-
PDF
- Split View
-
Views
-
Cite
Cite
Ioanna Kosmidou, Megan Durkin, Eileen Vella, Neisha DeJesus, Sofia Romero, Rosalyn Gamboa, Paul Jenkins, Brian Shaffer, Richard Steingart, Jennifer Liu, Clinical Outcomes in Hospitalized Patients with Cancer and New versus Preexistent Atrial Fibrillation, European Heart Journal - Quality of Care and Clinical Outcomes, Volume 10, Issue 8, December 2024, Pages 689–697, https://doi.org/10.1093/ehjqcco/qcad077
Close - Share Icon Share
Abstract
There is limited information on the prognostic impact of new onset versus preexistent atrial fibrillation (AF) in hospitalized patients with cancer.
We sought to determine the clinical impact of new onset AF (NOAF) compared with preexistent AF in hospitalized patients with cancer.
All patients with cancer hospitalized over the course of 1 year with clinically manifest new or preexistent AF were enrolled in the Memorial Sloan Kettering Cancer Center AF registry. The relationship of NOAF to the primary composite outcome of all cause death, cardiovascular (CV) rehospitalization, or cerebrovascular event (CVE), as well as secondary CV endpoints, were analysed using proportional hazards regression. Where applicable, the competing risk of death was accounted for using methodology described by Fine and Gray.
Among 606 patients included in the analysis, 313 (51.7%) had NOAF and 293 (48.3%) had preexistent AF. Patients with NOAF were younger and had less frequent prior history of CV disease compared with patients with preexistent AF. At follow-up, patients with NOAF had a higher adjusted hazard for the primary composite outcome versus patients with prior AF (hazard ratio [HR] 1.64, 95% confidence interval [CI] 1.27, 2.13, P = 0.002), as well as the secondary CV composite outcome of clinical AF recurrence, CV death, CV rehospitalization, or CVE (HR 2.17, 95% CI 1.57, 2.99, P < 0.0001).
In hospitalized patients with cancer and electrocardiographically manifest new versus preexistent AF, NOAF was associated with a higher risk for the primary composite outcome of all-cause death, CV rehospitalization, or CVE.
Abbreviations and Acronyms
- AF,
atrial fibrillation
- CV,
cardiovascular
- CVE,
cerebrovascular event
- MSKCC,
Memorial Sloan Kettering Cancer Center
- NOAF,
new onset atrial fibrillation
What is already known:
The overall incidence of new atrial fibrillation (AF) in patients with cancer is higher compared to the general population.
AF adversely impacts clinical and cardiovascular outcomes in patients with cancer.
AF is the most common arrhythmia affecting hospitalized patients with cancer.
What this study adds:
Newly diagnosed AF in the acute hospitalization setting of patients with cancer is associated with worse in hospital outcomes, less frequent utilization of antiarrhythmic and anticoagulant therapy at discharge, and more frequent cardiovascular therapy escalation requirement after discharge compared to hospitalized cancer patients with clinically manifest preexistent AF.
New AF was associated with a higher adjusted risk for adverse clinical and cardiovascular outcomes, including death and cardiovascular readmission, and similar adjusted risk of stroke compared with hospitalized cancer patients with a prior AF diagnosis.
Introduction
Atrial fibrillation (AF) is a leading cause of cardiovascular (CV) morbidity and mortality in the general population and increasingly recognized as a significant adverse prognostic factor among patients with cancer.1–4 Prior studies have suggested an increased incidence of AF among patients with cancer and a higher risk for adverse clinical and CV outcomes with new compared to preexistent AF.4–7 Nevertheless, the acute and post-discharge clinical impact of new onset AF (NOAF) diagnosed in the setting of acute hospitalization for cancer therapies or cancer-related adverse events remain poorly understood when compared to patients with a known history of AF. In the present analysis from the Memorial Sloan Kettering Cancer Center (MSKCC) AF registry, we sought to determine the in-hospital and long-term clinical and CV outcomes of hospitalized patients with cancer or cancer-related adverse events, comparing those with NOAF to those with electrocardiographically manifest preexistent AF.
Methods
Study design and endpoints
The MSKCC AF registry was a single centre prospective registry conducted at an academic institute specializing in cancer care and included all patients with a history of cancer admitted for at least 24 h with a concomitant index admission diagnosis of AF over the course of 1 year. Patients were eligible for inclusion if they had electrocardiographic evidence of AF during the index admission. The investigation was approved by the MSKCC Institutional Review Board. NOAF was defined as AF documented by cardiac electrocardiography within the index hospitalization in patients without a prior history of AF. Preexistent AF was defined as electrocardiographically manifest AF within the index hospitalization in patients with a documented prior history of AF. The primary study endpoint was defined a priori as the composite endpoint of all-cause death, cerebrovascular event (stroke or transient ischaemic attack, CVE) or CV rehospitalization. We further analysed a secondary CV composite outcome of clinical AF recurrence defined as (1) recurrent clinical AF or significant AF therapy escalation (including new anticoagulant, antiarrhythmic or rate control therapy, pacemaker implantation, AF ablation, left atrial appendage occlusion, direct current cardioversion, or AF-related rehospitalization), CV death, CVE, or CV rehospitalization. The other five secondary endpoints were all-cause death, CV death, CVE, CV rehospitalization, and clinical AF recurrence. Median follow-up was 7 ± 5.1 months.
Statistical methods
Continuous variables are reported as mean ± standard deviation and were compared using the Student's t test. Categorical variables are expressed as counts and percentages and were compared with the χ2 or Fisher exact test, as appropriate. Two of the seven study endpoints (time to the primary composite endpoint and time to all-cause death) were analysed using standard proportion hazards regression methodology. For the secondary composite endpoint, which included CV death as one of its four components, as well as for the individual secondary endpoint of time to CV death, non-CV death was treated as a competing risk. Finally, for analysis of the individual endpoints of CVE, clinical AF recurrence, and CV rehospitalization, all-cause death was treated as the competing event. All competing risks models utilized the subdistribution hazard function as described by Fine and Gray. The adjusted association between new or preexistent AF and adverse clinical outcomes was assessed using multivariable Cox proportional hazards regression accounting for the competing risk of death (where necessary) and included the following predefined clinically pertinent covariates: age, hypertension, prior coronary artery disease, prior congestive heart failure, prior CVE, cancer localization, cancer stage, and index surgery. Adjusted models for the individual endpoint of CVE were performed using a modified model that included age, CHA2DS2-Vasc score, and prior CVE. Alternate models including in hospital paroxysmal AF and CHA2DS2-Vasc score were also performed for the primary and secondary composite outcomes. The tenability of the proportional hazard's assumption was assessed via graphical methods. Specifically, plots of both the cumulative incidence and cumulative hazards functions were contrasted between new AF and prior AF groups to confirm that the functions did not cross and that there was no indication of meaningful group by time interaction. The results of these models were summarized using hazard ratios and corresponding 95% confidence intervals. A two-sided P < 0.05 was considered statistically significant for all hypothesis tests. All statistical analyses were performed with the use of SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline, echocardiographic, and cancer characteristics
Among 606 patients included, 313 patients had NOAF and 293 had preexistent AF. Baseline, echocardiographic, and cancer characteristics of patients with NOAF or preexistent AF are shown in Tables 1–3, respectively. Compared to patients with prior AF, patients with NOAF were younger, had lower body mass index, were more frequently current or former smokers, and had a significantly less frequent history of prior CV and cerebrovascular disease, as well as less frequent prior CV interventions (Table 1). Further, patients with NOAF had higher left ventricular ejection fraction and less frequent left atrial dilatation or evidence of valvular disease by echocardiography (Table 2). Cancer characteristics did not vary significantly between groups except for more frequent intraabdominal or pelvic cancer localization in patients with preexistent AF (Table 3). Surgical intervention during or up to 30 days prior to the index hospitalization was common in both groups and noted in approximately half of the patient population (Table 3). Medications are shown in Table 4; anticoagulant and antiarrhythmic therapy was more common in patient with preexistent AF compared with NOAF both at baseline and at discharge. Mean length of stay was 14.6 ± 17.6 days in patients with NOAF compared with 8.8 ± 8.7 days in patients with preexistent AF (P < 0.0001). AF as a primary diagnosis of admission was observed in 18/293 (5.8%) patients with NOAF and 10/293 (3.4%) patients with prior AF (P = 0.11). Index AF was more frequently paroxysmal in patients with NOAF compared with patients with preexistent AF (90.7% vs. 46.5%, P < 0.0001). A rhythm control strategy was used in the acute setting in 97/313 (31%) patients with NOAF compared to 48/293 (16.4%) patients with preexistent AF (P < 0.0001). Amiodarone was the main antiarrhythmic agent used in patients with NOAF managed with a rhythm control strategy (95/97, 97.9%) whereas 36/48 (75%) of patients with preexistent AF patients treated with a rhythm control strategy were managed with amiodarone. Direct current cardioversion was performed in two patients with NOAF; one patient underwent pharmacologic cardioversion with flecainide.
