-
PDF
- Split View
-
Views
-
Cite
Cite
Zhi Li, Haonan Sun, Yongchen Hao, Hangkuan Liu, Zhengyang Jin, Linjie Li, Chong Zhang, Min Ma, Tianming Teng, Xiongwen Chen, Yujun Shen, Ying Yu, Jing Liu, Arthur Mark Richards, Huay Cheem Tan, Dong Zhao, Xin Zhou, Qing Yang, on behalf of the CCC-ACS Investigators, Renin–angiotensin system inhibition and in-hospital mortality in acute coronary syndrome patients with advanced renal dysfunction: findings from CCC-ACS project and a nationwide electronic health record-based cohort in China, European Heart Journal - Quality of Care and Clinical Outcomes, Volume 9, Issue 8, December 2023, Pages 785–795, https://doi.org/10.1093/ehjqcco/qcad006
Close - Share Icon Share
Abstract
In acute coronary syndrome (ACS) patients without advanced renal dysfunction [estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2], early (within 24 h of admission) angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB) is the guideline-directed medical therapy. The clinical efficacy of early ACEI/ARB therapy among ACS patients with advanced renal dysfunction remains unclear.
Among 184 850 ACS patients hospitalized from July 2014 to December 2018 in the Chinese National Electronic Disease Surveillance System Platform (CNEDSSP) cohort and 113 650 ACS patients enrolled from November 2014 to December 2019 in the Improving Care for Cardiovascular Disease in China-ACS Project (CCC-ACS) cohort, we identified 3288 and 3916 ACS patients with admission eGFR < 30 mL/min/1.73 m2 [2647 patients treated with ACEI/ARB (36.7%)], respectively. After 1:1 propensity score matching (PSM) in each cohort, Kaplan–Meier analysis showed that early ACEI/ARB use was associated with a 39% [hazard ratio (HR): 0.61, 95% confidence interval (95% CI): 0.45–0.82] and a 34% (HR: 0.66, 95% CI: 0.46–0.95) reduction in in-hospital mortality in CNEDSSP and CCC-ACS cohorts, respectively, which was consistent in multiple sensitivity analyses. A random effect meta-analysis of the two cohorts after PSM revealed a 32% reduction (risk ratio: 0.68, 95% CI: 0.55–0.84) in in-hospital mortality among ACEI/ARB users.
Based on two nationwide cohorts in China in contemporary practice, we demonstrated that ACEI/ARB therapy initiated within 24 h of admission is associated with a reduction in in-hospital mortality in ACS patients with advanced renal dysfunction.
CCC-ACS project was registered at URL: https://www.clinicaltrials.gov. (Unique identifier: NCT02306616).

Association between renin–angiotensin system inhibition and in-hospital mortality in ACS patients with advanced renal dysfunction. ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; ACS, acute coronary syndrome; CI, confidence interval; eGFR, estimated glomerular filtration rate; EHR, electronic health record; HF, heart failure; HR, hazard ratio; STEMI, ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; MI, myocardial infarction; RR, risk ratio.
Introduction
It is estimated that >7 million people are diagnosed with acute coronary syndrome (ACS) each year worldwide, with an in-hospital mortality rate of ∼5%.1 Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB) is used as part of guideline-directed medical therapy after ACS to improve outcomes.2,3 However, ACEI/ARB use is not encouraged for ACS patients with advanced renal dysfunction in clinical guidelines. The Kidney Disease Improvement Global Outcomes Guideline suggested that temporary discontinuation of ACEI/ARB therapy may be considered in patients with an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, as it may deteriorate kidney function.4 The 2017 European Society of Cardiology (ESC) guideline for the diagnosis and treatment of ST-elevation myocardial infarction (STEMI) suggested that STEMI patients without contraindications should receive ACEI/ARB treatment routinely,2 and these contraindications included advanced renal dysfunction as described in the 2020 ESC non-ST-elevation acute coronary syndrome (NSTE-ACS) guideline.3
Despite the fact that ACEI/ARB therapy is associated with lower mortality for ACS patients regardless of renal function,5 there is inconsistent evidence regarding the benefit and risk of ACEI/ARB use in patients with advanced renal dysfunction. For example, in patients with eGFR < 30 mL/min/1.73 m2, some observational studies showed that chronic ACEI/ARB use was associated with lower risks for long-term dialysis and all-cause mortality,6,7 and discontinuation of ACEI/ARB was associated with increased risk of cardiovascular-renal events.8 Conversely, other studies focusing on this population demonstrated that ACEI/ARB use was associated with the deterioration of renal function,9 and discontinuation of ACEI/ARB may delay the need for dialysis in this population.10 Expert consensus therefore suggested that individualization of treatment plans with careful assessment of risks and benefits is needed when considering these agents in advanced renal dysfunction.11 The recently published randomized, controlled STOP-ACEi trial, which has long been anticipated to solve controversies in this area, demonstrated that discontinuation and continuation of ACEI/ARB yielded similar renal outcomes with respect to the initiation of renal-replacement therapy and that a numerically high but not statistical increase in cardiovascular events (secondary outcome) in the discontinuation group.12 Notably, the STOP-ACEi trial was not powered to test the differences in cardiovascular events and all-cause mortality.
ACS patients with advanced renal dysfunction had poor clinical outcomes.13,14 Moreover, advanced renal dysfunction is an important factor limiting the real-world practice of ACEI/ARB.15 Nonetheless, evidence for the impact of short-term ACEI/ARB therapy during hospitalization in ACS patients with advanced renal dysfunction is still lacking. In this study, we therefore used the Chinese National Electronic Disease Surveillance System Platform (CNEDSSP) database and the Improving Care for Cardiovascular Disease in China-ACS (CCC-ACS) project to explore the association between early ACEI/ARB use (within 24 h of admission) and in-hospital mortality in ACS patients with eGFR < 30 mL/min/1.73 m2.
Methods
Study design and population
The present analysis was a retrospective, multicentre, observational study based on the CNEDSSP and CCC-ACS projects.
The CNEDSSP was supported by the National Science and Technology Support Program (approval number 2005BA114A01) and initiated by the Statistical Information Center of the National Health Commission of China in 2005, with the aim of improving the quality of medical management by constructing an integrated platform of electronic health records (EHRs) from nationwide, representative hospitals in China. In total, 184 850 ACS inpatient cases included in 47 hospitals from July 2014 to December 2018 were identified based on International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes. For this study, information on demographics, clinical variables, procedures, and medications was collected. The data cleaning and management service was provided by Beijing 1 M Data Technology Corporation. This study protocol was approved by the Medical Ethics Committee of Tianjin Medical University General Hospital (approval number: IRB2022-YX-045-01) and exempted from informed consent.
