-
PDF
- Split View
-
Views
-
Cite
Cite
Yousif Ahmad, Mahesh V Madhavan, Gregg W Stone, Darrel P Francis, Raj Makkar, Deepak L Bhatt, James P Howard, Sodium-glucose cotransporter 2 inhibitors in patients with heart failure: a systematic review and meta-analysis of randomized trials, European Heart Journal - Quality of Care and Clinical Outcomes, Volume 8, Issue 4, July 2022, Pages 383–390, https://doi.org/10.1093/ehjqcco/qcab072
Close - Share Icon Share
Abstract
Sodium-glucose cotransporter 2 (SGLT-2) inhibitors have now been evaluated for the treatment of heart failure in several placebo-controlled randomized controlled trials (RCTs) across various ejection fraction ranges, but these trials were powered for composite outcomes rather than individual clinical endpoints. We therefore performed a meta-analysis to assess their safety and efficacy on all-cause mortality, cardiovascular mortality, and heart failure hospitalizations.
We performed a prospectively registered random-effects meta-analysis of all RCTs comparing SGLT-2 inhibitors to placebo in patients with heart failure. The pre-specified primary endpoint was all-cause mortality. Secondary endpoints included cardiovascular mortality, heart failure hospitalizations, and the composite of cardiovascular mortality or heart failure hospitalization. Four trials with 15 684 patients were eligible. The SGLT-2 inhibitor tested was empagliflozin in two trials, dapagliflozin in one trial, and sotagliflozin in one trial. The weighted-mean follow-up was 20.0 months. The hazard ratio (HR) for all-cause mortality was 0.91, 95% confidence interval (CI) 0.82–1.01, P = 0.071. There was a 12% reduction in cardiovascular mortality (HR 0.88, 95% CI 0.79 to 0.97, P = 0.012), and a 30% reduction in heart failure hospitalization (HR 0.70, 95% CI 0.64 to 0.77, P < 0.001).
SGLT-2 inhibitors significantly reduced cardiovascular mortality and heart failure hospitalizations in patients with heart failure. The effect appears consistent across three drugs studied in four trials. SGLT-2 inhibitors should become standard care for patients with heart failure.
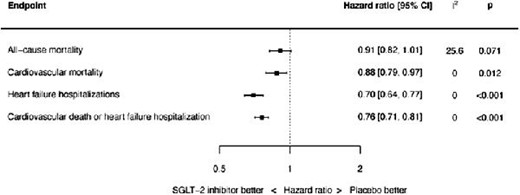
SGLT-2 inhibitors are associated with significant reductions in cardiovascular mortality, heart failure hospitalizations and the composite of cardiovascular death or heart failure hospitalization. Summary of point estimates of hazard ratios, 95% confidence intervals and P values shown. Random effects model used; all results also consistent with fixed effect analysis, with the exception of all-cause mortality reduction being significant by fixed effect (HR 0.91, 95% CI 0.84 to 1.00, P=0.040).
Introduction
Sodium-glucose cotransporter 2 (SGLT-2) inhibitors have now been evaluated for the treatment of heart failure (HF) in several placebo-controlled randomized trials across a spectrum of ejection fractions (EFs),1–4 but these studies were powered for composite outcomes rather than for individual clinical endpoints. Meta-analysis is a tool that can combine their results to determine effects on individual endpoints for which single trials are underpowered.
We therefore performed an updated meta-analysis of all randomized controlled trials (RCTs) focusing on individual clinical endpoints, to assess the safety and efficacy of SGLT-2 inhibitors in patients with HF. We also looked for a difference in response between reduced and preserved EF.
Methods
This study was prospectively registered at the International Prospective Register of Systematic Reviews (PROSPERO; CRD42021231485) and was performed in accordance with published guidance.5
Search strategy
We performed a systematic search of the MEDLINE, Cochrane Central Register of Controlled Trials, and Embase databases from December 2010 through September 2021 for all RCTs comparing SGLT-2 inhibitors to placebo for the treatment of HF. Our search strings are shown in the Supplementary material online, Table S1. We manually searched bibliographies of selected studies and meta-analyses to identify any other eligible trials. Abstracts were reviewed for suitability and full-text articles retrieved with conference abstracts also searched for relevant studies. Two independent authors performed the search and literature screening (Y.A. and J.H.), with disputes resolved by consensus following discussion.