Baseline characteristics of patients with cancer and new onset versus preexistent AF
| . | New onset AF (n = 313) . | Preexistent AF (n = 293) . | P-value . |
|---|---|---|---|
| Age (years) | 70.8 (11.5) | 74.6 (9.5) | <0.0001 |
| Sex (male) | 182/312 (58.3%) | 196/293 (66.9%) | 0.030 |
| Body mass index (kg/m2) | 27.2 (6.8) | 28.8 (6.0) | 0.005 |
| Race | |||
| Caucasian | 238/308 (77.3%) | 247/286 (86.4%) | 0.013 |
| African American | 30/308 (9.7%) | 14/286 (4.9%) | |
| Other | 40/308 (13.0%) | 25/286 (8.7%) | |
| Smoking status | |||
| Non-smoker | 166/311 (53.4%) | 184/288 (63.9%) | 0.008 |
| Current smoker | 30/311 (9.6%) | 13/288 (4.5%) | |
| Former smoker | 115/311 (37.0%) | 91/288 (31.6%) | |
| Prior myocardial infarct | 13/313 (4.2%) | 30/293 (10.2%) | 0.004 |
| Prior coronary artery disease | 44/311 (14.2%) | 76/293 (25.9%) | 0.0003 |
| Prior percutaneous coronary intervention | 29/311 (9.3%) | 49/292 (16.8%) | 0.006 |
| Prior coronary artery bypass grafting | 10/313 (3.2%) | 17/293 (5.8%) | 0.12 |
| Prior valve replacement | 5/313 (1.6%) | 28/291 (9.6%) | <0.0001 |
| Prior stroke or transient ischemic attack | 17/310 (5.5%) | 35/287 (12.2%) | 0.004 |
| Diabetes | 54/312 (17.3%) | 62/293 (21.2%) | 0.23 |
| Hypertension | 183/313 (58.5%) | 231/293 (78.8%) | <0.0001 |
| Congestive heart failure | 25/312 (8.0%) | 74/292 (25.3%) | <0.0001 |
| Obstructive sleep apnea | 22/304 (7.2%) | 26/293 (8.9%) | 0.09 |
| Chronic kidney disease | 36/312 (11.5%) | 45/292 (15.4%) | 0.16 |
| CHA2DS2Vasc score | 2.5 (1.4) | 3.3 (1.2) | <0.0001 |
| Anticoagulation indication | 201/313 (64.2%) | 267/290 (92.1%) | <0.0001 |
| . | New onset AF (n = 313) . | Preexistent AF (n = 293) . | P-value . |
|---|---|---|---|
| Age (years) | 70.8 (11.5) | 74.6 (9.5) | <0.0001 |
| Sex (male) | 182/312 (58.3%) | 196/293 (66.9%) | 0.030 |
| Body mass index (kg/m2) | 27.2 (6.8) | 28.8 (6.0) | 0.005 |
| Race | |||
| Caucasian | 238/308 (77.3%) | 247/286 (86.4%) | 0.013 |
| African American | 30/308 (9.7%) | 14/286 (4.9%) | |
| Other | 40/308 (13.0%) | 25/286 (8.7%) | |
| Smoking status | |||
| Non-smoker | 166/311 (53.4%) | 184/288 (63.9%) | 0.008 |
| Current smoker | 30/311 (9.6%) | 13/288 (4.5%) | |
| Former smoker | 115/311 (37.0%) | 91/288 (31.6%) | |
| Prior myocardial infarct | 13/313 (4.2%) | 30/293 (10.2%) | 0.004 |
| Prior coronary artery disease | 44/311 (14.2%) | 76/293 (25.9%) | 0.0003 |
| Prior percutaneous coronary intervention | 29/311 (9.3%) | 49/292 (16.8%) | 0.006 |
| Prior coronary artery bypass grafting | 10/313 (3.2%) | 17/293 (5.8%) | 0.12 |
| Prior valve replacement | 5/313 (1.6%) | 28/291 (9.6%) | <0.0001 |
| Prior stroke or transient ischemic attack | 17/310 (5.5%) | 35/287 (12.2%) | 0.004 |
| Diabetes | 54/312 (17.3%) | 62/293 (21.2%) | 0.23 |
| Hypertension | 183/313 (58.5%) | 231/293 (78.8%) | <0.0001 |
| Congestive heart failure | 25/312 (8.0%) | 74/292 (25.3%) | <0.0001 |
| Obstructive sleep apnea | 22/304 (7.2%) | 26/293 (8.9%) | 0.09 |
| Chronic kidney disease | 36/312 (11.5%) | 45/292 (15.4%) | 0.16 |
| CHA2DS2Vasc score | 2.5 (1.4) | 3.3 (1.2) | <0.0001 |
| Anticoagulation indication | 201/313 (64.2%) | 267/290 (92.1%) | <0.0001 |
AF = atrial fibrillation.
Values are mean ± standard deviation or n/N (%).