The CCC-ACS project is a collaborative effort by the American Heart Association and the Chinese Society of Cardiology to improve the quality of clinical management for ACS patients, which included 113 650 ACS patients enrolled in 241 hospitals from 1 November 2014 to 31 December 2019 in China. The rationale and study design have been published previously.16 The CCC-ACS project was approved by the institutional review board of Beijing Anzhen Hospital, Capital Medical University, with a waiver for informed consent. This study is registered at URL: https://clinicaltrial.gov (unique identifier: NCT02306616).
In our study, advanced renal dysfunction was defined as an eGFR < 30 mL/min/1.73 m2 on the first laboratory test after admission, which was calculated according to the equation by the Chronic Kidney Disease Epidemiology Collaboration.17 ACEI/ARB users were defined as patients who received ACEI/ARB treatment within 24 h of admission. Non-ACEI/ARB users were defined as patients who did not receive ACEI/ARB treatment within 24 h of admission.
Study outcome and covariates
The study outcome was in-hospital death. We used the following covariates for adjustment and/or propensity score matching (PSM), which could be classified into the following six domains: (i) demographic characteristics, including age, sex, and currently on dialysis; (ii) medical history, including myocardial infarction (MI), percutaneous coronary intervention (PCI), coronary bypass grafting (CABG), diabetes, dyslipidaemia, hypertension, atrial fibrillation, heart failure (HF), ischaemic stroke, haemorrhagic stroke, chronic obstructive pulmonary disease, and peripheral vascular disease; (iii) medical conditions on admission, including levels of systolic and diastolic blood pressure, heart rate, and Killip class; (iv) laboratory examinations on admission, including serum levels of low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, eGFR, and myocardial enzymes [creatine kinase-myocardial band (CK-MB) for CCC-ACS cohort and the proportion of increased myocardial enzymes for CNEDSSP cohort (as an EHR-based study, due to the existence of missing value of a certain cardiac enzymes (troponin I, T, or CK-MB), we used the proportion of higher than the upper reference limit of a certain maker)]; (v) treatment strategy upon the index admission, including aspirin, P2Y12 inhibitor, statin, and PCI; and (vi) ACS subtypes, including STEMI, non-ST-elevation myocardial infarction (NSTEMI), and unstable angina (UA).
Statistical analysis
We used PSM of a maximal ratio of 1:1 to balance the differences in baseline characteristics between ACEI/ARB users and patients not receiving early ACEI/ARB using a calliper of 0.02 of the propensity (Stata command ‘calipmatch’) for CNEDSSP cohort and CCC-ACS cohort, respectively. We described the baseline characteristics of the study population by treatment group before and after PSM. Continuous data with a normal distribution are presented as the means and standard deviation (SD). Non-parametric continuous data are presented as the median with the 25th to 75th percentiles. Categorical variables were reported as numbers and percentages. Differences between groups were assessed with absolute standardized difference (ASD). Between-group imbalances were considered to be ideal if the ASD for a given variable was <10%.18 The survival curves were determined individually by Kaplan–Meier analysis in the propensity score matched samples in CNEDSSP cohort and CCC-ACS cohort, with differences assessed by the log-rank test. We performed the following sensitivity analyses: (i) excluded patients who died within 24 h of admission; (ii) excluded patients with serious conditions on admission (cardiogenic shock, HF, or cardiac arrest); (iii) excluded patients with a history of PCI or CABG; (iv) excluded patients currently on dialysis; and (v) E-value to assess the robustness of the associations to unmeasured/uncontrolled confounders.19 Additionally, univariable Cox regression based on the propensity score matched cohorts, multivariable-adjusted (including all variables in Table 1), and inverse probability weighting (Stata command ‘teffects ipw’) Cox regression based on the total cohort was used as a sensitivity analysis to validate the primary findings. In addition, because the individual data for the CNEDSSP and CCC-ACS cohorts were not allowed for combination, we therefore performed meta-analysis based on the random effect model to combine the results derived from each matched cohort to verify the consistency and main effect size. Subsequently, we performed subgroup analyses based on the matched cohorts, including age (<65 years vs. ≥65 years), sex, ACS subtype (STEMI vs. NSTE-ACS), medical history (MI, diabetes and HF), and PCI or not during the index admission. Stata version 15.1 (StataCorp, College Station, TX, USA) was used for the analysis, and a two-tailed P < 0.05 was considered statistically significant.