Inclusion criteria
We only considered RCTs that reported clinical outcomes after randomization to either SGLT-2 inhibitors or placebo, in patients with HF. Observational studies were not included, nor were RCTs of patients with diabetes but no HF. We only considered entire trials focusing on HF, and not post-hoc reports of HF strata within trials that were not focusing on HF.
Endpoints
The pre-specified primary endpoint was all-cause mortality. Secondary pre-specified endpoints included cardiovascular mortality, all HF hospitalizations (i.e. recurrent events, and not time to first event), and the composite of cardiovascular mortality or HF hospitalization (assessed as time-to-event data). This composite was only assessed if it was specifically reported in the individual trials, i.e. summing of individual component outcomes was not performed. For HF hospitalizations, data for Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) excluding urgent visits were utilized from a subsequent publication.6 These data were not available for Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF), so for that trial urgent visits were also counted.
Data extraction
Independent abstraction of data was performed in duplicate by two individual authors (Y.A. and J.H.). Included trials were assessed using the Cochrane risk of bias tool.7
Data analysis
Random-effects meta-analyses were performed using the restricted maximum likelihood (REML) estimator. All analyses were performed based on the intention-to-treat principle. Hazard ratios (HRs) were used as the measure of effect due to the fact the included trials used time-to-event survival data and also to allow for variable follow-up duration across trials. We extracted the HRs with their associated 95% confidence intervals (95% CIs) and P-values. A random-effects meta-analysis was performed of the natural logarithm of the HRs and their associated standard errors using the REML estimator. The standard error was calculated by dividing the difference between the natural logarithms of the upper and lower 95% CIs by two times the appropriate normal score (1.96). Where the lower 95% CI approached zero, the standard error was calculated using only the difference between the natural logarithm of the upper 95% CI and the natural logarithm of the point estimate. Sensitivity analyses were performed using a fixed-effect model. The I2 statistic was used to assess heterogeneity.8 We also performed a jackknife sensitivity analysis excluding each trial in turn for all endpoints. Sensitivity analyses were performed for only patients with reduced EF and only patients with preserved EF, and interaction tests were performed to test for a significant interaction on the effects of SGLT-2 inhibitors. Pre-specified subgroup analyses for the composite of cardiovascular mortality and HF hospitalization were performed according to age (≥65 years), sex, body mass index (BMI) ≥ 30 kg/m2, renal dysfunction (estimated glomerular filtration rate (GFR) <60 mL/min), ethnicity, and baseline use of angiotensin receptor neprilysin inhibitors (ARNIs). Interactions between subgroups were assessed using a mixed effects meta-analytical model, with the subgroup characteristic in question as a moderator and the individual trial as a random effect.
Tests for publication bias would only be performed in the event of more than 10 trials being identified for inclusion.9 Mean values are expressed as mean ± SD unless otherwise stated. Statistical significance was set at P < 0.05. P-values are two-tailed and were not adjusted for multiplicity. The statistical programming environment R10 with the metafor package11 was used for all statistical analyses.
Results
Four trials randomizing a total of 15 684 patients were eligible, with 7841 randomized to SGLT-2 inhibitors and 7843 randomized to placebo.1–4 The search strategy and source of studies are shown in the Supplementary material online, Figure S1. The weighted-mean follow-up duration was 20.0 months. In DAPA-HF, 67.5% of patients were in New York Heart Association (NYHA) NYHA class II at baseline and 31.6% of patients were in NYHA class III. In Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR-REDUCED), 75% of patients were in NYHA class II and 24.4% were in NYHA class III. In EMPEROR-Preserved, 81.5% of patients were in NYHA class II and 18.1% were in NYHA class III. In SOLOIST-WHF, there were no data regarding NYHA class at baseline presented. DAPA-HF and EMPEROR-Reduced included ambulatory patients with EF of 40% or less, whereas SOLOIST-WHF studied patients with type 2 diabetes admitted with worsening HF requiring intravenous diuretics and encompassed patients with a range of EFs; EMPEROR-Preserved studied patients with chronic HF and an EF greater than 40%.