Baseline characteristics of patients with cancer and new onset versus preexistent AF
| . | New onset AF (n = 313) . | Preexistent AF (n = 293) . | P-value . |
|---|---|---|---|
| Age (years) | 70.8 (11.5) | 74.6 (9.5) | <0.0001 |
| Sex (male) | 182/312 (58.3%) | 196/293 (66.9%) | 0.030 |
| Body mass index (kg/m2) | 27.2 (6.8) | 28.8 (6.0) | 0.005 |
| Race | |||
| Caucasian | 238/308 (77.3%) | 247/286 (86.4%) | 0.013 |
| African American | 30/308 (9.7%) | 14/286 (4.9%) | |
| Other | 40/308 (13.0%) | 25/286 (8.7%) | |
| Smoking status | |||
| Non-smoker | 166/311 (53.4%) | 184/288 (63.9%) | 0.008 |
| Current smoker | 30/311 (9.6%) | 13/288 (4.5%) | |
| Former smoker | 115/311 (37.0%) | 91/288 (31.6%) | |
| Prior myocardial infarct | 13/313 (4.2%) | 30/293 (10.2%) | 0.004 |
| Prior coronary artery disease | 44/311 (14.2%) | 76/293 (25.9%) | 0.0003 |
| Prior percutaneous coronary intervention | 29/311 (9.3%) | 49/292 (16.8%) | 0.006 |
| Prior coronary artery bypass grafting | 10/313 (3.2%) | 17/293 (5.8%) | 0.12 |
| Prior valve replacement | 5/313 (1.6%) | 28/291 (9.6%) | <0.0001 |
| Prior stroke or transient ischemic attack | 17/310 (5.5%) | 35/287 (12.2%) | 0.004 |
| Diabetes | 54/312 (17.3%) | 62/293 (21.2%) | 0.23 |
| Hypertension | 183/313 (58.5%) | 231/293 (78.8%) | <0.0001 |
| Congestive heart failure | 25/312 (8.0%) | 74/292 (25.3%) | <0.0001 |
| Obstructive sleep apnea | 22/304 (7.2%) | 26/293 (8.9%) | 0.09 |
| Chronic kidney disease | 36/312 (11.5%) | 45/292 (15.4%) | 0.16 |
| CHA2DS2Vasc score | 2.5 (1.4) | 3.3 (1.2) | <0.0001 |
| Anticoagulation indication | 201/313 (64.2%) | 267/290 (92.1%) | <0.0001 |
| . | New onset AF (n = 313) . | Preexistent AF (n = 293) . | P-value . |
|---|---|---|---|
| Age (years) | 70.8 (11.5) | 74.6 (9.5) | <0.0001 |
| Sex (male) | 182/312 (58.3%) | 196/293 (66.9%) | 0.030 |
| Body mass index (kg/m2) | 27.2 (6.8) | 28.8 (6.0) | 0.005 |
| Race | |||
| Caucasian | 238/308 (77.3%) | 247/286 (86.4%) | 0.013 |
| African American | 30/308 (9.7%) | 14/286 (4.9%) | |
| Other | 40/308 (13.0%) | 25/286 (8.7%) | |
| Smoking status | |||
| Non-smoker | 166/311 (53.4%) | 184/288 (63.9%) | 0.008 |
| Current smoker | 30/311 (9.6%) | 13/288 (4.5%) | |
| Former smoker | 115/311 (37.0%) | 91/288 (31.6%) | |
| Prior myocardial infarct | 13/313 (4.2%) | 30/293 (10.2%) | 0.004 |
| Prior coronary artery disease | 44/311 (14.2%) | 76/293 (25.9%) | 0.0003 |
| Prior percutaneous coronary intervention | 29/311 (9.3%) | 49/292 (16.8%) | 0.006 |
| Prior coronary artery bypass grafting | 10/313 (3.2%) | 17/293 (5.8%) | 0.12 |
| Prior valve replacement | 5/313 (1.6%) | 28/291 (9.6%) | <0.0001 |
| Prior stroke or transient ischemic attack | 17/310 (5.5%) | 35/287 (12.2%) | 0.004 |
| Diabetes | 54/312 (17.3%) | 62/293 (21.2%) | 0.23 |
| Hypertension | 183/313 (58.5%) | 231/293 (78.8%) | <0.0001 |
| Congestive heart failure | 25/312 (8.0%) | 74/292 (25.3%) | <0.0001 |
| Obstructive sleep apnea | 22/304 (7.2%) | 26/293 (8.9%) | 0.09 |
| Chronic kidney disease | 36/312 (11.5%) | 45/292 (15.4%) | 0.16 |
| CHA2DS2Vasc score | 2.5 (1.4) | 3.3 (1.2) | <0.0001 |
| Anticoagulation indication | 201/313 (64.2%) | 267/290 (92.1%) | <0.0001 |
AF = atrial fibrillation.
Values are mean ± standard deviation or n/N (%).
Baseline echocardiographic characteristics of patients with cancer and new onset versus preexistent AF
| . | New onset AF (n = 288) . | Preexistent AF (n = 253) . | P-value . |
|---|---|---|---|
| Left ventricular ejection fraction (%) | 61.4 (10.3) | 58.6 (10.3) | 0.0014 |
| LVEF < 40% | 15 (5.4%) | 19 (7.6%) | 0.29 |
| Left atrial diameter (cm) | 3.8 (0.8) (288) | 4.7 (1.5) (253) | <0.0001 |
| Left atrial volume index (mL/m2) | 32.1 (14.3) (182) | 45.4 (21.3) (176) | <0.0001 |
| Left atrial enlargement | 115/268 (42.9%) | 163/230 (70.9%) | <0.0001 |
| Left ventricular hypertrophy | 65/214 (23.3%) | 75/144 (43.4%) | 0.007 |
| Left ventricular diastolic dysfunction | 30/138 (21.7%) | 23/75 (30.7%) | 0.21 |
| Pulmonary hypertension (PASP > 35 mmHg) | 61/206 (29.6%) | 72/191 (37.7%) | 0.09 |
| Moderate or severe valvular disease | 0.021 | ||
| Mitral valve regurgitation | 22/270 (8.1%) | 24/233 (10.3%) | |
| Tricuspid valve regurgitation | 7/270 (2.6%) | 15/233 (6.4%) | |
| Aortic valve regurgitation | – | 2/233 (0.9%) | |
| Mitral valve stenosis | – | 2/233 (0.9%) | |
| Aortic valve stenosis | 8/270 (3%) | 12/233 (5.2%) | |
| Mixed valvular disease | 2/270 (0.7%) | 1/233 (0.4%) | |
| Prosthetic valve | 1/270 (0.4%) | 5/233 (2.1%) |
| . | New onset AF (n = 288) . | Preexistent AF (n = 253) . | P-value . |
|---|---|---|---|
| Left ventricular ejection fraction (%) | 61.4 (10.3) | 58.6 (10.3) | 0.0014 |
| LVEF < 40% | 15 (5.4%) | 19 (7.6%) | 0.29 |
| Left atrial diameter (cm) | 3.8 (0.8) (288) | 4.7 (1.5) (253) | <0.0001 |
| Left atrial volume index (mL/m2) | 32.1 (14.3) (182) | 45.4 (21.3) (176) | <0.0001 |
| Left atrial enlargement | 115/268 (42.9%) | 163/230 (70.9%) | <0.0001 |
| Left ventricular hypertrophy | 65/214 (23.3%) | 75/144 (43.4%) | 0.007 |
| Left ventricular diastolic dysfunction | 30/138 (21.7%) | 23/75 (30.7%) | 0.21 |
| Pulmonary hypertension (PASP > 35 mmHg) | 61/206 (29.6%) | 72/191 (37.7%) | 0.09 |
| Moderate or severe valvular disease | 0.021 | ||
| Mitral valve regurgitation | 22/270 (8.1%) | 24/233 (10.3%) | |
| Tricuspid valve regurgitation | 7/270 (2.6%) | 15/233 (6.4%) | |
| Aortic valve regurgitation | – | 2/233 (0.9%) | |
| Mitral valve stenosis | – | 2/233 (0.9%) | |
| Aortic valve stenosis | 8/270 (3%) | 12/233 (5.2%) | |
| Mixed valvular disease | 2/270 (0.7%) | 1/233 (0.4%) | |
| Prosthetic valve | 1/270 (0.4%) | 5/233 (2.1%) |
AF = atrial fibrillation; LVEF = left ventricular ejection fraction; PASP = pulmonary artery systolic pressure.
Values are mean ± standard deviation (n) or n/N (%).
