the study.
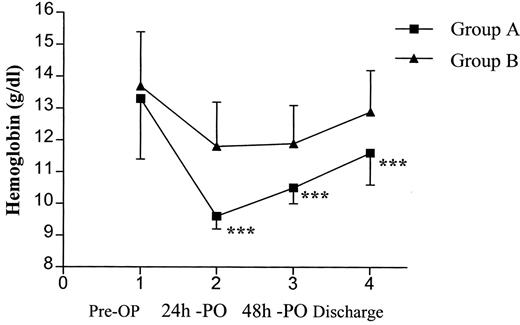
Graphical presentation of the Hb level between the groups, from admission to discharge. Pre-OP, preoperative; PO, postoperative.
4 Discussion
The purpose of transfusion is to reduce the risks associated with anaemia. The requirement of blood transfusion in surgical patients is related to the type of the surgical procedure, the accuracy of surgical haemostasis and the used surgical and anaesthesia techniques. It is also dependent on the clinical condition of the patient and particularly the cardiopulmonary system and the haematological status. To avoid unnecessary transfusion of RBCs, therefore, many factors must be considered for the determination of a safe transfusion trigger in surgical patients.
Because blood transfusion has been associated with decreased survival in patients undergoing surgery for cancer, probably due to the induced immunomodulation producing a more favourable host environment for the proliferation of tumour [10], many clinical studies have been designed to establish a lower transfusion trigger and therefore eliminate unnecessary transfusions. For critically ill patients, Hebert et al. [11] recommended that the Hb level of 7 g/dl could be considered as a transfusion trigger, with the possible exception of patients with acute myocardial infarction and unstable angina. Robertie and Gravlee [12] in an attempt to provide general transfusion guidelines, proposed a trigger of Hb 6 g/dl for well-compensated patients without perioperative heart disease, while this trigger was put up to Hb level 10 g/dl for older patients and those with postoperative complications with increased systemic oxygen demands or decreased cardiac reserve.
These new concepts and our awareness of the problems of transfusion reactions, disease transmission and potential immunosuppression have led us to revise our transfusion policy in thoracic surgery. Furthermore, our area is facing a limited availability of donated blood and blood products, while the incidence of severe trauma due to car traffic accidents remains high and overall, as a country, we usually import blood and blood products to meet up with our necessities. Using the proposed transfusion protocol (Table 1) as a general transfusion strategy, we have been able to, without increasing the overall morbidity and mortality, reduce the overall transfusion rate to 16% in this cohort of patients undergoing major lung resection, contrary to other studies where the transfusion rate ranged from 23.6–55% [4–9]. Among those 198 patients, the only parameters identified to be associated with increased transfusion requirements were a preoperative level of Hb≪11.5 g/dl as well as the total blood loss. Factors such as age, gender, previous thoracotomy and chest wall resection, reported by others [4,5,13], did not influence the need for transfusion in our study. In fact, the single independent variable predisposing to blood transfusion was the total blood loss. The highest demand for transfusion had patients undergoing pneumonectomy (19.8%), probably due to the increased incidence of history of tuberculosis in our area causing sufficient lung adhesions prone to postoperative bleeding in the empty postpneumonectomy space.
The most important result of our study, however, was that we found no difference in morbidity, hospital stay and mortality among patients with perioperative Hb of 8.5–10 g/dl, group A, and those with more than 10 g/dl, group B. We could, therefore, propose shifting the transfusion trigger from the currently widely accepted Hb and Hct (10/30) to 8.5 and 25, respectively, for stable patients undergoing lung resection. Nevertheless, we generally appreciate that the appropriate Hb level is the necessary one that meets the individual patient's tissue oxygen demands, but this is very complex to be precisely identified, particularly when there are no previous lung surgery reports on this particular issue. It is notable, as presented in Fig. 4, that there was a significant increase of the mean Hb at discharge for both groups compared to the perioperative level. It is appreciated that stable patients would have a mean increase of 2 g/dl during this period. This may be due to a certain degree of perioperative haemodilution from expanding crystalloid volume intake. Also, the routine use of epidural catheter for pain control could have contributed to that.