Other baseline characteristics of the included trials are shown in the Supplementary material online, Table S2, and the risk of bias is shown in the Supplementary material online, Table S3. The SGLT-2 inhibitor tested was empagliflozin in two trials, dapagliflozin in one trial, and sotagliflozin in one trial.
For all-cause mortality, the HR with SGLT-2 was 0.91, 95% CI 0.82–1.01, and P = 0.071 (see Figure 1). There was mild heterogeneity (I2 = 25.6%). The source of the heterogeneity was the EMPEROR-Preserved trial: when this trial was removed, the effect of SGLT-2 inhibitors on the hazard of all-cause mortality was HR 0.86, 95% CI 0.77–0.96, P = 0.009, and I2 = 0.0%.
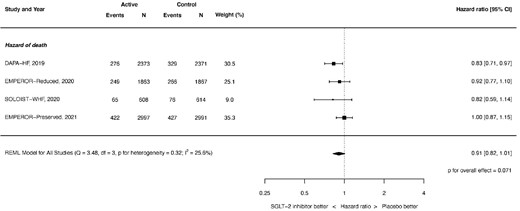
Hazard of all-cause mortality. REML, restricted maximum likelihood. Q = Cochran's Q level of heterogeneity; df = degrees of freedom.
Cardiovascular mortality was significantly reduced: HR 0.88, 95% CI 0.79–0.97, and P = 0.012 (see Figure 2). There was no heterogeneity (I2 = 0.0%).
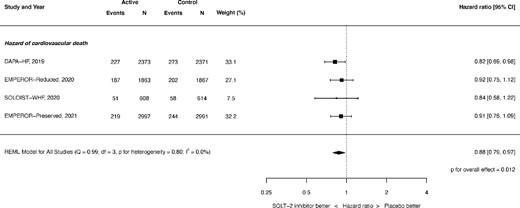
Hazard of cardiovascular mortality. REML, restricted maximum likelihood. Q = Cochran's Q level of heterogeneity; df = degrees of freedom.
HF hospitalizations were also significantly reduced: HR 0.70, 95% CI 0.64–0.77, and P < 0.001 (see Figure 3), with no heterogeneity observed (I2 = 0.0%).

Hazard of heart failure hospitalization. REML, restricted maximum likelihood. Q = Cochran's Q level of heterogeneity; df = degrees of freedom.
The composite of cardiovascular death or first HF hospitalization was significantly reduced: HR 0.76, 95% CI 0.71–0.81, and P < 0.001 (see Figure 4). There was no heterogeneity (I2 = 0.0%).

Hazard of composite of cardiovascular mortality or heart failure hospitalization. REML, restricted maximum likelihood. Q = Cochran's Q level of heterogeneity; df = degrees of freedom.
Impact of ejection fraction
There was no significant difference in hazard ratio (HR) for SGLT-2 inhibitors between the trial subgroups with reduced EF and those with preserved EF for the endpoint of cardiovascular death or first HF hospitalization (Pinteraction = 0.304, Figure 5). Within the individual subgroups, the HR was 0.74 (95% CI 0.68–0.80, P < 0.0001) for reduced EF, and 0.83 (95% CI 0.67–1.04, P = 0.1005) for preserved EF.

Hazard of composite of cardiovascular mortality or heart failure hospitalization stratified by reduced and preserved ejection fraction. REML, restricted maximum likelihood. Q = Cochran's Q level of heterogeneity; df = degrees of freedom.
Sensitivity analyses
When assessed by fixed effect analysis instead, the hazard ratio for all-cause mortality with SGLT-2 inhibitors met the criteria for statistical significance (HR 0.91, 95% CI 0.84–1.00, P = 0.040) and the HRs for the other endpoints remained broadly consistent (Supplementary material online, Figures S2–S5).
The results of the jackknife sensitivity analyses excluding each trial in turn are shown in Supplementary material online, Figures S6–S21.