Baseline characteristics by ACEI/ARB therapy after propensity score matching
| . | CNEDSSP cohort . | CCC-ACS cohort . | ||||
|---|---|---|---|---|---|---|
| Characteristics . | Non-early ACEI/ARB (n = 1114) . | Early ACEI/ARB (n = 1114) . | ASD, (%) . | Non-early ACEI/ARB (n = 1225) . | Early ACEI/ARB (n = 1225) . | ASD, (%) . |
| Age, years | 72.5 ± 11.4 | 72.1 ± 11.2 | 3.28 | 71.2 ± 11.8 | 71.6 ± 12.0 | 3.68 |
| Male sex, n (%) | 597 (53.6) | 591 (53.1) | 1.08 | 681 (55.6) | 689 (56.2) | 2.30 |
| ACS subtypes, n (%) | 1.02 | 2.70 | ||||
| STEMI | 227 (20.4) | 224 (20.1) | 530 (43.2) | 558 (45.5) | ||
| NSTEMI | 275 (24.7) | 272 (24.4) | 509 (41.5) | 464 (37.9) | ||
| UA | 612 (54.9) | 618 (55.5) | 187 (15.3) | 204 (16.6) | ||
| Currently on dialysis, n (%) | 98 (8.8) | 99 (8.9) | 0.32 | 91 (7.4) | 83 (6.8) | 1.00 |
| Medical history, n (%) | ||||||
| MI | 396 (35.5) | 388 (34.8) | 1.50 | 180 (14.7) | 180 (14.7) | 0.70 |
| PCI | 131 (11.8) | 139 (12.5) | 2.20 | 138 (11.3) | 137 (11.2) | 2.56 |
| CABG | 28 (2.5) | 29 (2.6) | 0.57 | 18 (1.5) | 15 (1.2) | 1.48 |
| Diabetes | 529 (47.5) | 530 (47.6) | 0.18 | 479 (39.1) | 483 (39.4) | 2.67 |
| Dyslipidaemia | 62 (5.6) | 66 (5.9) | 1.54 | 131 (10.7) | 127 (10.4) | 0.54 |
| Hypertension | 872 (78.3) | 871 (78.2) | 0.22 | 967 (78.9) | 968 (79.0) | 0.20 |
| Atrial fibrillation | 108 (9.7) | 100 (9.0) | 2.47 | 65 (5.3) | 73 (6.0) | 0.34 |
| Heart failure | 278 (25.0) | 276 (24.8) | 0.42 | 123 (10.0) | 125 (10.2) | 2.74 |
| Ischaemic stroke | 178 (16.0) | 176 (15.8) | 0.49 | 155 (12.7) | 158 (12.9) | 0.49 |
| Haemorrhagic stroke | 15 (1.3) | 18 (1.6) | 2.23 | 10 (0.8) | 14 (1.1) | 0 |
| Chronic obstructive pulmonary disease | 18 (1.6) | 20 (1.8) | 1.39 | 35 (2.9) | 37 (3.0) | 0.47 |
| Peripheral vascular disease | 44 (3.9) | 44 (3.9) | 0 | 32 (2.6) | 37 (3.0) | 0.97 |
| Medical conditions on admission | ||||||
| Heart rate (b.p.m.) | 79.5 ± 16.9 | 79.9 ± 20.0 | 2.08 | 82.2 ± 20.1 | 82.1 ± 18.5 | 0.35 |
| SBP (mmHg) | 138.2 ± 22.5 | 138.3 ± 21.4 | 0.47 | 138.3 ± 29.2 | 139.1 ± 25.6 | 1.24 |
| DBP (mmHg) | 77.1 ± 20.0 | 76.8 ± 14.1 | 1.88 | 79.0 ± 16.7 | 79.0 ± 15.6 | 1.50 |
| Killip class, n (%) | 0.49 | 1.19 | ||||
| Class I | 300 (26.9) | 311 (27.9) | 573 (46.8) | 544 (44.4) | ||
| Class II | 235 (21.1) | 241 (21.6) | 307 (25.0) | 343 (28.0) | ||
| Class III | 362 (32.5) | 323 (29.0) | 196 (16.0) | 212 (17.3) | ||
| Class IV | 217 (19.5) | 239 (21.4) | 150 (12.2) | 127 (10.3) | ||
| Laboratory examinations on admission | ||||||
| TG, mmol/L | 1.5 (1.1–1.9) | 1.5 (1.1–2.0) | 1.85 | 1.5 (1.0–2.2) | 1.5 (1.1–2.2) | 0.65 |
| LDL-C, mmol/L | 2.4 (1.9–2.8) | 2.4 (1.9–2.9) | 2.01 | 2.5 (1.9–3.2) | 2.5 (1.9–3.2) | 0.52 |
| HDL-C, mmol/L | 1.0 (0.8–1.1) | 1.0 (0.8–1.2) | 1.42 | 1.0 (0.8–1.3) | 1.0 (0.8–1.2) | 0.67 |
| eGFR (mL/min/1.73 m2) | 19.6 (12.2–25.4) | 20.3 (12.7–25.7) | 1.54 | 22.1 (15.5–26.3) | 22.9 (16.6–26.6) | 8.07 |
| CK-MB (U/L) or proportion of increased myocardial enzymesa | 403 (36.2) | 404 (36.3) | 0.19 | 22.3 (10.0–66.6) | 20.0 (8.9–60.4) | 2.22 |
| Treatment strategy upon the index admission, n (%) | ||||||
| Aspirin | 806 (72.4) | 819 (73.5) | 2.63 | 1112 (90.8) | 1114 (90.9) | 0 |
| P2Y12 inhibitor | 835 (75.2) | 845 (75.9) | 2.08 | 1129 (92.2) | 1129 (92.2) | 0.91 |
| Statin | 934 (83.8) | 948 (85.1) | 3.47 | 1162 (94.9) | 1172 (95.7) | 0.79 |
| PCI | 178 (16.0) | 165 (14.8) | 3.23 | 536 (43.8) | 549 (44.8) | 0.16 |
| . | CNEDSSP cohort . | CCC-ACS cohort . | ||||
|---|---|---|---|---|---|---|
| Characteristics . | Non-early ACEI/ARB (n = 1114) . | Early ACEI/ARB (n = 1114) . | ASD, (%) . | Non-early ACEI/ARB (n = 1225) . | Early ACEI/ARB (n = 1225) . | ASD, (%) . |
| Age, years | 72.5 ± 11.4 | 72.1 ± 11.2 | 3.28 | 71.2 ± 11.8 | 71.6 ± 12.0 | 3.68 |
| Male sex, n (%) | 597 (53.6) | 591 (53.1) | 1.08 | 681 (55.6) | 689 (56.2) | 2.30 |
| ACS subtypes, n (%) | 1.02 | 2.70 | ||||
| STEMI | 227 (20.4) | 224 (20.1) | 530 (43.2) | 558 (45.5) | ||
| NSTEMI | 275 (24.7) | 272 (24.4) | 509 (41.5) | 464 (37.9) | ||
| UA | 612 (54.9) | 618 (55.5) | 187 (15.3) | 204 (16.6) | ||
| Currently on dialysis, n (%) | 98 (8.8) | 99 (8.9) | 0.32 | 91 (7.4) | 83 (6.8) | 1.00 |
| Medical history, n (%) | ||||||
| MI | 396 (35.5) | 388 (34.8) | 1.50 | 180 (14.7) | 180 (14.7) | 0.70 |
| PCI | 131 (11.8) | 139 (12.5) | 2.20 | 138 (11.3) | 137 (11.2) | 2.56 |
