-
PDF
- Split View
-
Views
-
Cite
Cite
Iain Willits, Kim Keltie, Nicholas Linker, Mark de Belder, Robert Henderson, Hannah Patrick, Helen Powell, Lee Berry, Samuel G Urwin, Helen Cole, Andrew J Sims, Left atrial appendage occlusion in the UK: prospective registry and data linkage to Hospital Episode Statistics, European Heart Journal - Quality of Care and Clinical Outcomes, Volume 7, Issue 5, November 2021, Pages 468–475, https://doi.org/10.1093/ehjqcco/qcab042
Close - Share Icon Share
Abstract
Non-valvular atrial fibrillation (AF) greatly increases the risk of ischaemic stroke. For people with contraindications to oral anticoagulation, left atrial appendage occlusion (LAAO) provides a non-pharmacological management alternative. The aim of this study was to measure the procedural safety and longer-term effectiveness of LAAO for AF in a UK setting.
This was a prospective, single-armed registry of patients with AF for whom anticoagulation was unsuitable. Registry data were collected between October 2014 and April 2018 and linked to routine data sources for follow-up. Data from 583 LAAO procedures were entered into the registry, of which 537 (from 525 patients) were eligible for inclusion (median CHA2DS2-VASc score 4). A closure device was successfully implanted in 93.4% of cases, with a procedural success rate (device implanted without major complication) of 88.9%. Five patients (1.0%) died in hospital. During follow-up [median 729 (Q1:Q3, 523:913) days] 45 patients experienced neurological events; 33 of which were ischaemic. The ischaemic neurological event rate was 3.3 (1.6–5.0)% at 1 year (n = 387) and 7.0 (4.3–9.6)% at 2 years (n = 196). There were significant improvements in overall patient health (via Visual Analogue Scale) measured at 6 weeks and 6 months, but no significant improvements observed in patient utility over time.
The findings of our study suggest that LAAO is not without procedural risk, but that this risk may be justified in high-risk patients with AF who cannot take an anticoagulant. Moreover, the data do not provide support for more widespread use of LAAO as the complication rate was relatively high and would be difficult to justify in many patients with AF who tolerate anticoagulation.
Introduction
Non-valvular atrial fibrillation (AF) is common, with an estimated prevalence of 7.2% in people aged over 65 years.1 The disorder is associated with a high level of morbidity and excess mortality.2 Atrial fibrillation increases the risk of thromboembolic stroke by a factor of five,3 and strokes associated with AF tend to be more severe.4 Atrial fibrillation, symptomatic or otherwise, has been observed to have a negative effect on health-related quality of life (HRQoL), particularly in the anxiety domain.5 Management of AF and its complications poses a heavy financial burden, accounting for around 1% of the UK National Health Service (NHS) budget.6
The risk of AF-associated stroke can be mitigated by anticoagulation with warfarin or direct oral anticoagulants (DOACs).7,8 Warfarin has a narrow therapeutic window, requires frequent monitoring for effective and safe anticoagulation,2 and is associated with an increased risk of bleeding.9 Although DOACs do not require monitoring and reduce bleeding risk, they do not eliminate it.10 As a consequence, anticoagulation is not suitable for a proportion of people with AF who are predisposed to bleeding. Additionally, the adverse effects of anticoagulation are not tolerated by everyone and have a negative impact on HRQoL and treatment adherence.11
In approximately 90% of strokes in people with AF, the origin of the thromboembolism is the left atrial appendage.12 Left atrial appendage occlusion (LAAO) is a non-pharmacological intervention that aims to reduce the risk of thromboembolic stroke in patients with AF by mechanically blocking the entrance to the appendage. Thus, the need for oral anticoagulation is reduced or negated, and the procedure can be performed in patients who have contraindications to oral anticoagulation, or in whom oral anticoagulation has been ineffective (i.e. have had a thromboembolic event despite treatment).13
In 2013, LAAO was included within the NHS England Commissioning through Evaluation (CtE) Programme.14 This programme enabled a limited number of patients to access procedures whilst prospective safety and efficacy data were collected, to inform future commissioning decisions. Funding was initially made available from NHS England for the inclusion of around 450 patients in the registry. The aims of this study were to determine the safety and effectiveness of LAAO in patients with AF at risk of stroke in whom anticoagulation is unsuitable.
Methods
Design and ethics
This was a prospective observational study using a registry to capture characteristics of patients, procedures and outcomes, linked to two administrative datasets to validate the registry data and capture longer-term (2-year) outcomes. The registry was single-armed with no comparator or control group. Data were collected prospectively in accordance with best practice and reported using STROBE criteria.15,16 Follow-up measured a range of clinical and patient-reported outcome measures. All necessary ethical approvals for the data collection, data linkage and analyses were granted by the NHS Health Research Authority Confidentiality Advisory Group (Ref: 17/CAG/0153, CAG 10-07(b)/2014) and NHS Digital (Ref: DARS-NIC-151212-B5Z3R).
Patient selection, registry follow-up and outcomes
Ten hospitals across England contributed data. Candidate patients for LAAO were assessed for suitability at a multidisciplinary team meeting. Patients were eligible if they had AF, were at high risk of thromboembolic stroke (CHA2DS2-VASc score of 2 or more) but for whom oral anticoagulants were unsuitable or who had evidence of a thromboembolic event despite adequate oral anticoagulant therapy. Eligible reasons for treatment included previous bleed with/without anticoagulant therapy, embolic event in spite of oral anticoagulant, intolerant or poor control of oral anticoagulant, at risk of severe bleeding; non-eligible reasons included primary or secondary prophylaxis regardless of issues with anticoagulation, patient preference, other. Informed consent for the procedure was required.
Patients assessed as having appropriate left atrial appendage morphology and suitability for a trans-septal procedure underwent transoesophageal echocardiogram (TOE) guided LAAO. The LAAO procedure was undertaken using the WATCHMAN (Boston Scientific, Marlborough, MA, USA), AMPLATZER Cardiac Plug or AMPLATZER Amulet (St Jude Medical, ST Pauls, MN, USA), or Coherex WaveCrest (Biosense Webster, Irvine, CA, USA) devices. Procedural and in-hospital data were collected to determine safety and efficacy data. After discharge from hospital, follow-up data were collected during routine face-to-face or telephone appointments, scheduled at 6 weeks, 6 months, 1 year, and 2 years from the date of the procedure. The predefined primary outcome measure from the registry was procedural success (successful device implantation with no major complication) (Supplementary material online, Tables S1). Secondary outcome measures included in-hospital complications (classed as major or minor, Supplementary material online, Tables S1 and S2), presence and severity of leak post-procedure (confirmed by transoesophageal echocardiography: minor leak <1 mm, moderate 1–3 mm, major >3 mm, or multiple jets or free flow), post-discharge clinical failure (Supplementary material online, Table S3), and overall health scores [captured via the Visual Analogue Scale (VAS) pre-procedure and at each follow-up appointment, and HRQoL data using the EQ-5D-5L system converted into utility scores].