There was no significant interaction between any of the subgroups tested and the hazard of the composite of cardiovascular mortality or all HF hospitalizations (P = NS for all, Supplementary materials online, Table S4).
Serious adverse events
Adverse events, including hypotension and hypoglycaemia, were infrequent. They were no more common with SGLT-2 inhibitors than placebo (Table 1).
Serious adverse events in the randomized trials of sodium-glucose cotransporter 2 inhibitors vs. placebo in patients with heart failure
| . | DAPA HF . | EMPEROR-Reduced . | SOLOIST-WHF . | EMPEROR-Preserved . | ||||
|---|---|---|---|---|---|---|---|---|
| . | SGLT-2-inhibitor (2373) . | Placebo (2371) . | SGLT-2-inhibitor (1863) . | Placebo (1867) . | SGLT-2-inhibitor (605) . | Placebo (611) . | SGLT-2-inhibitor (2996) . | Placebo (2989) . |
| Severe hypoglycaemia | 4 (0.2%) | 4 (0.2%) | 27 (1.4%) | 28 (1.5%) | 9 (1.5%) | 2 (0.3%) | 73 (2.4%) | 78 (2.6%) |
| Amputation | 13 (0.5%) | 12 (0.5%) | 13 (0.7%) | 10 (0.5%) | 4 (0.7%) | 1 (0.2%) | 16 (0.5%) | 23 (0.8%) |
| Any renal adverse event | 141 (6.0%) | 158 (6.7%) | 175 (9.4%) | 192 (10.3%) | 70 (11.6%) | 75 (12.3%) | NR | NR |
| Hypotension | 7 (0.3%) | 11 (0.5%) | 176 (9.4%) | 163 (8.7%) | 36 (6.0%) | 28 (4.6%) | 311 (10.4%) | 257 (8.6%) |
| Volume depletion | 170 (7.2%) | 153 (6.5%) | 197 (10.6%) | 184 (9.9%) | 57 (9.4%) | 54 (8.8%) | NR | NR |
| Bone fractures | 48 (2.0%) | 47 (2.0%) | 45 (2.4%) | 42 (2.3%) | 12 (2.0%) | 9 (1.5%) | 134 (4.5%) | 126 (4.2%) |
| Diabetic ketoacidosis | 3 (0.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.3%) | 9 (1.5%) | 4 (0.1%) | 5 (0.2%) |
| . | DAPA HF . | EMPEROR-Reduced . | SOLOIST-WHF . | EMPEROR-Preserved . | ||||
|---|---|---|---|---|---|---|---|---|
| . | SGLT-2-inhibitor (2373) . | Placebo (2371) . | SGLT-2-inhibitor (1863) . | Placebo (1867) . | SGLT-2-inhibitor (605) . | Placebo (611) . | SGLT-2-inhibitor (2996) . | Placebo (2989) . |
| Severe hypoglycaemia | 4 (0.2%) | 4 (0.2%) | 27 (1.4%) | 28 (1.5%) | 9 (1.5%) | 2 (0.3%) | 73 (2.4%) | 78 (2.6%) |
| Amputation | 13 (0.5%) | 12 (0.5%) | 13 (0.7%) | 10 (0.5%) | 4 (0.7%) | 1 (0.2%) | 16 (0.5%) | 23 (0.8%) |
| Any renal adverse event | 141 (6.0%) | 158 (6.7%) | 175 (9.4%) | 192 (10.3%) | 70 (11.6%) | 75 (12.3%) | NR | NR |
| Hypotension | 7 (0.3%) | 11 (0.5%) | 176 (9.4%) | 163 (8.7%) | 36 (6.0%) | 28 (4.6%) | 311 (10.4%) | 257 (8.6%) |
| Volume depletion | 170 (7.2%) | 153 (6.5%) | 197 (10.6%) | 184 (9.9%) | 57 (9.4%) | 54 (8.8%) | NR | NR |
| Bone fractures | 48 (2.0%) | 47 (2.0%) | 45 (2.4%) | 42 (2.3%) | 12 (2.0%) | 9 (1.5%) | 134 (4.5%) | 126 (4.2%) |
| Diabetic ketoacidosis | 3 (0.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.3%) | 9 (1.5%) | 4 (0.1%) | 5 (0.2%) |
NR, not reported.