| CABG | 28 (2.5) | 29 (2.6) | 0.57 | 18 (1.5) | 15 (1.2) | 1.48 |
| Diabetes | 529 (47.5) | 530 (47.6) | 0.18 | 479 (39.1) | 483 (39.4) | 2.67 |
| Dyslipidaemia | 62 (5.6) | 66 (5.9) | 1.54 | 131 (10.7) | 127 (10.4) | 0.54 |
| Hypertension | 872 (78.3) | 871 (78.2) | 0.22 | 967 (78.9) | 968 (79.0) | 0.20 |
| Atrial fibrillation | 108 (9.7) | 100 (9.0) | 2.47 | 65 (5.3) | 73 (6.0) | 0.34 |
| Heart failure | 278 (25.0) | 276 (24.8) | 0.42 | 123 (10.0) | 125 (10.2) | 2.74 |
| Ischaemic stroke | 178 (16.0) | 176 (15.8) | 0.49 | 155 (12.7) | 158 (12.9) | 0.49 |
| Haemorrhagic stroke | 15 (1.3) | 18 (1.6) | 2.23 | 10 (0.8) | 14 (1.1) | 0 |
| Chronic obstructive pulmonary disease | 18 (1.6) | 20 (1.8) | 1.39 | 35 (2.9) | 37 (3.0) | 0.47 |
| Peripheral vascular disease | 44 (3.9) | 44 (3.9) | 0 | 32 (2.6) | 37 (3.0) | 0.97 |
| Medical conditions on admission | ||||||
| Heart rate (b.p.m.) | 79.5 ± 16.9 | 79.9 ± 20.0 | 2.08 | 82.2 ± 20.1 | 82.1 ± 18.5 | 0.35 |
| SBP (mmHg) | 138.2 ± 22.5 | 138.3 ± 21.4 | 0.47 | 138.3 ± 29.2 | 139.1 ± 25.6 | 1.24 |
| DBP (mmHg) | 77.1 ± 20.0 | 76.8 ± 14.1 | 1.88 | 79.0 ± 16.7 | 79.0 ± 15.6 | 1.50 |
| Killip class, n (%) | 0.49 | 1.19 | ||||
| Class I | 300 (26.9) | 311 (27.9) | 573 (46.8) | 544 (44.4) | ||
| Class II | 235 (21.1) | 241 (21.6) | 307 (25.0) | 343 (28.0) | ||
| Class III | 362 (32.5) | 323 (29.0) | 196 (16.0) | 212 (17.3) | ||
| Class IV | 217 (19.5) | 239 (21.4) | 150 (12.2) | 127 (10.3) | ||
| Laboratory examinations on admission | ||||||
| TG, mmol/L | 1.5 (1.1–1.9) | 1.5 (1.1–2.0) | 1.85 | 1.5 (1.0–2.2) | 1.5 (1.1–2.2) | 0.65 |
| LDL-C, mmol/L | 2.4 (1.9–2.8) | 2.4 (1.9–2.9) | 2.01 | 2.5 (1.9–3.2) | 2.5 (1.9–3.2) | 0.52 |
| HDL-C, mmol/L | 1.0 (0.8–1.1) | 1.0 (0.8–1.2) | 1.42 | 1.0 (0.8–1.3) | 1.0 (0.8–1.2) | 0.67 |
| eGFR (mL/min/1.73 m2) | 19.6 (12.2–25.4) | 20.3 (12.7–25.7) | 1.54 | 22.1 (15.5–26.3) | 22.9 (16.6–26.6) | 8.07 |
| CK-MB (U/L) or proportion of increased myocardial enzymesa | 403 (36.2) | 404 (36.3) | 0.19 | 22.3 (10.0–66.6) | 20.0 (8.9–60.4) | 2.22 |
| Treatment strategy upon the index admission, n (%) | ||||||
| Aspirin | 806 (72.4) | 819 (73.5) | 2.63 | 1112 (90.8) | 1114 (90.9) | 0 |
| P2Y12 inhibitor | 835 (75.2) | 845 (75.9) | 2.08 | 1129 (92.2) | 1129 (92.2) | 0.91 |
| Statin | 934 (83.8) | 948 (85.1) | 3.47 | 1162 (94.9) | 1172 (95.7) | 0.79 |
| PCI | 178 (16.0) | 165 (14.8) | 3.23 | 536 (43.8) | 549 (44.8) | 0.16 |
aCK-MB (U/L) for CCC-ACS project cohort or proportion exceeding 99% confidence interval of myocardial enzyme for CNEDSSP-ACS cohort.
ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; ACS, acute coronary syndrome; STEMI, ST-elevation myocardial infarction; NSTEMI, non-ST-elevation myocardial infarction; UA, unstable angina; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary bypass grafting; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; CK-MB, creatinine kinase subtype.
Baseline characteristics by ACEI/ARB therapy after propensity score matching
| . | CNEDSSP cohort . | CCC-ACS cohort . | ||||
|---|---|---|---|---|---|---|
| Characteristics . | Non-early ACEI/ARB (n = 1114) . | Early ACEI/ARB (n = 1114) . | ASD, (%) . | Non-early ACEI/ARB (n = 1225) . | Early ACEI/ARB (n = 1225) . | ASD, (%) . |
| Age, years | 72.5 ± 11.4 | 72.1 ± 11.2 | 3.28 | 71.2 ± 11.8 | 71.6 ± 12.0 | 3.68 |
| Male sex, n (%) | 597 (53.6) | 591 (53.1) | 1.08 | 681 (55.6) | 689 (56.2) | 2.30 |
| ACS subtypes, n (%) | 1.02 | 2.70 | ||||
| STEMI | 227 (20.4) | 224 (20.1) | 530 (43.2) | 558 (45.5) | ||
| NSTEMI | 275 (24.7) | 272 (24.4) | 509 (41.5) | 464 (37.9) | ||
| UA | 612 (54.9) | 618 (55.5) | 187 (15.3) | 204 (16.6) | ||
| Currently on dialysis, n (%) | 98 (8.8) | 99 (8.9) | 0.32 | 91 (7.4) | 83 (6.8) | 1.00 |
| Medical history, n (%) | ||||||
| MI | 396 (35.5) | 388 (34.8) | 1.50 | 180 (14.7) | 180 (14.7) | 0.70 |
| PCI | 131 (11.8) | 139 (12.5) | 2.20 | 138 (11.3) | 137 (11.2) | 2.56 |
| CABG | 28 (2.5) | 29 (2.6) | 0.57 | 18 (1.5) | 15 (1.2) | 1.48 |
| Diabetes | 529 (47.5) | 530 (47.6) | 0.18 | 479 (39.1) | 483 (39.4) | 2.67 |
| Dyslipidaemia | 62 (5.6) | 66 (5.9) | 1.54 | 131 (10.7) | 127 (10.4) | 0.54 |
| Hypertension | 872 (78.3) | 871 (78.2) | 0.22 | 967 (78.9) | 968 (79.0) | 0.20 |
| Atrial fibrillation | 108 (9.7) | 100 (9.0) | 2.47 | 65 (5.3) | 73 (6.0) | 0.34 |
| Heart failure | 278 (25.0) | 276 (24.8) | 0.42 | 123 (10.0) | 125 (10.2) | 2.74 |
| Ischaemic stroke | 178 (16.0) | 176 (15.8) | 0.49 | 155 (12.7) | 158 (12.9) | 0.49 |
| Haemorrhagic stroke | 15 (1.3) | 18 (1.6) | 2.23 | 10 (0.8) | 14 (1.1) | 0 |
| Chronic obstructive pulmonary disease | 18 (1.6) | 20 (1.8) | 1.39 | 35 (2.9) | 37 (3.0) | 0.47 |