Data linkage and outcomes
Data from enrolled patients were linked with Hospital Episode Statistics (HES) and Office of National Statistics (ONS) Mortality administrative datasets by NHS Digital.17 Data from HES included all inpatient activity with hospital discharge dates between 1 April 2008 and 1 March 2018. Data from ONS included all deaths reported until 1 March 2018. Records with conflicting demographic and administrative details between the linked data sources were flagged to indicate potential errors in matching (i.e. matching to incorrect patient) and excluded from subsequent long-term analysis. Primary outcomes from data linkage were mortality, neurological (ischaemic and haemorrhagic) events.
Statistics
Data and statistical analyses were performed using the programming language R.18 Patient demographics, pre-procedural clinical scores, and procedural details were reported via descriptive statistics. Univariate analyses were conducted for the defined outcome measures to test for significant association. For univariate tests, Bonferroni correction was used to adjust the level of significance for multiple comparisons.
Paired quality of life scores and utilities recorded in the registry were compared at each follow-up point against pre-operative scores using Fisher’s exact tests or t-tests where appropriate. Unadjusted event rates for mortality and neurological event data from the linked dataset were calculated as the number of events per 100 person years of follow-up using both registry and linked data. Kaplan–Meier analysis was applied to mortality (including in-hospital deaths) and neurological event outcomes using comprehensive follow-up from linked data.
Patient and public involvement
The commissioning through evaluation steering group included one lay representative.
Results
Registry data
A total of 583 LAAO procedure records from 569 unique patients were recorded in the registry between October 2014 and April 2018, Figure 1. Forty-six procedures did not meet the eligibility criteria of the CtE registry. Thus, 537 procedures from 523 patients were included for in-hospital analysis (breakdown by hospital provider, Supplementary material online, Table S4).
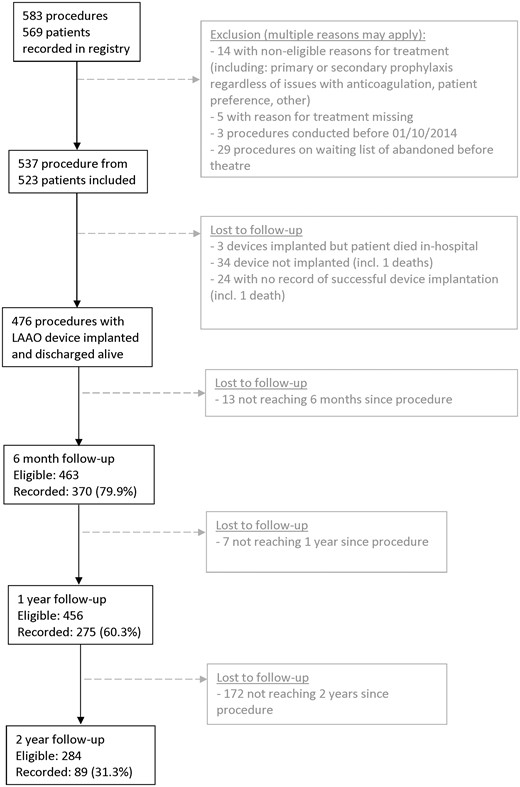
Baseline characteristics are reported in Table 1. The median CHA2DS2-VASc score was 4, and the median HAS-BLED score was 4. Most patients (423/521, 81%) had a previous major or life-threatening bleed whilst on oral anticoagulation; a previous bleed was documented as the primary reason for treatment in 353 procedures. At the time of enrolment, 42% were not receiving antiplatelet or anticoagulant medication, and only 15.7% were taking an oral anticoagulant (1.1% in combination with an antiplatelet, Table 1).
| Patient characteristic . | All LAAO procedures (n = 537) . |
|---|---|
| Female | 167 (31.2%) |
| Age, years | 75 |
| Median (Q1, Q3) (range) | (70, 80) (43–92) |
| BMI, kg/m2 | 27.3 |
| Median (Q1, Q3) (range) | (24.3, 31.2) (10.1–46.1) |
| Risk factors | |
| Diabetes | 140 (26.4%) |
| Hypertension | 374 (70.4%) |
| Congestive heart failure | 56 (10.6%) |
| Prior myocardial infarction | 101 (19.1%) |
| Peripheral vascular disease | 35 (6.8%) |
| Significant liver disease | 8 (1.5%) |
| Significant valve disease | 41 (7.9%) |
| Previous peripheral embolism | 9 (1.7%) |
| Neurological disease | |
| Yes, CVA | 266 (50.1%) |
| Yes, other | 87 (16.4%) |
| No | 178 (33.5%) |
| Clinical scores | |
| CHADS2 score | 3 |
| Median (Q1, Q3) (range) | (2, 4) (0–6) |
| CHA2DS2-VASc score | 4 |
| Median (Q1, Q3) (range) | (3, 5) (0–9) |
| HAS-BLED | 4 |
| Median (Q1, Q3) (range) | (3, 5) (1–6) |
| Previous significant bleed (major or life-threatening/disabling) | 423 (81.2%) |
| Previous rhythm historya | |
| No arrhythmias | 5 (0.9%) |
| Paroxysmal AF/flutter | 147 (27.7%) |
| Persistent AF/flutter | 88 (16.6%) |
| Permanent AF/flutter | 289 (54.4%) |
| Atrial tachycardia | 1 (0.2%) |
| Other symptomatic arrhythmia | 1 (0.2%) |
| Pre-operative heart rhythm | |
| Atrial fibrillation | 415 (77.3%) |
| Sinus rhythm | 98 (18.5%) |
| Atrial flutter | 9 (1.7%) |
| Paced rhythm | 7 (1.3%) |
| Other atrial tachycardia | 1 (0.2%) |
| Pre-procedural medications (pre-operative) | |
| Single antiplatelet | 139 (26.3%) |
| Dual antiplatelet | 28 (5.3%) |
| Anticoagulant alone | 77 (14.6%) |
| Antiplatelet(s) and anticoagulant(s) | 6 (1.1%) |
| Other | 56 (10.6%) |
| None | 222 (42.0%) |
| LV ejection fraction | |
| Good (>50%) | 411 (80.1%) |
| Moderate (30–50%) | 79 (15.4%) |
| Poor (<30%) | 23 (4.5%) |
| Patient characteristic . | All LAAO procedures (n = 537) . |
|---|---|
| Female | 167 (31.2%) |
| Age, years | 75 |
| Median (Q1, Q3) (range) | (70, 80) (43–92) |
| BMI, kg/m2 | 27.3 |
| Median (Q1, Q3) (range) | (24.3, 31.2) (10.1–46.1) |
| Risk factors | |
| Diabetes | 140 (26.4%) |
| Hypertension | 374 (70.4%) |
| Congestive heart failure | 56 (10.6%) |
| Prior myocardial infarction | 101 (19.1%) |
| Peripheral vascular disease | 35 (6.8%) |
| Significant liver disease | 8 (1.5%) |
| Significant valve disease | 41 (7.9%) |