Serious adverse events in the randomized trials of sodium-glucose cotransporter 2 inhibitors vs. placebo in patients with heart failure
| . | DAPA HF . | EMPEROR-Reduced . | SOLOIST-WHF . | EMPEROR-Preserved . | ||||
|---|---|---|---|---|---|---|---|---|
| . | SGLT-2-inhibitor (2373) . | Placebo (2371) . | SGLT-2-inhibitor (1863) . | Placebo (1867) . | SGLT-2-inhibitor (605) . | Placebo (611) . | SGLT-2-inhibitor (2996) . | Placebo (2989) . |
| Severe hypoglycaemia | 4 (0.2%) | 4 (0.2%) | 27 (1.4%) | 28 (1.5%) | 9 (1.5%) | 2 (0.3%) | 73 (2.4%) | 78 (2.6%) |
| Amputation | 13 (0.5%) | 12 (0.5%) | 13 (0.7%) | 10 (0.5%) | 4 (0.7%) | 1 (0.2%) | 16 (0.5%) | 23 (0.8%) |
| Any renal adverse event | 141 (6.0%) | 158 (6.7%) | 175 (9.4%) | 192 (10.3%) | 70 (11.6%) | 75 (12.3%) | NR | NR |
| Hypotension | 7 (0.3%) | 11 (0.5%) | 176 (9.4%) | 163 (8.7%) | 36 (6.0%) | 28 (4.6%) | 311 (10.4%) | 257 (8.6%) |
| Volume depletion | 170 (7.2%) | 153 (6.5%) | 197 (10.6%) | 184 (9.9%) | 57 (9.4%) | 54 (8.8%) | NR | NR |
| Bone fractures | 48 (2.0%) | 47 (2.0%) | 45 (2.4%) | 42 (2.3%) | 12 (2.0%) | 9 (1.5%) | 134 (4.5%) | 126 (4.2%) |
| Diabetic ketoacidosis | 3 (0.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.3%) | 9 (1.5%) | 4 (0.1%) | 5 (0.2%) |
| . | DAPA HF . | EMPEROR-Reduced . | SOLOIST-WHF . | EMPEROR-Preserved . | ||||
|---|---|---|---|---|---|---|---|---|
| . | SGLT-2-inhibitor (2373) . | Placebo (2371) . | SGLT-2-inhibitor (1863) . | Placebo (1867) . | SGLT-2-inhibitor (605) . | Placebo (611) . | SGLT-2-inhibitor (2996) . | Placebo (2989) . |
| Severe hypoglycaemia | 4 (0.2%) | 4 (0.2%) | 27 (1.4%) | 28 (1.5%) | 9 (1.5%) | 2 (0.3%) | 73 (2.4%) | 78 (2.6%) |
| Amputation | 13 (0.5%) | 12 (0.5%) | 13 (0.7%) | 10 (0.5%) | 4 (0.7%) | 1 (0.2%) | 16 (0.5%) | 23 (0.8%) |
| Any renal adverse event | 141 (6.0%) | 158 (6.7%) | 175 (9.4%) | 192 (10.3%) | 70 (11.6%) | 75 (12.3%) | NR | NR |
| Hypotension | 7 (0.3%) | 11 (0.5%) | 176 (9.4%) | 163 (8.7%) | 36 (6.0%) | 28 (4.6%) | 311 (10.4%) | 257 (8.6%) |
| Volume depletion | 170 (7.2%) | 153 (6.5%) | 197 (10.6%) | 184 (9.9%) | 57 (9.4%) | 54 (8.8%) | NR | NR |
| Bone fractures | 48 (2.0%) | 47 (2.0%) | 45 (2.4%) | 42 (2.3%) | 12 (2.0%) | 9 (1.5%) | 134 (4.5%) | 126 (4.2%) |
| Diabetic ketoacidosis | 3 (0.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.3%) | 9 (1.5%) | 4 (0.1%) | 5 (0.2%) |
NR, not reported.