| Peripheral vascular disease | 44 (3.9) | 44 (3.9) | 0 | 32 (2.6) | 37 (3.0) | 0.97 |
| Medical conditions on admission | ||||||
| Heart rate (b.p.m.) | 79.5 ± 16.9 | 79.9 ± 20.0 | 2.08 | 82.2 ± 20.1 | 82.1 ± 18.5 | 0.35 |
| SBP (mmHg) | 138.2 ± 22.5 | 138.3 ± 21.4 | 0.47 | 138.3 ± 29.2 | 139.1 ± 25.6 | 1.24 |
| DBP (mmHg) | 77.1 ± 20.0 | 76.8 ± 14.1 | 1.88 | 79.0 ± 16.7 | 79.0 ± 15.6 | 1.50 |
| Killip class, n (%) | 0.49 | 1.19 | ||||
| Class I | 300 (26.9) | 311 (27.9) | 573 (46.8) | 544 (44.4) | ||
| Class II | 235 (21.1) | 241 (21.6) | 307 (25.0) | 343 (28.0) | ||
| Class III | 362 (32.5) | 323 (29.0) | 196 (16.0) | 212 (17.3) | ||
| Class IV | 217 (19.5) | 239 (21.4) | 150 (12.2) | 127 (10.3) | ||
| Laboratory examinations on admission | ||||||
| TG, mmol/L | 1.5 (1.1–1.9) | 1.5 (1.1–2.0) | 1.85 | 1.5 (1.0–2.2) | 1.5 (1.1–2.2) | 0.65 |
| LDL-C, mmol/L | 2.4 (1.9–2.8) | 2.4 (1.9–2.9) | 2.01 | 2.5 (1.9–3.2) | 2.5 (1.9–3.2) | 0.52 |
| HDL-C, mmol/L | 1.0 (0.8–1.1) | 1.0 (0.8–1.2) | 1.42 | 1.0 (0.8–1.3) | 1.0 (0.8–1.2) | 0.67 |
| eGFR (mL/min/1.73 m2) | 19.6 (12.2–25.4) | 20.3 (12.7–25.7) | 1.54 | 22.1 (15.5–26.3) | 22.9 (16.6–26.6) | 8.07 |
| CK-MB (U/L) or proportion of increased myocardial enzymesa | 403 (36.2) | 404 (36.3) | 0.19 | 22.3 (10.0–66.6) | 20.0 (8.9–60.4) | 2.22 |
| Treatment strategy upon the index admission, n (%) | ||||||
| Aspirin | 806 (72.4) | 819 (73.5) | 2.63 | 1112 (90.8) | 1114 (90.9) | 0 |
| P2Y12 inhibitor | 835 (75.2) | 845 (75.9) | 2.08 | 1129 (92.2) | 1129 (92.2) | 0.91 |
| Statin | 934 (83.8) | 948 (85.1) | 3.47 | 1162 (94.9) | 1172 (95.7) | 0.79 |
| PCI | 178 (16.0) | 165 (14.8) | 3.23 | 536 (43.8) | 549 (44.8) | 0.16 |
| . | CNEDSSP cohort . | CCC-ACS cohort . | ||||
|---|---|---|---|---|---|---|
| Characteristics . | Non-early ACEI/ARB (n = 1114) . | Early ACEI/ARB (n = 1114) . | ASD, (%) . | Non-early ACEI/ARB (n = 1225) . | Early ACEI/ARB (n = 1225) . | ASD, (%) . |
| Age, years | 72.5 ± 11.4 | 72.1 ± 11.2 | 3.28 | 71.2 ± 11.8 | 71.6 ± 12.0 | 3.68 |
| Male sex, n (%) | 597 (53.6) | 591 (53.1) | 1.08 | 681 (55.6) | 689 (56.2) | 2.30 |
| ACS subtypes, n (%) | 1.02 | 2.70 | ||||
| STEMI | 227 (20.4) | 224 (20.1) | 530 (43.2) | 558 (45.5) | ||
| NSTEMI | 275 (24.7) | 272 (24.4) | 509 (41.5) | 464 (37.9) | ||
| UA | 612 (54.9) | 618 (55.5) | 187 (15.3) | 204 (16.6) | ||
| Currently on dialysis, n (%) | 98 (8.8) | 99 (8.9) | 0.32 | 91 (7.4) | 83 (6.8) | 1.00 |
| Medical history, n (%) | ||||||
| MI | 396 (35.5) | 388 (34.8) | 1.50 | 180 (14.7) | 180 (14.7) | 0.70 |
| PCI | 131 (11.8) | 139 (12.5) | 2.20 | 138 (11.3) | 137 (11.2) | 2.56 |
| CABG | 28 (2.5) | 29 (2.6) | 0.57 | 18 (1.5) | 15 (1.2) | 1.48 |
| Diabetes | 529 (47.5) | 530 (47.6) | 0.18 | 479 (39.1) | 483 (39.4) | 2.67 |
| Dyslipidaemia | 62 (5.6) | 66 (5.9) | 1.54 | 131 (10.7) | 127 (10.4) | 0.54 |
| Hypertension | 872 (78.3) | 871 (78.2) | 0.22 | 967 (78.9) | 968 (79.0) | 0.20 |
| Atrial fibrillation | 108 (9.7) | 100 (9.0) | 2.47 | 65 (5.3) | 73 (6.0) | 0.34 |
| Heart failure | 278 (25.0) | 276 (24.8) | 0.42 | 123 (10.0) | 125 (10.2) | 2.74 |
| Ischaemic stroke | 178 (16.0) | 176 (15.8) | 0.49 | 155 (12.7) | 158 (12.9) | 0.49 |
| Haemorrhagic stroke | 15 (1.3) | 18 (1.6) | 2.23 | 10 (0.8) | 14 (1.1) | 0 |
| Chronic obstructive pulmonary disease | 18 (1.6) | 20 (1.8) | 1.39 | 35 (2.9) | 37 (3.0) | 0.47 |
| Peripheral vascular disease | 44 (3.9) | 44 (3.9) | 0 | 32 (2.6) | 37 (3.0) | 0.97 |
| Medical conditions on admission | ||||||
| Heart rate (b.p.m.) | 79.5 ± 16.9 | 79.9 ± 20.0 | 2.08 | 82.2 ± 20.1 | 82.1 ± 18.5 | 0.35 |
| SBP (mmHg) | 138.2 ± 22.5 | 138.3 ± 21.4 | 0.47 | 138.3 ± 29.2 | 139.1 ± 25.6 | 1.24 |
| DBP (mmHg) | 77.1 ± 20.0 | 76.8 ± 14.1 | 1.88 | 79.0 ± 16.7 | 79.0 ± 15.6 | 1.50 |
| Killip class, n (%) | 0.49 | 1.19 | ||||
| Class I | 300 (26.9) | 311 (27.9) | 573 (46.8) | 544 (44.4) | ||
| Class II | 235 (21.1) | 241 (21.6) | 307 (25.0) | 343 (28.0) | ||
| Class III | 362 (32.5) | 323 (29.0) | 196 (16.0) | 212 (17.3) | ||
| Class IV | 217 (19.5) | 239 (21.4) | 150 (12.2) | 127 (10.3) | ||
| Laboratory examinations on admission | ||||||
| TG, mmol/L | 1.5 (1.1–1.9) | 1.5 (1.1–2.0) | 1.85 | 1.5 (1.0–2.2) | 1.5 (1.1–2.2) | 0.65 |
| LDL-C, mmol/L | 2.4 (1.9–2.8) | 2.4 (1.9–2.9) | 2.01 | 2.5 (1.9–3.2) | 2.5 (1.9–3.2) | 0.52 |
| HDL-C, mmol/L | 1.0 (0.8–1.1) | 1.0 (0.8–1.2) | 1.42 | 1.0 (0.8–1.3) | 1.0 (0.8–1.2) | 0.67 |
| eGFR (mL/min/1.73 m2) | 19.6 (12.2–25.4) | 20.3 (12.7–25.7) | 1.54 | 22.1 (15.5–26.3) | 22.9 (16.6–26.6) | 8.07 |
| CK-MB (U/L) or proportion of increased myocardial enzymesa | 403 (36.2) | 404 (36.3) | 0.19 | 22.3 (10.0–66.6) | 20.0 (8.9–60.4) | 2.22 |