Baseline echocardiographic characteristics of patients with cancer and new onset versus preexistent AF
| . | New onset AF (n = 288) . | Preexistent AF (n = 253) . | P-value . |
|---|---|---|---|
| Left ventricular ejection fraction (%) | 61.4 (10.3) | 58.6 (10.3) | 0.0014 |
| LVEF < 40% | 15 (5.4%) | 19 (7.6%) | 0.29 |
| Left atrial diameter (cm) | 3.8 (0.8) (288) | 4.7 (1.5) (253) | <0.0001 |
| Left atrial volume index (mL/m2) | 32.1 (14.3) (182) | 45.4 (21.3) (176) | <0.0001 |
| Left atrial enlargement | 115/268 (42.9%) | 163/230 (70.9%) | <0.0001 |
| Left ventricular hypertrophy | 65/214 (23.3%) | 75/144 (43.4%) | 0.007 |
| Left ventricular diastolic dysfunction | 30/138 (21.7%) | 23/75 (30.7%) | 0.21 |
| Pulmonary hypertension (PASP > 35 mmHg) | 61/206 (29.6%) | 72/191 (37.7%) | 0.09 |
| Moderate or severe valvular disease | 0.021 | ||
| Mitral valve regurgitation | 22/270 (8.1%) | 24/233 (10.3%) | |
| Tricuspid valve regurgitation | 7/270 (2.6%) | 15/233 (6.4%) | |
| Aortic valve regurgitation | – | 2/233 (0.9%) | |
| Mitral valve stenosis | – | 2/233 (0.9%) | |
| Aortic valve stenosis | 8/270 (3%) | 12/233 (5.2%) | |
| Mixed valvular disease | 2/270 (0.7%) | 1/233 (0.4%) | |
| Prosthetic valve | 1/270 (0.4%) | 5/233 (2.1%) |
| . | New onset AF (n = 288) . | Preexistent AF (n = 253) . | P-value . |
|---|---|---|---|
| Left ventricular ejection fraction (%) | 61.4 (10.3) | 58.6 (10.3) | 0.0014 |
| LVEF < 40% | 15 (5.4%) | 19 (7.6%) | 0.29 |
| Left atrial diameter (cm) | 3.8 (0.8) (288) | 4.7 (1.5) (253) | <0.0001 |
| Left atrial volume index (mL/m2) | 32.1 (14.3) (182) | 45.4 (21.3) (176) | <0.0001 |
| Left atrial enlargement | 115/268 (42.9%) | 163/230 (70.9%) | <0.0001 |
| Left ventricular hypertrophy | 65/214 (23.3%) | 75/144 (43.4%) | 0.007 |
| Left ventricular diastolic dysfunction | 30/138 (21.7%) | 23/75 (30.7%) | 0.21 |
| Pulmonary hypertension (PASP > 35 mmHg) | 61/206 (29.6%) | 72/191 (37.7%) | 0.09 |
| Moderate or severe valvular disease | 0.021 | ||
| Mitral valve regurgitation | 22/270 (8.1%) | 24/233 (10.3%) | |
| Tricuspid valve regurgitation | 7/270 (2.6%) | 15/233 (6.4%) | |
| Aortic valve regurgitation | – | 2/233 (0.9%) | |
| Mitral valve stenosis | – | 2/233 (0.9%) | |
| Aortic valve stenosis | 8/270 (3%) | 12/233 (5.2%) | |
| Mixed valvular disease | 2/270 (0.7%) | 1/233 (0.4%) | |
| Prosthetic valve | 1/270 (0.4%) | 5/233 (2.1%) |
AF = atrial fibrillation; LVEF = left ventricular ejection fraction; PASP = pulmonary artery systolic pressure.
Values are mean ± standard deviation (n) or n/N (%).
Cancer characteristics of patients with cancer and new onset versus preexistent AF
| . | New onset AF (n = 313) . | Preexistent AF (n = 293) . | P-value . |
|---|---|---|---|
| Active cancer | 297/311 (95.5%) | 269/289 (93.1%) | 0.20 |
| Cancer localization | 0.048 | ||
| Thoracic | 73/299 (24.2%) | 53/293 (18.1%) | |
| Abdominal/pelvic | 121/299 (22.7%) | 142/293 (48.7%) | |
| Hematologic | 68/299 (22.7%) | 52/293 (17.7%) | |
| Other | 37/299 (12.4%) | 46/293 (15.7%) | |
| Cancer organ/system | 0.27 | ||
| Breast | 9/313 (2.9%) | 12/293 (4.1%) | |
| Colorectal | 30/313 (9.6%) | 28/293 (9.6%) | |
| Dermatologic | 8/313 (2.6%) | 14/293 (4.8%) | |
| Endocrine | 11/313 (3.5%) | 7/293 (2.4%) | |
| Esophageal | 17/313 (5.4%) | 11/293 (3.8%) | |
| Gastric | 8/313 (2.6%) | 8/293 (2.7%) | |
| Genitourinary | 48/313 (15.3%) | 66/293 (22.5%) | |
| Hematologic | 66/313 (21.1%) | 51/293 (17.4%) | |
| Liver and cholangiocarcinoma | 8/313 (2.6%) | 13/293 (4.4%) | |
| Lung | 62/313 (19.8%) | 40/293 (13.7%) | |
| Musculoskeletal | 1/313 (0.3%) | 2/293 (0.7%) | |
| Nervous system | 3/313 (1%) | 1/293 (0.3%) | |
| Oropharyngeal | 9/313 (2.9%) | 7/293 (2.4%) | |
| Pancreatic | 16/313 (5.1%) | 21/293 (7.2%) | |
| Sarcoma | 12/313 (3.8%) | 8/293 (2.7%) | |
| Other/unspecified primary | 5/313 (1.6%) | 4/293 (1.4%) | |
| Cancer stage | 0.08 | ||
| Local or resectable | 80/276 (29.0%) | 103/271 (38%) | |
| Locally advanced or relapsed | 71/276 (25.7%) | 59/271 (21.8%) | |
| Metastatic or diffuse | 125/276 (45.3%) | 109/271 (40.2%) | |
| Index surgery | 144/303 (47.5%) | 126/291 (43.3%) | 0.30 |
| Surgery within past 30 days from index admission | 154/310 (49.7%) | 134/291 (46.1%) | 0.37 |
| Active cancer therapies | 183/304 (60.2%) | 156/285 (54.7%) | 0.18 |
| . | New onset AF (n = 313) . | Preexistent AF (n = 293) . | P-value . |
|---|---|---|---|
| Active cancer | 297/311 (95.5%) | 269/289 (93.1%) | 0.20 |
| Cancer localization | 0.048 | ||
| Thoracic | 73/299 (24.2%) | 53/293 (18.1%) | |
| Abdominal/pelvic | 121/299 (22.7%) | 142/293 (48.7%) | |
| Hematologic | 68/299 (22.7%) | 52/293 (17.7%) | |
| Other | 37/299 (12.4%) | 46/293 (15.7%) | |
| Cancer organ/system | 0.27 | ||
| Breast | 9/313 (2.9%) | 12/293 (4.1%) | |
| Colorectal | 30/313 (9.6%) | 28/293 (9.6%) | |
| Dermatologic | 8/313 (2.6%) | 14/293 (4.8%) | |
| Endocrine | 11/313 (3.5%) | 7/293 (2.4%) | |
| Esophageal | 17/313 (5.4%) | 11/293 (3.8%) | |
| Gastric | 8/313 (2.6%) | 8/293 (2.7%) | |
| Genitourinary | 48/313 (15.3%) | 66/293 (22.5%) | |
| Hematologic | 66/313 (21.1%) | 51/293 (17.4%) | |
| Liver and cholangiocarcinoma | 8/313 (2.6%) | 13/293 (4.4%) | |
| Lung | 62/313 (19.8%) | 40/293 (13.7%) | |
| Musculoskeletal | 1/313 (0.3%) | 2/293 (0.7%) | |
| Nervous system | 3/313 (1%) | 1/293 (0.3%) | |
| Oropharyngeal | 9/313 (2.9%) | 7/293 (2.4%) | |
| Pancreatic | 16/313 (5.1%) | 21/293 (7.2%) | |
| Sarcoma | 12/313 (3.8%) | 8/293 (2.7%) | |
| Other/unspecified primary | 5/313 (1.6%) | 4/293 (1.4%) | |
| Cancer stage | 0.08 | ||
| Local or resectable | 80/276 (29.0%) | 103/271 (38%) | |
| Locally advanced or relapsed | 71/276 (25.7%) | 59/271 (21.8%) | |
| Metastatic or diffuse | 125/276 (45.3%) | 109/271 (40.2%) | |
| Index surgery | 144/303 (47.5%) | 126/291 (43.3%) | 0.30 |
| Surgery within past 30 days from index admission | 154/310 (49.7%) | 134/291 (46.1%) | 0.37 |
| Active cancer therapies | 183/304 (60.2%) | 156/285 (54.7%) | 0.18 |
AF = atrial fibrillation.
Values are mean ± standard deviation (n) or n/N (%).