Discussion
This meta-analysis finds that in RCTs of HF, across a range of EFs, SGLT-2 inhibitors significantly reduce cardiovascular mortality, recurrent HF hospitalizations, and the composite of cardiovascular mortality or first HF hospitalization at a mean follow-up time of 20.0 months. There was no indication of a significant difference in effect between patients with reduced and preserved EF (Pinteraction = 0.304).
Consistency
The point estimates for the outcomes of cardiovascular mortality, recurrent HF hospitalizations, and the composite of cardiovascular mortality or first HF hospitalization are remarkably consistent across the four trials of three drugs. This manifests as the absence of heterogeneity for these endpoints. Subgroup analyses show consistent effects across all tested subgroups.
SGLT-2 inhibitors may therefore have a class effect of benefit in patients with HF. In support of this possibility, the randomized trials of ertugliflozin12 and canagliflozin,13 in patients with diabetes but no HF, have shown reduction in HF and cardiovascular death. Our analysis extends previous meta-analytic work14 by including the SOLOIST-WHF and EMPEROR-Preserved trials.
Mechanism remains unknown
Our analysis cannot shine any light on the mechanism by which SGLT-2 inhibitors benefit patients with HF. Some proposed mechanisms relate to the direct cardiac action of SGLT-2 inhibitors, including improved myocardial ionic homeostasis15 and improved myocardial energetics,16 thereby improving cardiac efficiency.17 Another postulated mechanism relates osmotic diuresis and natriuresis.18,19 Elevations in serum hematocrit levels in patients taking SGLT-2 inhibitors were interpreted as supportive evidence for a predominantly diuretic effect underlying their clinical benefit.20 However, in this study neither volume depletion nor hypotension was more common in patients treated with SGLT-2 inhibitors compared with placebo. A recent detailed analysis from the EMPEROR-Reduced trial did not support diuresis as the prevalent factor for the clinical benefits seen with empagliflozin.21 Further studies are required to determine the mechanism of the salutary benefits seen with SGLT-2 inhibitors in HF. Nonetheless, given the present data demonstrating both a reduction in cardiovascular mortality and reduced HF hospitalizations, their adoption in the guidelines and routine use in patients with HF should not be delayed. The 2021 European Society of Cardiology guidelines now recommend the use of either empagliflozin or dapagliflozin as a class I recommendation for all patients with HF and reduced EF.22
New trial data
Despite its early termination, the SOLOIST-WHF trial provides several important innovations.23 First, it showed that an SGLT-2 inhibitor can be safely and beneficially initiated while a patient is hospitalized for worsening HF. Second, it provided the first evidence of improved outcomes in patients with HF and preserved EF, although as a result of the early termination of this study only 256 such patients were enrolled. The SCORED trial (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk)24 also provides some supportive evidence for a consistent benefit in patients with preserved EF. Interestingly, in the SCORED trial a post-hoc analysis suggested sotagliflozin was associated with a reduced risk of both myocardial infarction and stroke, although this must be interpreted with caution as the CIs for these post-hoc analyses were not adjusted for multiplicity.
EMPEROR-Preserved exclusively enrolled patients with an EF above 40%. It found a reduction in the composite of cardiovascular mortality and HF hospitalization, although there was no significant effect on all-cause mortality. The magnitude of benefit for the reduction in the primary endpoint appeared to be greatest in patients with an EF between 40 and 50%, creating suspicion that the positive results were driven by patients who in fact had reduced EF. Ultimately, the role of SGLT-2 inhibitors in patients with HF and preserved EF will further be evaluated in additional ongoing dedicated trials of this patient cohort (i.e. Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure (DELIVER), a trial randomizing 6236 patients with HF and preserved EF to dapagliflozin or placebo; ClinicalTrials.gov identifier NCT03619213).
The event rate for first HF hospitalization in the placebo arm was greatest in SOLOIST-WHF, indicating that either the presence of diabetes or the recent hospitalization was associated with an increased risk of HF hospitalization (48.4% in SOLOIST-WHF; 18.1% in EMPEROR-Preserved; 29.6% in EMPEROR-Reduced; and 19.8% in DAPA-HF). This increased event rate was not seen for all-cause mortality in the placebo arm of SOLOIST-WHF compared with the other trials, however (12.4% in SOLOIST-WHF; 14.2% in EMPEROR-Preserved; 14.2% in EMPEROR-Reduced; and 13.9% in DAPA-HF).