| Treatment strategy upon the index admission, n (%) | ||||||
| Aspirin | 806 (72.4) | 819 (73.5) | 2.63 | 1112 (90.8) | 1114 (90.9) | 0 |
| P2Y12 inhibitor | 835 (75.2) | 845 (75.9) | 2.08 | 1129 (92.2) | 1129 (92.2) | 0.91 |
| Statin | 934 (83.8) | 948 (85.1) | 3.47 | 1162 (94.9) | 1172 (95.7) | 0.79 |
| PCI | 178 (16.0) | 165 (14.8) | 3.23 | 536 (43.8) | 549 (44.8) | 0.16 |
aCK-MB (U/L) for CCC-ACS project cohort or proportion exceeding 99% confidence interval of myocardial enzyme for CNEDSSP-ACS cohort.
ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; ACS, acute coronary syndrome; STEMI, ST-elevation myocardial infarction; NSTEMI, non-ST-elevation myocardial infarction; UA, unstable angina; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary bypass grafting; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; CK-MB, creatinine kinase subtype.
Results
Baseline characteristics between CNEDSSP and CCC-ACS cohorts
In CNEDSSP cohort, we excluded 160 488 patients with eGFR ≥ 30 mL/min/1.73 m2 and 21 074 patients with suspected ACS whose diagnosis was not confirmed upon admission. Accordingly, we excluded 108 447 and 1287 corresponding patients in CCC-ACS cohort. By applying our exclusion criteria (Figure 1), we focused our analysis on 3288 patients with admission eGFR < 30 mL/min/1.73 m2 in CNEDSSP cohort and 3916 counterparts in CCC-ACS cohort. Among 7204 individuals, 2647 (36.7%) patients received ACEI/ARB therapy within 24 h after admission. During the hospital stay with a mean duration of 10.1 days, 605 in-hospital deaths (8.4%) were recorded in the two cohorts, including 145 patients receiving early ACEI/ARB (5.5%) and 460 patients not receiving early ACEI/ARB (10.1%). The average age of the two total cohorts was 71.9 years, with 55.8% being males, and 584 patients (8.1%) were currently on dialysis. In addition, the baseline characteristics of the two cohorts were slightly different (Supplementary material online, Figures S1 and S2). In CNEDSSP cohort, NSTE-ACS accounted for 79.2%, whereas ACS subtype was mainly composed of STEMI in CCC-ACS cohort, accounting for 46.6% (Supplementary material online, Figure S1A). Compared with CCC-ACS cohort, patients in CNEDSSP cohort had a higher Killip class (Supplementary material online, Figure S2A), which may be related to a higher proportion of medical history of MI or HF (Supplementary material online, Figure S1B). In addition, the proportion of PCI in CCC-ACS cohort was higher, while most patients of CNEDSSP cohort received conservative treatment (Supplementary material online, Figure S2B). Compared with non-ACEI/ARB users, individuals who received ACEI/ARB therapy were more likely to be hypertensive, and were more likely to receive aspirin, P2Y12 inhibitor, statin, and PCI during hospitalization (Supplementary material online, Table S1).
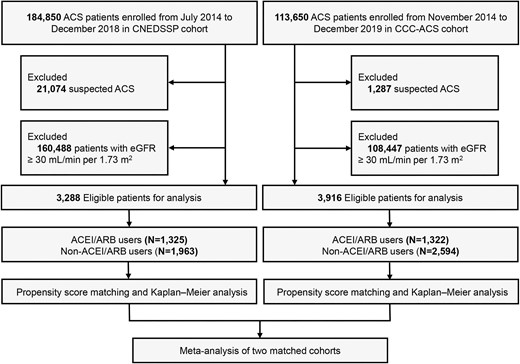
Study flow chart. ACS, acute coronary syndrome; eGFR, estimated glomerular filtration rate; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
Association between ACEI/ARB early use and in-hospital mortality
Table 1 shows the comparisons of clinical features between ACEI/ARB users and non-users after 1:1 PSM. The clinical features of these two groups in two cohorts were well-balanced after PSM, with ASD <10% for all covariates (Supplementary material online, Figure S3). In matched cohorts, 180 [8.1%, (12.6% for STEMI, 6.9% for NSTE-ACS)] in-hospital deaths in CNEDSSP cohort and 139 [5.7%, (6.6% for STEMI, 3.9% for NSTE-ACS)] in CCC-ACS cohort were recorded. Figure 2A shows the Kaplan‒Meier survival curves of CNEDSSP cohort for ACEI/ARB users and non-ACEI/ARB users in the propensity score-matched cohort, with ACEI/ARB use showing an association with lower in-hospital mortality [hazard ratio (HR): 0.61, 95% confidence interval (95% CI): 0.45–0.82; P < 0.01]. This result was consistently observed in the CCC-ACS cohort (HR: 0.66, 95% CI: 0.46–0.95; P = 0.03) (Figure 2B). The meta-analysis combining the results of the matched CNEDSSP cohort and matched CCC-ACS cohort (Figure 2C) revealed that early ACEI/ARB use was associated with a 32% reduction in in-hospital mortality for ACS patients with eGFR < 30 mL/min/1.73 m2 [risk ratio (RR): 0.68, 95% CI: 0.55–0.84]. In a separate analysis, ACEI and ARB users were all associated with similar magnitude reductions in in-hospital mortality (Supplementary material online, Figure S4), further suggesting that there was no class effect between ACEI and ARB in terms of improving the in-hospital outcome in ACS patients with advanced renal dysfunction.