| Previous peripheral embolism | 9 (1.7%) |
| Neurological disease | |
| Yes, CVA | 266 (50.1%) |
| Yes, other | 87 (16.4%) |
| No | 178 (33.5%) |
| Clinical scores | |
| CHADS2 score | 3 |
| Median (Q1, Q3) (range) | (2, 4) (0–6) |
| CHA2DS2-VASc score | 4 |
| Median (Q1, Q3) (range) | (3, 5) (0–9) |
| HAS-BLED | 4 |
| Median (Q1, Q3) (range) | (3, 5) (1–6) |
| Previous significant bleed (major or life-threatening/disabling) | 423 (81.2%) |
| Previous rhythm historya | |
| No arrhythmias | 5 (0.9%) |
| Paroxysmal AF/flutter | 147 (27.7%) |
| Persistent AF/flutter | 88 (16.6%) |
| Permanent AF/flutter | 289 (54.4%) |
| Atrial tachycardia | 1 (0.2%) |
| Other symptomatic arrhythmia | 1 (0.2%) |
| Pre-operative heart rhythm | |
| Atrial fibrillation | 415 (77.3%) |
| Sinus rhythm | 98 (18.5%) |
| Atrial flutter | 9 (1.7%) |
| Paced rhythm | 7 (1.3%) |
| Other atrial tachycardia | 1 (0.2%) |
| Pre-procedural medications (pre-operative) | |
| Single antiplatelet | 139 (26.3%) |
| Dual antiplatelet | 28 (5.3%) |
| Anticoagulant alone | 77 (14.6%) |
| Antiplatelet(s) and anticoagulant(s) | 6 (1.1%) |
| Other | 56 (10.6%) |
| None | 222 (42.0%) |
| LV ejection fraction | |
| Good (>50%) | 411 (80.1%) |
| Moderate (30–50%) | 79 (15.4%) |
| Poor (<30%) | 23 (4.5%) |
aTo be offered the procedure, all patients were required by an multidisciplinary team to have documented AF.
| Patient characteristic . | All LAAO procedures (n = 537) . |
|---|---|
| Female | 167 (31.2%) |
| Age, years | 75 |
| Median (Q1, Q3) (range) | (70, 80) (43–92) |
| BMI, kg/m2 | 27.3 |
| Median (Q1, Q3) (range) | (24.3, 31.2) (10.1–46.1) |
| Risk factors | |
| Diabetes | 140 (26.4%) |
| Hypertension | 374 (70.4%) |
| Congestive heart failure | 56 (10.6%) |
| Prior myocardial infarction | 101 (19.1%) |
| Peripheral vascular disease | 35 (6.8%) |
| Significant liver disease | 8 (1.5%) |
| Significant valve disease | 41 (7.9%) |
| Previous peripheral embolism | 9 (1.7%) |
| Neurological disease | |
| Yes, CVA | 266 (50.1%) |
| Yes, other | 87 (16.4%) |
| No | 178 (33.5%) |
| Clinical scores | |
| CHADS2 score | 3 |
| Median (Q1, Q3) (range) | (2, 4) (0–6) |
| CHA2DS2-VASc score | 4 |
| Median (Q1, Q3) (range) | (3, 5) (0–9) |
| HAS-BLED | 4 |
| Median (Q1, Q3) (range) | (3, 5) (1–6) |
| Previous significant bleed (major or life-threatening/disabling) | 423 (81.2%) |
| Previous rhythm historya | |
| No arrhythmias | 5 (0.9%) |
| Paroxysmal AF/flutter | 147 (27.7%) |
| Persistent AF/flutter | 88 (16.6%) |
| Permanent AF/flutter | 289 (54.4%) |
| Atrial tachycardia | 1 (0.2%) |
| Other symptomatic arrhythmia | 1 (0.2%) |
| Pre-operative heart rhythm | |
| Atrial fibrillation | 415 (77.3%) |
| Sinus rhythm | 98 (18.5%) |
| Atrial flutter | 9 (1.7%) |
| Paced rhythm | 7 (1.3%) |
| Other atrial tachycardia | 1 (0.2%) |
| Pre-procedural medications (pre-operative) | |
| Single antiplatelet | 139 (26.3%) |
| Dual antiplatelet | 28 (5.3%) |
| Anticoagulant alone | 77 (14.6%) |
| Antiplatelet(s) and anticoagulant(s) | 6 (1.1%) |
| Other | 56 (10.6%) |
| None | 222 (42.0%) |
| LV ejection fraction | |
| Good (>50%) | 411 (80.1%) |
| Moderate (30–50%) | 79 (15.4%) |
| Poor (<30%) | 23 (4.5%) |
| Patient characteristic . | All LAAO procedures (n = 537) . |
|---|---|
| Female | 167 (31.2%) |
| Age, years | 75 |
| Median (Q1, Q3) (range) | (70, 80) (43–92) |
| BMI, kg/m2 | 27.3 |
| Median (Q1, Q3) (range) | (24.3, 31.2) (10.1–46.1) |
| Risk factors | |
| Diabetes | 140 (26.4%) |
| Hypertension | 374 (70.4%) |
| Congestive heart failure | 56 (10.6%) |
| Prior myocardial infarction | 101 (19.1%) |
| Peripheral vascular disease | 35 (6.8%) |
| Significant liver disease | 8 (1.5%) |
| Significant valve disease | 41 (7.9%) |
| Previous peripheral embolism | 9 (1.7%) |
| Neurological disease | |
| Yes, CVA | 266 (50.1%) |
| Yes, other | 87 (16.4%) |
| No | 178 (33.5%) |
| Clinical scores | |
| CHADS2 score | 3 |
| Median (Q1, Q3) (range) | (2, 4) (0–6) |
| CHA2DS2-VASc score | 4 |
| Median (Q1, Q3) (range) | (3, 5) (0–9) |
| HAS-BLED | 4 |
| Median (Q1, Q3) (range) | (3, 5) (1–6) |
| Previous significant bleed (major or life-threatening/disabling) | 423 (81.2%) |
| Previous rhythm historya | |
| No arrhythmias | 5 (0.9%) |
| Paroxysmal AF/flutter | 147 (27.7%) |
| Persistent AF/flutter | 88 (16.6%) |
| Permanent AF/flutter | 289 (54.4%) |
| Atrial tachycardia | 1 (0.2%) |
| Other symptomatic arrhythmia | 1 (0.2%) |
| Pre-operative heart rhythm | |
| Atrial fibrillation | 415 (77.3%) |
| Sinus rhythm | 98 (18.5%) |
| Atrial flutter | 9 (1.7%) |
| Paced rhythm | 7 (1.3%) |
| Other atrial tachycardia | 1 (0.2%) |
| Pre-procedural medications (pre-operative) | |
| Single antiplatelet | 139 (26.3%) |
| Dual antiplatelet | 28 (5.3%) |
| Anticoagulant alone | 77 (14.6%) |
| Antiplatelet(s) and anticoagulant(s) | 6 (1.1%) |
| Other | 56 (10.6%) |
| None | 222 (42.0%) |
| LV ejection fraction | |
| Good (>50%) | 411 (80.1%) |
| Moderate (30–50%) | 79 (15.4%) |
| Poor (<30%) | 23 (4.5%) |
aTo be offered the procedure, all patients were required by an multidisciplinary team to have documented AF.