In clinical practice, the degree of anaemia, not the blood losses, plays the most important role in the decision to administer RBCs. Humans can tolerate anaemia well and the signs and symptoms of severe anaemia often occur when the level of Hb is extremely low [14]. The measurements of tissue oxygenation are the more accurate predictors to determine the minimum acceptable Hb level for every separate patient [15]. Deem et al. [16] found in a rabbit model that a 2/3 reduction of Hct had no effect on pulmonary CO2 gas exchange, as long as the breathing mechanisms remain intact. Weiskopf et al. [17] proposed that acute normovolemic haemodilution to Hb 5 g/dl in healthy humans can be tolerated without signs of tissue hypoxia, but Nelson et al. [18] reported that postoperative Hct level less than 28% was associated with myocardial ischemia and morbid cardiac events in intensive care high-risk vascular patients. We did not note any difference in postoperative cardiac events including atrial fibrillation, which was equally observed in both groups of our study. Since a perioperative transfusion trigger of Hb 8 g/dl has been proposed for surgical patients with known stable coronary artery disease undergoing surgery [12], we believe we are reasonable in suggesting a transfusion trigger of Hb 8.5 g/dl in the perioperative period after lung resection, since we have also showed that the Hb level will raise by 2 g/dl by the time of discharge.
Pulmonary disease presents an important factor that prevents adequate compensation to severe anaemia. Decreased ventilation and/or oxygen diffusion associated with the diminished oxygen carrying capacity in the anaemic patient lead to inadequate oxygen delivery to tissues. Schonhofer et al. [19] stated that anaemia in patients with lung disease may increase the work of breathing and therefore, blood transfusion may improve the pulmonary function. It is anticipated that the majority of patients proceeding to surgery have a certain chronic pulmonary disease or will have one following lung resection. In our study, it appeared that a perioperative Hb level of 8.5–10 g/dl did not have any adverse effect in either the cardiac or the respiratory function postoperatively, as complications were similarly distributed in the two study groups.
It is clear that the transfusion of RBCs may not only not help but in fact do harm to the critically ill patients [20], but it is also clear that no single measure can replace good clinical judgment as the basis of decision making regarding perioperative transfusion [15]. Based on our clinical data, we currently believe that the optimal surgical blood schedule for any major lung resection should be two units of RBCs, provided there is no coexisting chronic anaemia, and we expect to transfuse one out of five patients approximately.
Among the limitations of our study are: (1) this trial is not a prospected randomized one; (2) we have not presented precise data regarding factors and markers reflecting the tissue oxygenation, such as mixed venous oxygen (PvO2), mixed venous oxygen saturation (SvO2) or oxygen consumption (VO2). To achieve such a data would require more invasive monitoring plus pulmonary artery catheter insertion, which is not included in our routine perioperative surgical management.
In conclusion, it is difficult at the present to postulate a certain transfusion policy for patients undergoing lung surgery. Using a rather restrictive transfusion strategy, as the protocol presented here, perioperative transfusion trigger can be shifted to Hb 8.5 g/dl. Our study suggested that this elective perioperative anaemia could be well tolerated in stable patients. However, further studies are needed to elucidate the appropriate transfusion trigger as well as to evaluate the further outcome of patients subjected to perioperative elective anaemia after major lung resection.
References
- anemia
- hemorrhage
- heart diseases
- atelectasis
- arterial blood gas
- carcinoma
- creatinine
- forced expiratory volume function
- hemoglobin
- blood transfusion
- bronchoscopy
- non-small-cell lung carcinoma
- intensive care unit
- pneumonectomy
- preoperative care
- respiratory distress syndrome, adult
- surgical procedures, operative
- thoracoplasty
- thoracotomy
- morbidity
- mortality
- gender
- thoracic surgery specialty
- lower respiratory tract infections
- lung volume reduction
- transfusion
- lobectomy
- sputum retention
- univariate analysis
- transfusion threshold