Limitations
This analysis used only published trial results, and not individual patient data that are held by the various trial teams and their commercial sponsors.
Although our analysis found consistent treatment effects across the three drugs with little evidence of statistical heterogeneity, the results cannot automatically be extended to other SGLT-2 inhibitors.
The trial populations were different. DAPA-HF and EMPEROR-Reduced primarily studied ambulatory patients with reduced EF, whereas SOLOIST-WHF studied patients with type 2 diabetes admitted with worsening HF requiring intravenous diuretics and encompassed patients with a range of EFs; and EMPEROR-Preserved studied patients with chronic HF and an EF of greater than 40%. Strictly speaking, this could be considered a strength of this analysis because despite the variation in patient population, there was a consistent observed effect on the endpoints.
For the HF hospitalization endpoint, the SOLOIST-WHF trial also included urgent visits for HF. However, these events occurred in a minority of patients. Moreover, the SOLOIST-WHF primary endpoint analysis was similar regardless of whether the urgent visits for HF were included.2
Sotagliflozin (the agent studied in SOLOIST-WHF) inhibits the SGLT-1 receptor in addition to SGLT-2. Nonetheless, the results with this agent were consistent with empagliflozin and dapagliflozin, suggesting that the effect from inhibiting the SGLT-2 receptor is the predominant mechanism in improving outcomes in patients with HF.
Our ejection fraction stratified analysis could only use the categories reported by the trials, which were not uniform. In the SOLOIST-WHF trial, patients with an EF ≥ 50% were classified as preserved EF in this analysis and those with EF < 40% were classified as reduced EF in this analysis. In the EMPEROR-Preserved trial, patients with an EF ≥ 60% were classified as preserved EF in this analysis, whereas those with an EF < 50% were classified as reduced EF. EMPEROR-Reduced and DAPA-HF only included patients with EF ≤ 40%.
Finally, our analysis includes only randomized trials that can only study the fraction of patients who meet strict eligibility criteria. While such an approach can be criticized as limiting applicability, it is the only reliable method of accounting for measured and unmeasured confounders and provide a true estimate of effect of therapy.
Conclusions
The RCT evidence shows that SGLT-2 inhibitors significantly reduced cardiovascular mortality, HF hospitalizations, and the composite of cardiovascular mortality or HF hospitalization in patients with HF. The results seem to be consistent regardless of EF across four trials of three drugs. SGLT-2 inhibitors should thus become standard care for patients with HF.
Funding
M.V.M. was supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute to Columbia University Irving Medical Center (T32 HL007854).
Conflict of interest
G.W.S. has received speaker or other honoraria from Terumo, Cook; has served as a consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Abiomed, Ancora, Elucid Bio, Occlutech, CorFlow, Reva, Matrizyme, MAIA Pharmaceuticals, Vascular Dynamics, Shockwave, V-Wave, Cardiomech, and Gore; and has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, and Valfix, MedFocus family of funds. D.L.B discloses the following relationships: advisory board—Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, and Regado Biosciences; board of directors—Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; chair—American Heart Association Quality Oversight Committee; data monitoring committees—Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), and Population Health Research Institute; honoraria—American College of Cardiology (senior associate editor, Clinical Trials and News, ACC.org; vice-chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (editor-in-chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (editor-in-chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor; associate editor), K2P (co-chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (chief medical editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (secretary/treasurer), and WebMD (CME steering committees); other—Clinical Cardiology (deputy editor), NCDR-ACTION Registry Steering Committee (chair), and VA CART Research and Publications Committee (chair); research funding—Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Lilly, Medtronic, MyoKardia, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, and The Medicines Company; royalties—Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); site co-investigator—Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), and Svelte; trustee—American College of Cardiology; unfunded research—FlowCo, Merck, and Takeda. All other authors report no disclosures.
Data availability
The data underlying this article are available in the article and in its online supplementary material.