Procedural information is reported in Table 2. An Amplatzer device was used in 56% of procedures, the Watchman device in 37% of cases. The procedure took a median of 75 min (Q1:Q3, 57–110 min). A device was successfully implanted in 93.4 [95% confidence interval (CI) 90.9–95.4]% of procedures. A major complication occurred in 5.4 (95% CI 3.6–7.7)% of procedures and the procedural success rate was 88.9 (95% CI 85.8–91.5)%. Five patients died in hospital (with the cause of death reported as air embolism with cerebral oedema, sepsis, cardiac tamponade, left atrial perforation, and one cause unreported). In those in whom a device was implanted (n = 479), 92.5% of procedures had no leak, 6.9% had a minor leak, and 0.6% had a moderate or major leak. Clinical failure after discharge was reported in 40 procedures [9.0 (95% CI 6.5–12.1)%] following TOE examination during follow-up (between 6 weeks and 2 years); the device was found not to be in situ in 26 cases, there was a small leak in 37, a large leak in 7, and neurological events were reported post-procedurally in 12; 66.7% were judged to be ischaemic in nature.
Procedural details and in-hospital complications of people included in the registry
| Procedural characteristics . | All LAAO procedures (n = 537) . |
|---|---|
| Device used (n = 463) | |
| WATCHMAN (Boston Scientific) | 172 (37.1%) |
| AMPLATZER Cardiac plug (St Jude Medical) | 35 (7.6%) |
| AMPLATZER Amulet (St Jude Medical) | 223 (48.2%) |
| Coherex WaveCrest | 3 (0.6%) |
| Not specified | 30 (6.5%) |
| Device size, mm, median (Q1, Q3) (range) | 25 (22, 27) (14–35) |
| Procedural details | |
| Fluoroscopy time, min, median (Q1, Q3) (range) (n = 493) | 10 (7, 15) (5–120) |
| X-ray dose, mGray.cm2, median (Q1, Q3) (range) (n = 478) | 1690 (585, 3094) (10–20 000) |
| Contrast dose, mL, median (Q1, Q3) (range) (n = 427) | 70 (40, 102.5) (10–350) |
| Procedural duration, min, median (Q1, Q3) (range) (n = 530) | 76 (58, 110) (2–300) |
| Procedural success and complications, n [% (95%CI)] | |
| Technical success ratea | 479 [93.4 (90.9 to 95.4) %] |
| Procedural success rateb | 456 [88.9 (85.8 to 91.5) %] |
| Device implanted without a leak | 443 [86.4 (83.1 to 89.2) %] |
| Extended length of stay (>1 night) | 115 [22.4 (18.8 to 26.2) %] |
| Major complicationc | 29 [5.4 (3.6 to 7.7) %] |
| Minor complicationd | 24 [4.5 (2.9 to 6.6) %] |
| Death | 5 [0.9 (0.3 to 2.2) %] |
| Clinical failure (after discharge, patients with follow-up onlye), n [% (95%CI)] | |
| Total | 40 [9.0 (6.5 to 12.1) %] |
| Device not in situ | 26 [5.9 (3.9 to 8.5) %] |
| LAA not sealed (large leak) | 7 [1.6 (0.6 to 3.2) %] |
| Neurological event | 8 [1.8 (0.8 to 3.5) %] |
| Procedural characteristics . | All LAAO procedures (n = 537) . |
|---|---|
| Device used (n = 463) | |
| WATCHMAN (Boston Scientific) | 172 (37.1%) |
| AMPLATZER Cardiac plug (St Jude Medical) | 35 (7.6%) |
| AMPLATZER Amulet (St Jude Medical) | 223 (48.2%) |
| Coherex WaveCrest | 3 (0.6%) |
| Not specified | 30 (6.5%) |
| Device size, mm, median (Q1, Q3) (range) | 25 (22, 27) (14–35) |
| Procedural details | |
| Fluoroscopy time, min, median (Q1, Q3) (range) (n = 493) | 10 (7, 15) (5–120) |
| X-ray dose, mGray.cm2, median (Q1, Q3) (range) (n = 478) | 1690 (585, 3094) (10–20 000) |
| Contrast dose, mL, median (Q1, Q3) (range) (n = 427) | 70 (40, 102.5) (10–350) |
| Procedural duration, min, median (Q1, Q3) (range) (n = 530) | 76 (58, 110) (2–300) |
| Procedural success and complications, n [% (95%CI)] | |
| Technical success ratea | 479 [93.4 (90.9 to 95.4) %] |
| Procedural success rateb | 456 [88.9 (85.8 to 91.5) %] |
| Device implanted without a leak | 443 [86.4 (83.1 to 89.2) %] |
| Extended length of stay (>1 night) | 115 [22.4 (18.8 to 26.2) %] |
| Major complicationc | 29 [5.4 (3.6 to 7.7) %] |
| Minor complicationd | 24 [4.5 (2.9 to 6.6) %] |
| Death | 5 [0.9 (0.3 to 2.2) %] |
| Clinical failure (after discharge, patients with follow-up onlye), n [% (95%CI)] | |
| Total | 40 [9.0 (6.5 to 12.1) %] |
| Device not in situ | 26 [5.9 (3.9 to 8.5) %] |
| LAA not sealed (large leak) | 7 [1.6 (0.6 to 3.2) %] |
| Neurological event | 8 [1.8 (0.8 to 3.5) %] |
95% CI, 95% confidence interval; LAA, left atrial appendage; n, number; Q, quartile.
Device successfully implanted.
Device successfully implanted in absence of major complications.
Death (n = 5), neurological event (n = 4), pericardial infusion requiring intervention (n = 11), embolization (n = 4), surgical intervention (n = 10), major vascular injury (n = 5), major bleed (n = 10), MI (n = 2), stage 2 or 3 AKI (n = 3), endocarditis (n = 2).
Device malfunction (n = 1), malposition (n = 0), minor vascular injury (n = 3), pericardial effusion—conservative treatment (n = 6), significant oesophageal damage (n = 0), procedure-related arrhythmia (n = 5), minor bleed (n = 8), peripheral embolism (n = 0), stage 1 AKI (n = 1).
Not all data fields were complete for every patient at baseline and follow-up. The percentages presented in this table are calculated using the number of patients with each characteristic reported as the denominator.