Cancer characteristics of patients with cancer and new onset versus preexistent AF
| . | New onset AF (n = 313) . | Preexistent AF (n = 293) . | P-value . |
|---|---|---|---|
| Active cancer | 297/311 (95.5%) | 269/289 (93.1%) | 0.20 |
| Cancer localization | 0.048 | ||
| Thoracic | 73/299 (24.2%) | 53/293 (18.1%) | |
| Abdominal/pelvic | 121/299 (22.7%) | 142/293 (48.7%) | |
| Hematologic | 68/299 (22.7%) | 52/293 (17.7%) | |
| Other | 37/299 (12.4%) | 46/293 (15.7%) | |
| Cancer organ/system | 0.27 | ||
| Breast | 9/313 (2.9%) | 12/293 (4.1%) | |
| Colorectal | 30/313 (9.6%) | 28/293 (9.6%) | |
| Dermatologic | 8/313 (2.6%) | 14/293 (4.8%) | |
| Endocrine | 11/313 (3.5%) | 7/293 (2.4%) | |
| Esophageal | 17/313 (5.4%) | 11/293 (3.8%) | |
| Gastric | 8/313 (2.6%) | 8/293 (2.7%) | |
| Genitourinary | 48/313 (15.3%) | 66/293 (22.5%) | |
| Hematologic | 66/313 (21.1%) | 51/293 (17.4%) | |
| Liver and cholangiocarcinoma | 8/313 (2.6%) | 13/293 (4.4%) | |
| Lung | 62/313 (19.8%) | 40/293 (13.7%) | |
| Musculoskeletal | 1/313 (0.3%) | 2/293 (0.7%) | |
| Nervous system | 3/313 (1%) | 1/293 (0.3%) | |
| Oropharyngeal | 9/313 (2.9%) | 7/293 (2.4%) | |
| Pancreatic | 16/313 (5.1%) | 21/293 (7.2%) | |
| Sarcoma | 12/313 (3.8%) | 8/293 (2.7%) | |
| Other/unspecified primary | 5/313 (1.6%) | 4/293 (1.4%) | |
| Cancer stage | 0.08 | ||
| Local or resectable | 80/276 (29.0%) | 103/271 (38%) | |
| Locally advanced or relapsed | 71/276 (25.7%) | 59/271 (21.8%) | |
| Metastatic or diffuse | 125/276 (45.3%) | 109/271 (40.2%) | |
| Index surgery | 144/303 (47.5%) | 126/291 (43.3%) | 0.30 |
| Surgery within past 30 days from index admission | 154/310 (49.7%) | 134/291 (46.1%) | 0.37 |
| Active cancer therapies | 183/304 (60.2%) | 156/285 (54.7%) | 0.18 |
| . | New onset AF (n = 313) . | Preexistent AF (n = 293) . | P-value . |
|---|---|---|---|
| Active cancer | 297/311 (95.5%) | 269/289 (93.1%) | 0.20 |
| Cancer localization | 0.048 | ||
| Thoracic | 73/299 (24.2%) | 53/293 (18.1%) | |
| Abdominal/pelvic | 121/299 (22.7%) | 142/293 (48.7%) | |
| Hematologic | 68/299 (22.7%) | 52/293 (17.7%) | |
| Other | 37/299 (12.4%) | 46/293 (15.7%) | |
| Cancer organ/system | 0.27 | ||
| Breast | 9/313 (2.9%) | 12/293 (4.1%) | |
| Colorectal | 30/313 (9.6%) | 28/293 (9.6%) | |
| Dermatologic | 8/313 (2.6%) | 14/293 (4.8%) | |
| Endocrine | 11/313 (3.5%) | 7/293 (2.4%) | |
| Esophageal | 17/313 (5.4%) | 11/293 (3.8%) | |
| Gastric | 8/313 (2.6%) | 8/293 (2.7%) | |
| Genitourinary | 48/313 (15.3%) | 66/293 (22.5%) | |
| Hematologic | 66/313 (21.1%) | 51/293 (17.4%) | |
| Liver and cholangiocarcinoma | 8/313 (2.6%) | 13/293 (4.4%) | |
| Lung | 62/313 (19.8%) | 40/293 (13.7%) | |
| Musculoskeletal | 1/313 (0.3%) | 2/293 (0.7%) | |
| Nervous system | 3/313 (1%) | 1/293 (0.3%) | |
| Oropharyngeal | 9/313 (2.9%) | 7/293 (2.4%) | |
| Pancreatic | 16/313 (5.1%) | 21/293 (7.2%) | |
| Sarcoma | 12/313 (3.8%) | 8/293 (2.7%) | |
| Other/unspecified primary | 5/313 (1.6%) | 4/293 (1.4%) | |
| Cancer stage | 0.08 | ||
| Local or resectable | 80/276 (29.0%) | 103/271 (38%) | |
| Locally advanced or relapsed | 71/276 (25.7%) | 59/271 (21.8%) | |
| Metastatic or diffuse | 125/276 (45.3%) | 109/271 (40.2%) | |
| Index surgery | 144/303 (47.5%) | 126/291 (43.3%) | 0.30 |
| Surgery within past 30 days from index admission | 154/310 (49.7%) | 134/291 (46.1%) | 0.37 |
| Active cancer therapies | 183/304 (60.2%) | 156/285 (54.7%) | 0.18 |
AF = atrial fibrillation.
Values are mean ± standard deviation (n) or n/N (%).
Antiarrhythmic, antiplatelet and anticoagulant medications at baseline and discharge in patients with cancer and new onset versus preexistent AF
| . | New onset AF (n = 313) . | Preexistent AF (n = 293) . | P value . |
|---|---|---|---|
| Baseline | |||
| Anticoagulant medications | 35/313 (11.2%) | 219/293 (74.7%) | <0.0001 |
| Apixaban | 12 (3.8%) | 139 (47.4%) | |
| Dabigatran | – | 2 (0.7%) | |
| LMWH | 9 (2.9%) | 7 (0.4%) | |
| Rivaroxaban | 14 (4.5%) | 47 (16%) | |
| Warfarin | – | 16 (5.5%) | |
| Antiplatelet medications | 63/306 (20.6%) | 57/293 (19.4%) | 0.73 |
| Aspirin | 52 (16.1%) | 46 (15.7%) | |
| Aspirin/clopidogrel | 6 (1.9%) | – | |
| Ticagrelor | – | 2 (0.6%) | |
| Clopidogrel | 5 (1.6%) | 9 (2.2%) | |
| Antiarrhythmic medications | 1/313 (0.3%) | 28/293 (9.6%) | <0.0001 |
| Amiodarone | 1 (0.3%) | 8 (2.7%) | |
| Dronedarone | – | 5 (1.7%) | |
| Sotalol | – | 5 (1.7%) | |
| Dofetilide | – | 2 (0.7%) | |
| Flecainide | – | 2 (0.7%) | |
| Discharge | |||
| Anticoagulant medications | 105/272 (38.6%) | 198/279 (70.1%) | <0.0001 |
| Apixaban | 49 (18%) | 118 (42.3%) | |
| Dabigatran | – | 2 (0.7%) | |
| LMWH | 365(12.9%) | 26 (9.4%) | |
| Rivaroxaban | 20 (7.4%) | 42 (15.1%) | |
| Warfarin | 1 (0.4%) | 10 (3.6%) | |
| Antiplatelet medications | 46/272 (16.9%) | 42/279 (15%) | 0.28 |
| Aspirin | 40 (14.7%) | 41 (14.7%) | |
| Clopidogrel | 5 (1.8%) | 1 (0.4%) | |
| Aspirin/clopidogrel | 1 (0.4%) | – | |
| Antiarrhythmic medications | 52/272 (19.1%) | 35/279 (12.3%) | 0.024 |
| Amiodarone | 44 (16.2%) | 22 (7.9%) | |