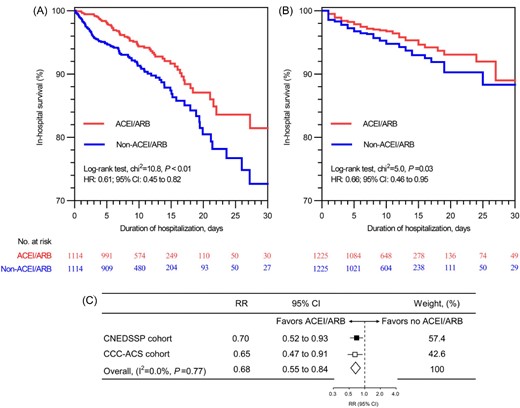
Association between early ACEI/ARB use and in-hospital mortality among ACS patients with advanced renal dysfunction. (A) Kaplan–Meier survival analysis in propensity score—matched CNEDSSP cohort; (B) Kaplan–Meier survival analysis in propensity score—matched CCC-ACS cohort; (C) Meta-analysis of the results combined matched CNEDSSP cohort and matched CCC-ACS cohort. ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; HR, hazard ratio; RR, risk ratio; 95% CI, 95% confidence interval.
Sensitivity analysis
As shown in Supplementary material online, Figure S5, the association between early ACEI/ARB use and in-hospital mortality remained significant when patients who died within 24 h of admission, or those with severe conditions (cardiogenic shock, HF, or cardiac arrest) on admission, with a history of PCI or CABG, and those currently not on dialysis were excluded in the matched cohort based on Cox regression models. The E-value for the association between ACEI/ARB use and in-hospital mortality derived from the meta-analysis of the main effect was 2.30 (Supplementary material online, Figure S6), indicating that an unmeasured/uncontrolled confounder with the same association with both the exposure (ACEI/ARB use) and outcome (death) might negate the current finding. The results based on the meta-analysis (Figure 3) and the total cohort by multiple statistical methods (Supplementary material online, Figure S7) also confirmed the above findings.
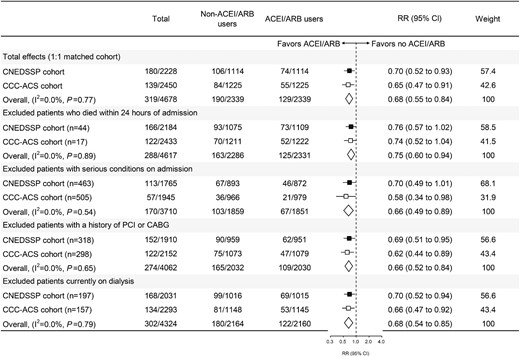
Meta-analysis and sensitivity analysis of propensity score—matched CNEDSSP cohort and CCC-ACS cohort. ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; HR, hazard ratio; 95% CI, 95% confidence interval; PCI, percutaneous coronary intervention; CABG, coronary bypass grafting. Serious condition included one of the following admission conditions, which contained cardiogenic shock, heart failure, or cardiac arrest.
Subgroup analysis
In general, subgroup analyses across age subgroup (≥65 years old and <65 years old), sex, ACS subtypes (STEMI and NSTE-ACS), with or without histories of MI, diabetes, HF, and PCI during the index admission yielded similar trends for the protective association between ACEI/ARB use and in-hospital death (Figure 4). Notably, the effect size of ACEI/ARB-associated benefit in in-hospital deaths was attenuated in patients presenting with NSTE-ACS (Figure 4), indicating that the primary finding may be driven by the improvement of adverse outcome in STEMI patients with advanced renal dysfunction.
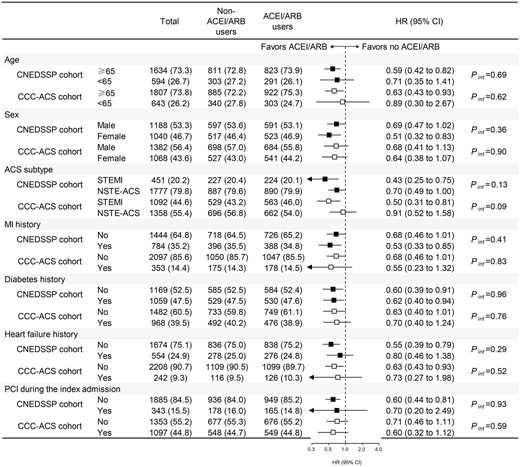
Subgroup analysis based on matched CNEDSSP cohort and matched CCC-ACS cohort. ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; ACS, acute coronary syndrome; STEMI, ST-elevation myocardial infarction; NSTE-ACS, non-ST-elevation acute coronary syndrome; MI, myocardial infarction; PCI, percutaneous coronary intervention; Pint, P value for interaction; HR, hazard ratio; 95% CI, 95% confidence interval.
Discussion
In two nationwide cohorts (an EHR-based and an ACS registry-based) in China, we demonstrated that early (initiated within the first 24 h after hospitalization) ACEI/ARB therapy was associated with a 32% reduction in in-hospital deaths among ACS patients with an admission eGFR < 30 mL/min/1.73 m2. These findings were consistent in multiple sensitivity analyses. Although our findings were based on the observational data, which are therefore hypothesis-generating, considering the consistent benefit across multiple subgroups, to improve clinical outcome, the early ACEI/ARB therapy should be encouraged in ACS patients with concurrent advanced renal dysfunction.