Procedural details and in-hospital complications of people included in the registry
| Procedural characteristics . | All LAAO procedures (n = 537) . |
|---|---|
| Device used (n = 463) | |
| WATCHMAN (Boston Scientific) | 172 (37.1%) |
| AMPLATZER Cardiac plug (St Jude Medical) | 35 (7.6%) |
| AMPLATZER Amulet (St Jude Medical) | 223 (48.2%) |
| Coherex WaveCrest | 3 (0.6%) |
| Not specified | 30 (6.5%) |
| Device size, mm, median (Q1, Q3) (range) | 25 (22, 27) (14–35) |
| Procedural details | |
| Fluoroscopy time, min, median (Q1, Q3) (range) (n = 493) | 10 (7, 15) (5–120) |
| X-ray dose, mGray.cm2, median (Q1, Q3) (range) (n = 478) | 1690 (585, 3094) (10–20 000) |
| Contrast dose, mL, median (Q1, Q3) (range) (n = 427) | 70 (40, 102.5) (10–350) |
| Procedural duration, min, median (Q1, Q3) (range) (n = 530) | 76 (58, 110) (2–300) |
| Procedural success and complications, n [% (95%CI)] | |
| Technical success ratea | 479 [93.4 (90.9 to 95.4) %] |
| Procedural success rateb | 456 [88.9 (85.8 to 91.5) %] |
| Device implanted without a leak | 443 [86.4 (83.1 to 89.2) %] |
| Extended length of stay (>1 night) | 115 [22.4 (18.8 to 26.2) %] |
| Major complicationc | 29 [5.4 (3.6 to 7.7) %] |
| Minor complicationd | 24 [4.5 (2.9 to 6.6) %] |
| Death | 5 [0.9 (0.3 to 2.2) %] |
| Clinical failure (after discharge, patients with follow-up onlye), n [% (95%CI)] | |
| Total | 40 [9.0 (6.5 to 12.1) %] |
| Device not in situ | 26 [5.9 (3.9 to 8.5) %] |
| LAA not sealed (large leak) | 7 [1.6 (0.6 to 3.2) %] |
| Neurological event | 8 [1.8 (0.8 to 3.5) %] |
| Procedural characteristics . | All LAAO procedures (n = 537) . |
|---|---|
| Device used (n = 463) | |
| WATCHMAN (Boston Scientific) | 172 (37.1%) |
| AMPLATZER Cardiac plug (St Jude Medical) | 35 (7.6%) |
| AMPLATZER Amulet (St Jude Medical) | 223 (48.2%) |
| Coherex WaveCrest | 3 (0.6%) |
| Not specified | 30 (6.5%) |
| Device size, mm, median (Q1, Q3) (range) | 25 (22, 27) (14–35) |
| Procedural details | |
| Fluoroscopy time, min, median (Q1, Q3) (range) (n = 493) | 10 (7, 15) (5–120) |
| X-ray dose, mGray.cm2, median (Q1, Q3) (range) (n = 478) | 1690 (585, 3094) (10–20 000) |
| Contrast dose, mL, median (Q1, Q3) (range) (n = 427) | 70 (40, 102.5) (10–350) |
| Procedural duration, min, median (Q1, Q3) (range) (n = 530) | 76 (58, 110) (2–300) |
| Procedural success and complications, n [% (95%CI)] | |
| Technical success ratea | 479 [93.4 (90.9 to 95.4) %] |
| Procedural success rateb | 456 [88.9 (85.8 to 91.5) %] |
| Device implanted without a leak | 443 [86.4 (83.1 to 89.2) %] |
| Extended length of stay (>1 night) | 115 [22.4 (18.8 to 26.2) %] |
| Major complicationc | 29 [5.4 (3.6 to 7.7) %] |
| Minor complicationd | 24 [4.5 (2.9 to 6.6) %] |
| Death | 5 [0.9 (0.3 to 2.2) %] |
| Clinical failure (after discharge, patients with follow-up onlye), n [% (95%CI)] | |
| Total | 40 [9.0 (6.5 to 12.1) %] |
| Device not in situ | 26 [5.9 (3.9 to 8.5) %] |
| LAA not sealed (large leak) | 7 [1.6 (0.6 to 3.2) %] |
| Neurological event | 8 [1.8 (0.8 to 3.5) %] |
95% CI, 95% confidence interval; LAA, left atrial appendage; n, number; Q, quartile.
Device successfully implanted.
Device successfully implanted in absence of major complications.
Death (n = 5), neurological event (n = 4), pericardial infusion requiring intervention (n = 11), embolization (n = 4), surgical intervention (n = 10), major vascular injury (n = 5), major bleed (n = 10), MI (n = 2), stage 2 or 3 AKI (n = 3), endocarditis (n = 2).
Device malfunction (n = 1), malposition (n = 0), minor vascular injury (n = 3), pericardial effusion—conservative treatment (n = 6), significant oesophageal damage (n = 0), procedure-related arrhythmia (n = 5), minor bleed (n = 8), peripheral embolism (n = 0), stage 1 AKI (n = 1).
Not all data fields were complete for every patient at baseline and follow-up. The percentages presented in this table are calculated using the number of patients with each characteristic reported as the denominator.
Data linked to HES/ONS
A total of 460 patients (88%) were matched to HES datasets and passed internal quality checks (Supplementary material online, Figure S1) and were available for longitudinal outcomes. No significant differences in patient characteristics were observed between patients in the registry and those matched and validated with HES. During follow-up [median of 729 (Q1:Q3, 523:913) days], there were 50 deaths and 45 neurological events (of which 30 were ischaemic, 12 were haemorrhagic, and 3 were unknown), Table 3. There were 81 combined events (composite outcome combining all-cause mortality or neurological event). The Kaplan–Meier combined event rates (neurological event or death) at 1 and 2 years were 8.1 (95% 5.5–10.6)% (n = 387) and 17.4 (95% CI 13.4–21.2)% (n = 196), respectively, Figure 2. Neurological event and mortality rates are reported separately within Supplementary material online, FiguresS2 and S3, respectively.
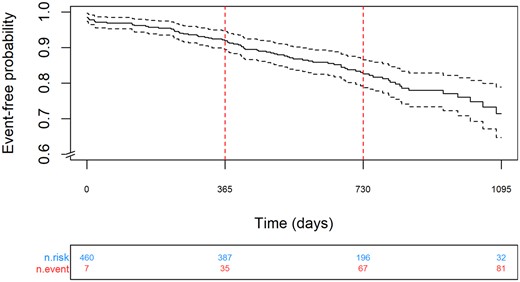
Kaplan–Meier analysis of neurological event or death over 2 years follow-up.