| Dofetilide | – | 1 (0.4%) | |
| Dronedarone | 1 (0.4%) | 4 (1.4%) | |
| Flecainide | 1 (0.4%) | 3 (1.1%) | |
| Sotalol | – | 4 (1.4%) |
| . | New onset AF (n = 313) . | Preexistent AF (n = 293) . | P value . |
|---|---|---|---|
| Baseline | |||
| Anticoagulant medications | 35/313 (11.2%) | 219/293 (74.7%) | <0.0001 |
| Apixaban | 12 (3.8%) | 139 (47.4%) | |
| Dabigatran | – | 2 (0.7%) | |
| LMWH | 9 (2.9%) | 7 (0.4%) | |
| Rivaroxaban | 14 (4.5%) | 47 (16%) | |
| Warfarin | – | 16 (5.5%) | |
| Antiplatelet medications | 63/306 (20.6%) | 57/293 (19.4%) | 0.73 |
| Aspirin | 52 (16.1%) | 46 (15.7%) | |
| Aspirin/clopidogrel | 6 (1.9%) | – | |
| Ticagrelor | – | 2 (0.6%) | |
| Clopidogrel | 5 (1.6%) | 9 (2.2%) | |
| Antiarrhythmic medications | 1/313 (0.3%) | 28/293 (9.6%) | <0.0001 |
| Amiodarone | 1 (0.3%) | 8 (2.7%) | |
| Dronedarone | – | 5 (1.7%) | |
| Sotalol | – | 5 (1.7%) | |
| Dofetilide | – | 2 (0.7%) | |
| Flecainide | – | 2 (0.7%) | |
| Discharge | |||
| Anticoagulant medications | 105/272 (38.6%) | 198/279 (70.1%) | <0.0001 |
| Apixaban | 49 (18%) | 118 (42.3%) | |
| Dabigatran | – | 2 (0.7%) | |
| LMWH | 365(12.9%) | 26 (9.4%) | |
| Rivaroxaban | 20 (7.4%) | 42 (15.1%) | |
| Warfarin | 1 (0.4%) | 10 (3.6%) | |
| Antiplatelet medications | 46/272 (16.9%) | 42/279 (15%) | 0.28 |
| Aspirin | 40 (14.7%) | 41 (14.7%) | |
| Clopidogrel | 5 (1.8%) | 1 (0.4%) | |
| Aspirin/clopidogrel | 1 (0.4%) | – | |
| Antiarrhythmic medications | 52/272 (19.1%) | 35/279 (12.3%) | 0.024 |
| Amiodarone | 44 (16.2%) | 22 (7.9%) | |
| Dofetilide | – | 1 (0.4%) | |
| Dronedarone | 1 (0.4%) | 4 (1.4%) | |
| Flecainide | 1 (0.4%) | 3 (1.1%) | |
| Sotalol | – | 4 (1.4%) |
LMWH = low molecular weight heparin; POAF = postoperative atrial fibrillation.
Values are n/N (%).
Antiarrhythmic, antiplatelet and anticoagulant medications at baseline and discharge in patients with cancer and new onset versus preexistent AF
| . | New onset AF (n = 313) . | Preexistent AF (n = 293) . | P value . |
|---|---|---|---|
| Baseline | |||
| Anticoagulant medications | 35/313 (11.2%) | 219/293 (74.7%) | <0.0001 |
| Apixaban | 12 (3.8%) | 139 (47.4%) | |
| Dabigatran | – | 2 (0.7%) | |
| LMWH | 9 (2.9%) | 7 (0.4%) | |
| Rivaroxaban | 14 (4.5%) | 47 (16%) | |
| Warfarin | – | 16 (5.5%) | |
| Antiplatelet medications | 63/306 (20.6%) | 57/293 (19.4%) | 0.73 |
| Aspirin | 52 (16.1%) | 46 (15.7%) | |
| Aspirin/clopidogrel | 6 (1.9%) | – | |
| Ticagrelor | – | 2 (0.6%) | |
| Clopidogrel | 5 (1.6%) | 9 (2.2%) | |
| Antiarrhythmic medications | 1/313 (0.3%) | 28/293 (9.6%) | <0.0001 |
| Amiodarone | 1 (0.3%) | 8 (2.7%) | |
| Dronedarone | – | 5 (1.7%) | |
| Sotalol | – | 5 (1.7%) | |
| Dofetilide | – | 2 (0.7%) | |
| Flecainide | – | 2 (0.7%) | |
| Discharge | |||
| Anticoagulant medications | 105/272 (38.6%) | 198/279 (70.1%) | <0.0001 |
| Apixaban | 49 (18%) | 118 (42.3%) | |
| Dabigatran | – | 2 (0.7%) | |
| LMWH | 365(12.9%) | 26 (9.4%) | |
| Rivaroxaban | 20 (7.4%) | 42 (15.1%) | |
| Warfarin | 1 (0.4%) | 10 (3.6%) | |
| Antiplatelet medications | 46/272 (16.9%) | 42/279 (15%) | 0.28 |
| Aspirin | 40 (14.7%) | 41 (14.7%) | |
| Clopidogrel | 5 (1.8%) | 1 (0.4%) | |
| Aspirin/clopidogrel | 1 (0.4%) | – | |
| Antiarrhythmic medications | 52/272 (19.1%) | 35/279 (12.3%) | 0.024 |
| Amiodarone | 44 (16.2%) | 22 (7.9%) | |
| Dofetilide | – | 1 (0.4%) | |
| Dronedarone | 1 (0.4%) | 4 (1.4%) | |
| Flecainide | 1 (0.4%) | 3 (1.1%) | |
| Sotalol | – | 4 (1.4%) |
| . | New onset AF (n = 313) . | Preexistent AF (n = 293) . | P value . |
|---|---|---|---|
| Baseline | |||
| Anticoagulant medications | 35/313 (11.2%) | 219/293 (74.7%) | <0.0001 |
| Apixaban | 12 (3.8%) | 139 (47.4%) | |
| Dabigatran | – | 2 (0.7%) | |
| LMWH | 9 (2.9%) | 7 (0.4%) | |
| Rivaroxaban | 14 (4.5%) | 47 (16%) | |
| Warfarin | – | 16 (5.5%) | |
| Antiplatelet medications | 63/306 (20.6%) | 57/293 (19.4%) | 0.73 |
| Aspirin | 52 (16.1%) | 46 (15.7%) | |
| Aspirin/clopidogrel | 6 (1.9%) | – | |
| Ticagrelor | – | 2 (0.6%) | |
| Clopidogrel | 5 (1.6%) | 9 (2.2%) | |
| Antiarrhythmic medications | 1/313 (0.3%) | 28/293 (9.6%) | <0.0001 |
| Amiodarone | 1 (0.3%) | 8 (2.7%) | |
| Dronedarone | – | 5 (1.7%) | |
| Sotalol | – | 5 (1.7%) | |
| Dofetilide | – | 2 (0.7%) | |
| Flecainide | – | 2 (0.7%) | |
| Discharge | |||
| Anticoagulant medications | 105/272 (38.6%) | 198/279 (70.1%) | <0.0001 |
| Apixaban | 49 (18%) | 118 (42.3%) | |
| Dabigatran | – | 2 (0.7%) | |
| LMWH | 365(12.9%) | 26 (9.4%) | |
| Rivaroxaban | 20 (7.4%) | 42 (15.1%) | |
| Warfarin | 1 (0.4%) | 10 (3.6%) | |
| Antiplatelet medications | 46/272 (16.9%) | 42/279 (15%) | 0.28 |
| Aspirin | 40 (14.7%) | 41 (14.7%) | |
| Clopidogrel | 5 (1.8%) | 1 (0.4%) | |
| Aspirin/clopidogrel | 1 (0.4%) | – | |
| Antiarrhythmic medications | 52/272 (19.1%) | 35/279 (12.3%) | 0.024 |
| Amiodarone | 44 (16.2%) | 22 (7.9%) | |
| Dofetilide | – | 1 (0.4%) | |
| Dronedarone | 1 (0.4%) | 4 (1.4%) | |
| Flecainide | 1 (0.4%) | 3 (1.1%) | |
| Sotalol | – | 4 (1.4%) |
LMWH = low molecular weight heparin; POAF = postoperative atrial fibrillation.