ACS patients with eGFR < 30 mL/min/1.73 m2 are at high risk of in-hospital mortality; however, the implementation of evidence-based therapies in this population is inadequate.20,21 In our study period (2014–2019), we found that in-hospital mortality was improved (8.4% in this study) as compared with a US nationwide registry that enrolled ACS patients during 2007 [12.4–31.8% for in-hospital mortality for MI patients with stage 4 to 5 chronic kidney disease (CKD), respectively].22 The present study included two study cohorts with slightly differences in cohort characteristics: Firstly, the CNEDSSP cohort is an HER-based population, whose STEMI proportion (21%) is close to those reported in epidemiological study;1 in the CCC-ACS project, MI cases have reporting priority, and the hospital will report STEMI only if the number of cases in a participating hospital is small,16 which led to a higher proportion of STEMI (46%) and PCI (42%) cases; secondly, the proportion of patients with Killip class III/IV and the prevalence of prior histories for HF and MI were higher in the CNEDSSP cohort than in the CCC-ACS cohort. These differences may influence the medication choice by the treating physicians. For example, 77.6% patients in CNEDSSP cohort and 88.1% in CCC-ACS cohort received antithrombotic therapy; the latter was similar to the proportion of antiplatelet therapy in other studies that enrolled MI patients with advanced renal dysfunction (∼83%).23,24 Considering the recent observational evidence that potent P2Y12 inhibition via prasugrel and ticagrelor was associated with a lower risk of death in ACS patients with eGFR < 60 mL/min/1.73 m2,25,26 future work is warranted to optimize the antiplatelet regimen in ACS patients with stage 4 to 5 CKD. In terms of the percentage of PCI performed in ACS patients during hospitalization, our two cohorts were higher than a study based on US National Inpatient Sample data from 2007 to 2012 [258 MI patients (17.5%) vs. 29.3%].27 Despite the finding from the ISCHEMIA-CKD study fact that invasive strategy (85% PCI) did not reduce the risk of death and non-fatal MI in stable coronary disease patients with eGFR < 30 mL/min/1.73 m2,28 the high-level evidence concerning clinical outcomes of ACS patients with advanced renal dysfunction remains limited.
To our knowledge, our work for the first time demonstrated a protective association between early ACEI/ARB use and in-hospital mortality for patients with admission eGFR < 30 mL/min/1.73 m2 in the acute phase of ACS. A previous study addressed the association between discharge ACEI/ARB use and 3-year out-of-hospital survival among MI patients across all renal function stratifications in the SWEDEHEART registry.29 The authors demonstrated an ∼20% overall reduction in 3-year mortality in patients treated with ACEI/ARB, and this beneficial association was sustained among MI patients with eGFR < 30 mL/min/1.73 m2 (HR: 0.79, 95% CI: 0.71–0.87).29 In the above study, there was a time-dependent increasing trend for the discontinuation of ACEI/ARB, with 14%, 24%, and 30% after 1, 2, and 3 years, respectively.29 This finding is in agreement with other reports that discontinuation of ACEI/ARB is a relatively common phenomenon in ACS patients with the comorbidity of advanced renal dysfunction,30–32 which may be associated with a higher risk of mortality.33 In the present study, ∼63% of patients did not receive ACEI/ARB treatment. Given the protective association of early ACEI/ARB use and in-hospital death in the present study, as well as the post-discharge long-term mortality benefit of ACEI/ARB use demonstrated in previous work,29 specific efforts should be made in the future to address the underuse of ACEI/ARB both in the acute phase and chronic phase of MI onset among patients with advanced renal dysfunction.
Notably, we observed that patients with STEMI upon indexed admission were more likely to benefit from early ACEI/ARB therapy, whereas for NSTE-ACS patients, this survival benefit was attenuated without statistical significance. The 2020 European guideline recommended that ACEI/ARB should be taken into consideration in NSTE-ACS patients with LVEF < 40% unless they have advanced renal dysfunction (IA recommendation).3 It is therefore reasonable that, in NSTE-ACS patients, the reversal role of ACEI/ARB in adverse ventricular remodeling, which is mainly due to its impact on the healing and on the haemodynamic alteration in the infarcted left ventricle, is less significant. Given the relatively low-risk characteristics of NSTE-ACS patients, the prescription would be individualized according to each patient's characteristics. In addition, a previous study showed that ACEI use was associated with lower long-term mortality after MI compared with ARB,34 whereas inconsistent results were reported among patients with predialytic stage 5 CKD, which demonstrated a mortality benefit of ARB over ACEI.35 In the present study, ACEI and ARB use were both associated with similar magnitude reductions in in-hospital mortality. Therefore, if indicated, we did not find evidence to favour one over the other during the first 24 h following admission.
The strength of our study was the inclusion of two nationwide cohorts in China, with a large sample size to represent the entire country, as well as the statistical power to assess outcomes in specific subgroups. In addition, the two cohorts had relatively different yet complementary baseline characteristics that would represent the full spectrum of ACS patients with advanced renal dysfunction. Moreover, the consistency in the main findings from the two cohorts and the meta-analysis of combined results further confirmed the robustness of our findings. Admittedly, our study had the following limitations: First, as an observational study, we could not establish a causal relationship between ACEI/ARB treatment and in-hospital mortality. Second, although we used statistical methods such as PSM and inverse probability weighting to control confounding factors, we could not exclude the influence of confounding factors that were not included in the database. For example, in the present study, early ACEI/ARB use during admission was associated with a 32% reduction in the hazard of in-hospital death, which is higher than the 21% reduction in the SWEDEHEART registry.29 Although the higher mortality rate in the acute phase of ACS contributes to the discrepancy of ACEI/ARB-associated reduction in in-hospital mortality compared with the SWEDEHEART registry, this suggests that the relatively large effect size reported in our study might have been due to unmeasured confounding. Additionally, we acknowledged that we had no information on the volume of contrast used in patients treated with PCI, despite that contrast-induced nephropathy was not associated with worsened clinical outcome in this population.36 Third, as our analysis was based on observational data, considering the comorbidity nature of our study population, the exact causes of death were unclear unless by an adjudication committee. As our study population was identified during their hospitalization in the cardiology department, most of deaths could be ascribed to cardiovascular causes. Future out-of-hospital follow-up work is therefore required to examine the competing effect of ACEI/ARB use on reason of death in this population. Forth, our study was only based on the Chinese population, and further research is needed from other countries and different ethnic groups.
Conclusion
Based on a nationwide EHR-based cohort and on a nationwide ACS registry in contemporary practice, our results provide evidence that ACEI/ARB treatment initiated within 24 h of admission is associated with a reduced risk of in-hospital mortality in ACS patients with admission eGFR < 30 mL/min/1.73 m2.
Funding
This work was supported by the National Natural Science Foundation of China (82270349 and 72274133), the Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-069C), the Tianjin Municipal Science and Technology Commission (19JCQNJC11500), and the Double First-Class Project of Tianjin Medical University (SYL001-303078100820 and SYL002-303078100821).
Conflict of interest
The authors declare that they have no conflict of interest.
Data availability statement
The data, analytic methods, and study materials will be made available for onsite audit by third parties for purposes of reproducing the results or replicating the procedure.
References
Author notes
The first two authors contributed equally to this study.