Mortality, overall neurological event rate, ischaemic event rate, haemorrhagic event rate, and combined rate from linked data
| . | All-cause mortality . | Total neurological events . | Ischaemic events . | Haemorrhagic events . | Total neurological events combined with all-cause mortality . |
|---|---|---|---|---|---|
| No. of patients followed | 460 | 460 | 460 | 460 | 460 |
| Mean (SD) length of follow-up, days | 712 (289) | 648 (318) | 690 (309) | 706 (302) | 648 (318) |
| Median (Q1; Q3) length of follow-up, days | 729 (523–913) | 689 (471–869) | 689 (471–869) | 689 (471–869) | 689 (471–869) |
| No. of events | 50 | 45a | 30 | 12 | 81 |
| Total follow-up, person years (range) | 897 (0–1233 days) | 8817 (0–1227 days) | 817 (0–1227 days) | 817 (0–1227 days) | 817 (0–1227 days) |
1-year event-free probability (95% CI) (number at risk) | 95.3 (93.3–97.3) (n = 416) | 94.9 (92.8–97.0) (n = 387) | 96.7 (95.0–98.4) (n = 387) | 98.6 (97.4–99.7) (n = 387) | 91.9 (89.4–94.5) (n = 387) |
2-year event-free probability (95% CI) (number at risk) | 90.4 (87.4–93.4) (n = 229) | 89.5 (86.5–92.7) (n = 196) | 93.0 (90.4–95.7) (n = 196) | 97.1 (95.4–98.8) (n = 196) | 82.6 (78.8–86.6) (n = 196) |
| Total event rate, per 100 person years follow-up (95% CI) | 5.6 (4.1–7.3) | 5.5 (4.0–7.4) | 3.7 (2.5–5.2) | 1.5 (0.8–2.6) | 9.9 (7.9–12.3) |
| . | All-cause mortality . | Total neurological events . | Ischaemic events . | Haemorrhagic events . | Total neurological events combined with all-cause mortality . |
|---|---|---|---|---|---|
| No. of patients followed | 460 | 460 | 460 | 460 | 460 |
| Mean (SD) length of follow-up, days | 712 (289) | 648 (318) | 690 (309) | 706 (302) | 648 (318) |
| Median (Q1; Q3) length of follow-up, days | 729 (523–913) | 689 (471–869) | 689 (471–869) | 689 (471–869) | 689 (471–869) |
| No. of events | 50 | 45a | 30 | 12 | 81 |
| Total follow-up, person years (range) | 897 (0–1233 days) | 8817 (0–1227 days) | 817 (0–1227 days) | 817 (0–1227 days) | 817 (0–1227 days) |
1-year event-free probability (95% CI) (number at risk) | 95.3 (93.3–97.3) (n = 416) | 94.9 (92.8–97.0) (n = 387) | 96.7 (95.0–98.4) (n = 387) | 98.6 (97.4–99.7) (n = 387) | 91.9 (89.4–94.5) (n = 387) |
2-year event-free probability (95% CI) (number at risk) | 90.4 (87.4–93.4) (n = 229) | 89.5 (86.5–92.7) (n = 196) | 93.0 (90.4–95.7) (n = 196) | 97.1 (95.4–98.8) (n = 196) | 82.6 (78.8–86.6) (n = 196) |
| Total event rate, per 100 person years follow-up (95% CI) | 5.6 (4.1–7.3) | 5.5 (4.0–7.4) | 3.7 (2.5–5.2) | 1.5 (0.8–2.6) | 9.9 (7.9–12.3) |
CI, confidence interval; Q, quartile.
Including three neurological events of unknown type.
Mortality, overall neurological event rate, ischaemic event rate, haemorrhagic event rate, and combined rate from linked data
| . | All-cause mortality . | Total neurological events . | Ischaemic events . | Haemorrhagic events . | Total neurological events combined with all-cause mortality . |
|---|---|---|---|---|---|
| No. of patients followed | 460 | 460 | 460 | 460 | 460 |
| Mean (SD) length of follow-up, days | 712 (289) | 648 (318) | 690 (309) | 706 (302) | 648 (318) |
| Median (Q1; Q3) length of follow-up, days | 729 (523–913) | 689 (471–869) | 689 (471–869) | 689 (471–869) | 689 (471–869) |
| No. of events | 50 | 45a | 30 | 12 | 81 |
| Total follow-up, person years (range) | 897 (0–1233 days) | 8817 (0–1227 days) | 817 (0–1227 days) | 817 (0–1227 days) | 817 (0–1227 days) |
1-year event-free probability (95% CI) (number at risk) | 95.3 (93.3–97.3) (n = 416) | 94.9 (92.8–97.0) (n = 387) | 96.7 (95.0–98.4) (n = 387) | 98.6 (97.4–99.7) (n = 387) | 91.9 (89.4–94.5) (n = 387) |
2-year event-free probability (95% CI) (number at risk) | 90.4 (87.4–93.4) (n = 229) | 89.5 (86.5–92.7) (n = 196) | 93.0 (90.4–95.7) (n = 196) | 97.1 (95.4–98.8) (n = 196) | 82.6 (78.8–86.6) (n = 196) |
| Total event rate, per 100 person years follow-up (95% CI) | 5.6 (4.1–7.3) | 5.5 (4.0–7.4) | 3.7 (2.5–5.2) | 1.5 (0.8–2.6) | 9.9 (7.9–12.3) |
| . | All-cause mortality . | Total neurological events . | Ischaemic events . | Haemorrhagic events . | Total neurological events combined with all-cause mortality . |
|---|---|---|---|---|---|
| No. of patients followed | 460 | 460 | 460 | 460 | 460 |
| Mean (SD) length of follow-up, days | 712 (289) | 648 (318) | 690 (309) | 706 (302) | 648 (318) |
| Median (Q1; Q3) length of follow-up, days | 729 (523–913) | 689 (471–869) | 689 (471–869) | 689 (471–869) | 689 (471–869) |
| No. of events | 50 | 45a | 30 | 12 | 81 |
| Total follow-up, person years (range) | 897 (0–1233 days) | 8817 (0–1227 days) | 817 (0–1227 days) | 817 (0–1227 days) | 817 (0–1227 days) |
1-year event-free probability (95% CI) (number at risk) | 95.3 (93.3–97.3) (n = 416) | 94.9 (92.8–97.0) (n = 387) | 96.7 (95.0–98.4) (n = 387) | 98.6 (97.4–99.7) (n = 387) | 91.9 (89.4–94.5) (n = 387) |
2-year event-free probability (95% CI) (number at risk) | 90.4 (87.4–93.4) (n = 229) | 89.5 (86.5–92.7) (n = 196) | 93.0 (90.4–95.7) (n = 196) | 97.1 (95.4–98.8) (n = 196) | 82.6 (78.8–86.6) (n = 196) |
| Total event rate, per 100 person years follow-up (95% CI) | 5.6 (4.1–7.3) | 5.5 (4.0–7.4) | 3.7 (2.5–5.2) | 1.5 (0.8–2.6) | 9.9 (7.9–12.3) |
CI, confidence interval; Q, quartile.