Values are n/N (%).
Clinical outcomes
Unadjusted clinical outcomes are presented in Figure 1, Table 5, and Supplementary Table S1. NOAF was associated with increased cumulative hazard of the composite outcome of death from any cause, CVE, or CV rehospitalization (hazard ratio [HR] 1.48, 95% confidence interval [CI] 1.15, 1.91, P = 0.0002) (Table 5 and Figure 1A) as well as the individual endpoint of all-cause death; similarly, patients with NOAF had a higher unadjusted cumulative incidence of the secondary CV composite outcome of clinical AF recurrence, CV death, CVE or CV rehospitalization (Table 5 and Figure 1B), as well as the individual endpoint of clinical AF recurrence, after accounting for the competing risk of death (Table 5). Following adjustment and after accounting for the competing risk of death, patients with NOAF had a significantly higher risk of the primary composite outcome of death, CVE or CV rehospitalization, the CV composite outcome of clinical AF recurrence, CV death, CVE or CV rehospitalization, as well as the individual endpoints of CV death, CV rehospitalization, and clinical AF (Figure 2). Further adjustment including the type of in hospital AF (paroxysmal vs. persistent) and the CHA2DS2-Vasc score did not alter the observed higher risk for the primary composite outcome (HR 1.71, 95% CI 1.24, 2.36, P = 0.0011) or secondary composite outcome (HR 1.55, 95% CI 1.07, 2.29, P = 0.024) observed in patients with NOAF compared with patients with preexistent AF. There was no difference in the overall adjusted risk of stroke between the two groups (HR 2.2, 95% CI 0.86, 5.73, P = 0.09). When examining types of CV rehospitalizations, AF-related rehospitalizations were more common in patient with NOAF compared with patients with preexistent AF (11.2% vs. 3.8% respectively, P < 0.0001); after accounting for the competing risk of death, there was a trend towards an increased overall adjusted risk for AF-related rehospitalization in patients with NOAF compared with patients with preexistent AF but this did not reach statistical significance (HR 0.72, 95% CI 0.97, 3.05, P = 0.063).
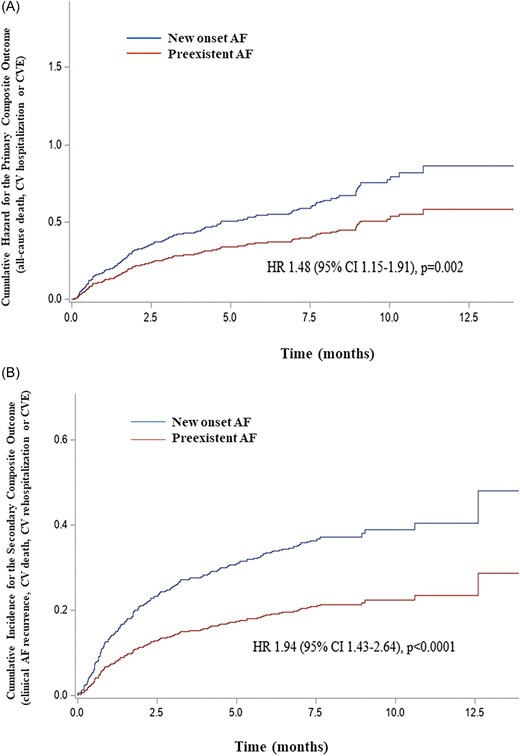
(A) Cumulative hazard of the primary composite outcome of all-cause death, CV rehospitalization, or CVE in hospitalized patients with cancer and clinically manifest new onset or preexistent AF. The unadjusted hazard for the primary composite outcome of all-cause death, CV rehospitalization, or CVE was higher in hospitalized patients with cancer and manifest NOAF compared with preexistent clinically manifest AF. (B) Cumulative hazard of the secondary CV composite outcome of clinical AF recurrence, CV death, CV rehospitalization, or CVE in hospitalized patients with cancer and clinically manifest new onset or preexistent AF. After accounting for the competing risk of death, the unadjusted hazard for the secondary CV composite outcome of clinical AF recurrence, CV death, CV rehospitalization, or CVE was higher in hospitalized patients with cancer and manifest NOAF compared with preexistent clinically manifest AF. AF = atrial fibrillation; NOAF = new onset atrial fibrillation; CV = cardiovascular; CVE = cerebrovascular event; HR = hazard ratio; CI = confidence interval.
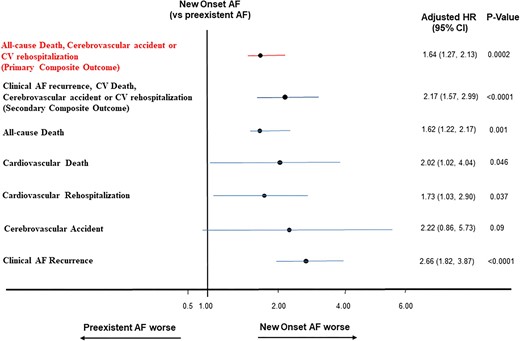
Adjusted risk of adverse outcomes in hospitalized patients with cancer and clinically manifest new onset or preexistent AF. NOAF conferred a higher adjusted hazard risk for the primary composite outcome of all-cause death, CV rehospitalization, or CVE, the secondary CV composite outcome of clinical AF recurrence, CV death, CV rehospitalization or CVE and the individual outcomes of CV rehospitalization, all-cause and CV death, but not CVE, after accounting for the competing risk of death, in hospitalized patients with cancer and manifest NOAF compared with preexistent clinically manifest AF. AF = atrial fibrillation; NOAF = new onset atrial fibrillation; CV = cardiovascular; CVE = cerebrovascular event; HR = hazard ratio; CI = confidence interval.
Unadjusted cumulative incidence hazard function in patients with cancer and new onset versus preexistent AF
| . | New onset AF (versus preexistent AF) . | |
|---|---|---|
| . | (HR, 95% CI) . | P value . |
| Primary composite outcome (all-cause death, CVE, or CV rehospitalization) | 1.48 (1.15, 1.91) | 0.002 |
| Secondary composite outcome (clinical AF recurrence, CV death, CVE, or CV rehospitalization) | 1.94 (1.43, 2.64) | <0.0001 |
| All-cause death | 1.48 (1.12, 1.97) | 0.007 |
| Cardiovascular death | 1.46 (0.76, 2.81) | 0.25 |
| Cerebrovascular accident | 2.22 (0.86, 5.73) | 0.09 |
| CV rehospitalization | 1.45 (0.92, 2.48) | 0.074 |
| Clinical AF recurrence | 2.62 (1.80, 3.81) | <0.0001 |
| . | New onset AF (versus preexistent AF) . | |
|---|---|---|
| . | (HR, 95% CI) . | P value . |
| Primary composite outcome (all-cause death, CVE, or CV rehospitalization) | 1.48 (1.15, 1.91) | 0.002 |
| Secondary composite outcome (clinical AF recurrence, CV death, CVE, or CV rehospitalization) | 1.94 (1.43, 2.64) | <0.0001 |
| All-cause death | 1.48 (1.12, 1.97) | 0.007 |
| Cardiovascular death | 1.46 (0.76, 2.81) | 0.25 |
| Cerebrovascular accident | 2.22 (0.86, 5.73) | 0.09 |
| CV rehospitalization | 1.45 (0.92, 2.48) | 0.074 |
| Clinical AF recurrence | 2.62 (1.80, 3.81) | <0.0001 |
AF = atrial fibrillation; CI = confidence interval; CV= cardiovascular; CVE = cerebrovascular event; HR = hazard ratio.