Including three neurological events of unknown type.
The baseline patient utility score was recorded pre-procedure in 272 procedures; median 0.82 (Q1:Q3, 0.68:1.00). No significant change in utility was observed during follow-up (Supplementary material online, Table S5). Overall patient health (measured via VAS) at baseline was recorded in 232 procedures; median 75 (Q1:Q3, 50:90). A significant increase in VAS was observed at 6 weeks; mean change of 6.04 (SD 15.66), n = 103 pairs, but not thereafter. Use of medications over time is described in Table 4.
| . | N . | Antiplatelet only . | Anticoagulant . | Other (incl. none) . | Fisher’s test . |
|---|---|---|---|---|---|
| P-value (pairs) . | |||||
| Pre-procedure | 472 | 150 (31.8%) | 84 (17.8%) | 238 (50.4%) | Reference |
| At discharge | 464 | 348 (75.0%) | 64 (13.8%) | 52 (11.2%) | P < 0.0001 (n = 457) |
| 6 weeks | 344 | 249 (72.4%) | 35 (10.2%) | 60 (17.4%) | P < 0.0001 (n = 337) |
| 6 months | 309 | 177 (57.3%) | 12 (3.9%) | 120 (38.8%) | P < 0.15 (n = 302) |
| 1 year | 224 | 121 (54.0%) | 8 (3.6%) | 95 (42.4%) | P = 0.01 (n = 224) |
| 2 years | 61 | 37 (60.7%) | 2 (3.3%) | 22 (36.1%) | P = 0.51 (n = 61) |
| . | N . | Antiplatelet only . | Anticoagulant . | Other (incl. none) . | Fisher’s test . |
|---|---|---|---|---|---|
| P-value (pairs) . | |||||
| Pre-procedure | 472 | 150 (31.8%) | 84 (17.8%) | 238 (50.4%) | Reference |
| At discharge | 464 | 348 (75.0%) | 64 (13.8%) | 52 (11.2%) | P < 0.0001 (n = 457) |
| 6 weeks | 344 | 249 (72.4%) | 35 (10.2%) | 60 (17.4%) | P < 0.0001 (n = 337) |
| 6 months | 309 | 177 (57.3%) | 12 (3.9%) | 120 (38.8%) | P < 0.15 (n = 302) |
| 1 year | 224 | 121 (54.0%) | 8 (3.6%) | 95 (42.4%) | P = 0.01 (n = 224) |
| 2 years | 61 | 37 (60.7%) | 2 (3.3%) | 22 (36.1%) | P = 0.51 (n = 61) |
| . | N . | Antiplatelet only . | Anticoagulant . | Other (incl. none) . | Fisher’s test . |
|---|---|---|---|---|---|
| P-value (pairs) . | |||||
| Pre-procedure | 472 | 150 (31.8%) | 84 (17.8%) | 238 (50.4%) | Reference |
| At discharge | 464 | 348 (75.0%) | 64 (13.8%) | 52 (11.2%) | P < 0.0001 (n = 457) |
| 6 weeks | 344 | 249 (72.4%) | 35 (10.2%) | 60 (17.4%) | P < 0.0001 (n = 337) |
| 6 months | 309 | 177 (57.3%) | 12 (3.9%) | 120 (38.8%) | P < 0.15 (n = 302) |
| 1 year | 224 | 121 (54.0%) | 8 (3.6%) | 95 (42.4%) | P = 0.01 (n = 224) |
| 2 years | 61 | 37 (60.7%) | 2 (3.3%) | 22 (36.1%) | P = 0.51 (n = 61) |
| . | N . | Antiplatelet only . | Anticoagulant . | Other (incl. none) . | Fisher’s test . |
|---|---|---|---|---|---|
| P-value (pairs) . | |||||
| Pre-procedure | 472 | 150 (31.8%) | 84 (17.8%) | 238 (50.4%) | Reference |
| At discharge | 464 | 348 (75.0%) | 64 (13.8%) | 52 (11.2%) | P < 0.0001 (n = 457) |
| 6 weeks | 344 | 249 (72.4%) | 35 (10.2%) | 60 (17.4%) | P < 0.0001 (n = 337) |
| 6 months | 309 | 177 (57.3%) | 12 (3.9%) | 120 (38.8%) | P < 0.15 (n = 302) |
| 1 year | 224 | 121 (54.0%) | 8 (3.6%) | 95 (42.4%) | P = 0.01 (n = 224) |
| 2 years | 61 | 37 (60.7%) | 2 (3.3%) | 22 (36.1%) | P = 0.51 (n = 61) |
Discussion
This study has reported the results of the real-world safety and efficacy of LAAO from a multicentre, prospective, observational registry, with patient selection and treatment reflecting UK practice within the NHS of England. It is the largest study performed on LAAO in the UK to date. The results of this registry partly informed NHS England’s decision to commission LAAO routinely.14 In our cohort of 537 procedures, successful implantation of the LAAO device (in the absence of major complications) was achieved in 88.9 (95% CI 85.8–91.5)% of patients. There were no significant improvements in the reported HRQoL, and no significant improvement in VAS beyond 6 weeks. The proportion free of ischaemic neurological events at 1 year was 96.7 (95% CI 95.0–98.4)%.
Currently, the only experimental evidence to support the efficacy and safety of LAAO for the prevention of stroke in patients with AF comes from two randomized controlled trials (RCTs) which investigated the use of LAAO using the WATCHMAN system, Supplementary material online, Table S6. The PROTECT AF trial demonstrated non-inferiority in its primary composite outcome in patients receiving LAAO compared with those taking warfarin,19 and this effect lasted for at least 3.8 years.20 However, it did not demonstrate non-inferiority in reduction in ischaemic strokes, and there were material issues with peri-procedural safety. In contrast, the subsequent PREVAIL trial demonstrated improved safety, but failed to demonstrate non-inferiority in clinical outcomes compared with warfarin after 12 months of follow-up.21 These studies are not necessarily generalizable to settings where LAAO is reserved as a second-line option for patients with contraindications to oral anticoagulants, because they were conducted in populations that were candidates for these drugs, and oral anticoagulation was used in the intervention arm for 45 days.
Whilst published comparative data are limited and not generalizable, there have been several observational studies that reflect the use of LAAO in the population enrolled in the current study, Supplementary material online, Table S7. These included the ACP registry (n = 1053, AMPLATZER devices),22 the EWOLUTION registry (WATCHMAN device, n = 1021),23 and most applicable to this study, one set in the UK NHS in a similar population, Betts et al. (n = 371),24 which included multiple device types, and had a similar follow-up (mean 24.7 months). Comparison of outcomes from different registry studies is unreliable and confounded by differences in baseline characteristics, stroke risk, bleeding risk, medical/device management strategies, operator experience levels, and outcome definitions, Supplementary material online, Table S7. This applies particularly to our registry, which enrolled patients in whom anticoagulation was unsuitable (due to intolerance, previous significant bleed, high bleeding risk) or who had evidence of a thromboembolic event despite adequate oral anticoagulant therapy. Data on the medical management of this specific group of AF patients are not available in the published literature.
Left atrial appendage occlusion has a high technical success rate. The PROTECT AF trial reported a success rate of 91%,19 which increased to 95% in the PREVAIL trial,21 an improvement that was partly attributed to a learning curve effect as both studies were conducted by the same clinical teams, Supplementary material online, Table S6. The EWOLUTION registry reported successful implantation in 98.5% of patients, with a complete seal reported in 91.4%.23 The ACP registry reported a technical success rate of 97.3%.22 Betts et al.24 reported a procedural success rate of 92.5%, acute major events in 3.5% [including five (1.35%) device embolizations and one (0.25%) death]. Whilst some studies have reported nominally superior LAAO implantation success rate when compared in the current study, the definition of success varies.
Five in-hospital procedural deaths occurred in our study (0.9%); this is similar to other published trials which have reported procedural mortality rates between 0% and 0.8%.25 Our study also identified 26 instances (5.9%) where the device was reported to have embolized. This failure rate has to be considered against the expected rate of thromboembolic events in those patients who could not take oral anticoagulants (as predicted by the risk scores) and also the risks of a major bleed in those who continued to take an oral anticoagulant (which is very difficult to define in a cohort of patients in whom their physician has deemed that they can no longer take such therapy).
In this defined high-risk population (median CHA2DS2VASc of 4), LAAO in a routine NHS setting (i.e. not part of an RCT) was associated with non-trivial risk, including in-hospital death in 0.9% and late device embolism in 5.9%. Notwithstanding the limitations of uncontrolled registry data, these complication rates raise concern and might be considered unacceptable in most patients with AF who are able to take an anticoagulant (especially DOAC) and are at relatively low risk of stroke and of bleeding. Our data suggest that LAAO devices should not be considered as an alternative to anticoagulation, and this is supported by failure to demonstrate non-inferiority in the PROTECT AF and PREVAIL RCTs comparing LAAO to anticoagulation. The ongoing ASAP-TOO trial may provide valuable comparative data in this population.26
The main limitation of this study was that it was single-armed, and comparisons had to be made with results published in the literature where patient eligibility may have differed,27 and outcomes may also have been influenced by a learning curve effect. The follow-up of 2 years was relatively short, and most patients were not eligible for assessment at this time point because of the timeframe of the study. Patients were additionally lost to follow-up in the registry as care was transferred away from the centre that carried out the procedure. Whilst data linkage allowed collection of mortality and neurological outcomes occurring in NHS hospitals across England, it did not allow complete follow-up for other important clinical or patient-orientated outcomes. Additionally, linkage does not identify patients who moved from England after their procedure and were unknowingly lost to follow-up; this will be rare but suggests that the outcomes reported are likely to be lower limits.
In conclusion, this was the largest study to date on the safety and efficacy of LAAO in a UK NHS setting. The results show that LAAO is successfully implanted in about 90% of patients in whom it is attempted, which is consistent with other studies. The incidence of thromboembolic events was numerically lower compared with historic data from epidemiological studies (CHADS2 and CHA2DS2-VASc scores, although these are not directly comparable). The results support the view, consistent with the 2020 European Heat Rhythm Association/European Association of Percutaneous Cardiovascular Interventions (EHRA/EAPCI) consensus statement,25 that LAAO is an appropriate option to reduce the risk of ischaemic stroke in people with AF for whom oral anticoagulation is contraindicated or carries a high risk of bleeding. Further comparative research is warranted to understand the full extent of the protection afforded by LAAO in patients unable to take anticoagulants.
Supplementary material
Supplementary material is available at European Heart Journal – Quality of Care and Clinical Outcomes online.
We are grateful for the support of staff at centres performing LAAO procedures and completing data entry to the online registry: Barts Health NHS Trust, University College of London Hospital NHS Foundation Trust; Brighton & Sussex University Hospitals NHS Trust; Guy’s and St Thomas’ NHS Foundation Trust; Kings College Hospital NHS Foundation Trust; Leeds Teaching Hospitals NHS Trust; Liverpool Heart & Chest Hospital NHS Foundation Trust; Oxford University Hospitals NHS Trust; Royal Papworth Hospital NHS Foundation Trust; The Newcastle upon Tyne Hospital NHS Foundation Trust; South Tees Hospitals NHS Foundation Trust; University Hospitals Birmingham NHS Foundation Trust; University Hospitals Leicester NHS Trust; University Hospital of North Staffordshire NHS Trust.
Funding
Newcastle upon Tyne Hospitals NHS Foundation Trust, the employing institution of I.W., K.K., H.C., and A.J.S., is contracted as External Assessment Centre to the NICE Medical Technologies Evaluation Programme (MTEP) and is contracted by Academic Health Science Network North East and North Cumbria to develop methodologies and case studies relating to ‘evaluation in practice’ in the context of using routine healthcare datasets and, where appropriate, clinical registries, to assess outcomes and adoption of novel interventions.
Conflict of interest: A.J.S. reports grants from NIHR and Wellcome Trust and outside the submitted work. K.K. reports grants from NIHR outside the submitted work. H.Pa., H.Po. and L.B. are employed by NICE and were contracted by NHS England to oversee the Commissioning through Evaluation scheme. There are no other financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Data availability
There are ethical and legal restrictions on sharing de-identified data. The identifiable registry data were collected by the National Institute of Cardiovascular Outcomes and Research (NICOR) on behalf of NHS England. NICOR provided these data to NHS Digital for linkage to the Hospital Episodes Statistics database and the national mortality data set (ONS). The resultant linked, pseudonymized, dataset was provided to the authors (in Newcastle upon Tyne Hospitals) for analysis. Because the data were pseudonymized rather than fully anonymized, they are considered by NHS Digital to be sensitive due to the risk of re-identification. The Health Research Authority (HRA) Confidentiality Advisory Group (application details provided in the manuscript) gave permission to link the datasets and imposed the terms of its use. These included not permitting public sharing, and a requirement to destroy it after use. Registry data are available from NHS England’s Specialized Services (https://www.england.nhs.uk/commissioning/spec-services/ (15 June 2021)), or via NICOR (https://www.nicor.org.uk/ (15 June 2021)
) and HES and ONS data from NHS Digital (https://digital.nhs.uk/ (15 June 2021)). As per the terms of use, a copy of the linked data is no longer held by The Newcastle upon Tyne Hospitals NHS Foundation Trust.
Acknowledgements
LAAO data collected through the Commissioning through Evaluation programme were provided by the National Institute for Cardiovascular Outcomes Research (NICOR). HES data held by NHS Digital (formerly the UK NHS Health and Social Care Information Centre, HSCIC) have been used to help complete the analysis © 2020. Reused with the permission of NHS Digital/HSCIC. All rights reserved.