Unadjusted cumulative incidence hazard function in patients with cancer and new onset versus preexistent AF
| . | New onset AF (versus preexistent AF) . | |
|---|---|---|
| . | (HR, 95% CI) . | P value . |
| Primary composite outcome (all-cause death, CVE, or CV rehospitalization) | 1.48 (1.15, 1.91) | 0.002 |
| Secondary composite outcome (clinical AF recurrence, CV death, CVE, or CV rehospitalization) | 1.94 (1.43, 2.64) | <0.0001 |
| All-cause death | 1.48 (1.12, 1.97) | 0.007 |
| Cardiovascular death | 1.46 (0.76, 2.81) | 0.25 |
| Cerebrovascular accident | 2.22 (0.86, 5.73) | 0.09 |
| CV rehospitalization | 1.45 (0.92, 2.48) | 0.074 |
| Clinical AF recurrence | 2.62 (1.80, 3.81) | <0.0001 |
| . | New onset AF (versus preexistent AF) . | |
|---|---|---|
| . | (HR, 95% CI) . | P value . |
| Primary composite outcome (all-cause death, CVE, or CV rehospitalization) | 1.48 (1.15, 1.91) | 0.002 |
| Secondary composite outcome (clinical AF recurrence, CV death, CVE, or CV rehospitalization) | 1.94 (1.43, 2.64) | <0.0001 |
| All-cause death | 1.48 (1.12, 1.97) | 0.007 |
| Cardiovascular death | 1.46 (0.76, 2.81) | 0.25 |
| Cerebrovascular accident | 2.22 (0.86, 5.73) | 0.09 |
| CV rehospitalization | 1.45 (0.92, 2.48) | 0.074 |
| Clinical AF recurrence | 2.62 (1.80, 3.81) | <0.0001 |
AF = atrial fibrillation; CI = confidence interval; CV= cardiovascular; CVE = cerebrovascular event; HR = hazard ratio.
During the index hospitalization, 41 (13.5%) patients with NOAF died compared with 14 (4.8%) patients with preexistent AF (P = 0.031); among those that died, CV death occurred in 7/41 (17.1%) patients with NOAF and 2/14 (14.3%) patients with preexistent AF (P = 0.31). During the index admission, 5/313 (1.6%) patients with NOAF had a CVE compared to none in patients with preexistent AF (P ≤ 0.0001). Among the patients who were alive at discharge, 81.9% of NOAF patients were in sinus rhythm on discharge compared with 45.6% of patients with preexistent AF (P < 0.0001). Following discharge, new antiarrhythmic drug therapy was prescribed at follow-up in 25/271 (9.2%) patients with NOAF and 10/279 (3.6%) patients with preexistent AF (P = 0.005). De novo implementation of anticoagulant therapy or left atrial appendage occlusion was noted at follow-up in 39/272 (14.3%) of patients with NOAF compared with 19/279 (6.8%) patients with prior AF (P = 0.004). Cardiac rhythm monitoring was performed in 40/246 (16.3%) of patients with NOAF, as well as in 14/99 (14.1%) of patients with a prior history of AF, who were noted to be in sinus rhythm at discharge (P = 0.013). Recurrent AF was detected in 44.2% of monitored patients with NOAF and 47% of monitored patients with preexistent AF (P = 0.25). Following discharge, 9/272 (3.3%) of patients with NOAF suffered a CVE compared with 6/273 (2.2%) patients with prior AF (P = 0.28). Among these cases, 7/9 patients with NOAF and 5/6 patients with prior AF were not on anticoagulant therapy prior to the event.
Discussion
In the present study from the MSKCC AF registry, which included patients with cancer hospitalized for at least 24 h with documented new or preexistent AF on electrocardiography, we showed that (i) patients with NOAF were younger, less obese, and had less frequent prior cardiac history compared with patients with preexistent AF; (ii) in-hospital adverse outcomes, including death and stroke, were more frequent in patients with NOAF; (iii) the adjusted cumulative risk for the composite outcome of death, CV rehospitalization or CVE, the secondary CV composite outcome of clinical AF recurrence, CV death, CVE, and CV rehospitalization as well as the individual endpoints of death, CV death and CV rehospitalization were higher in patients with NOAF compared with patients with preexistent AF; and (iv) patients with NOAF had more frequent clinical AF recurrence requiring addition of antiarrhythmic and anticoagulant therapy at follow-up, compared with patients with preexistent AF.
To the best of our knowledge, the present study is the first study to assess the clinical implications of newly diagnosed AF occurring in patients with cancer in the acute hospitalization setting, as compared to recurrent AF in cancer patients with a prior history of AF; further, the present study addressed the association of NOAF and the requirement for CV therapy escalation as well as the risk for CV readmission as compared with patients with a prior AF diagnosis. The present analyses were prespecified to reflect current clinical practice and are thus widely applicable. Prior studies have reported an overall increased incidence of AF in patients with cancer compared to the general population and long-term adverse impact of new diagnosis of AF.3,6,8,9 Importantly, whether AF triggered in the setting of acute illness, such as infection, surgery, or initiation of cancer therapies, is of long-term clinical significance and should be managed per current guidelines in the general population has not been previously clearly elucidated. Results from the present study affirm that NOAF is a significant marker of short- and long-term adverse prognosis. It also confers significantly elevated risk for adverse CV outcomes compared with preexistent and electrocardiographically manifest AF diagnosis in the setting of acute hospitalization of patients with cancer.
In the present study, the adjusted risk for individual CV outcomes, such as CV death and CV readmissions, was significantly elevated in patients with NOAF. Recent advances in the field of oncology have led to a dramatic improvement in the overall survival of patients with cancer; in turn, as the life expectancy of patients with cancer has increased, their risk of adverse CV outcomes has also increased and often exceeds the cancer-related risk.3,10 As such, recognizing the prognostic implications of AF, the most common arrhythmia diagnosed in the acute inpatient setting among patients with cancer is essential and underscores the need for implementation of aggressive monitoring and standard preventive and therapeutic strategies, similar to the strategies typically applied to patients with a prior AF diagnosis.11,12 Alternatively, AF that occurs de novo may be an indirect, rather than direct, marker of increased atherothrombotic burden and immunologic disturbances that typically coexist or overlap in patients with cancer.10 Nonetheless, the increased risk for CV readmission and escalation of AF therapies in patients with in-hospital NOAF suggests that early initiation of cardioprotective and antithrombotic therapies after discharge may reduce CV morbidity and mortality and also improve quality of life.
Several observations related to presentation, management, and outcomes of either new or preexistent AF are noteworthy. First, patients with NOAF were younger, had less frequent concurrent CV disease, and had fewer structural abnormalities by echocardiographic imaging, underscoring the difficulty in determining cancer patients at risk for recurrent AF or other CV events, such as stroke and CV readmissions. Length of hospital stay was longer in patients with NOAF and these patients also more frequently required antiarrhythmic therapy, implicating AF as a significant culprit for prolonged hospitalizations. Second, approximately half of patients with a prior history of AF were noted to be in persistent or permanent AF; nonetheless, utilization of antiarrhythmic therapy was low and noted in approximately 20% of patients with known paroxysmal AF. Similarly, antiarrhythmic therapy on discharge was relevantly limited in both groups, though both antiarrhythmic and anticoagulant therapy initiation after discharge (but not at discharge) was more frequently noted in patients with NOAF, typically occurring after recurrent AF episodes or AF-related adverse events, such as stroke. Underutilization of anticoagulant therapy in patients with cancer is often limited by elevated bleeding risk or at end of life11,13,14; however, a further contributing factor in the underutilization of anticoagulation relates to the ongoing controversy as to the risk for recurrent AF and associated adverse outcomes following an in hospital AF episode. The present study suggested a similar risk for CVEs in patients with NOAF compared with patients with established thromboembolic risk related to AF. Importantly, most CVEs after discharge occurred in patients who were not on anticoagulant therapy at the time; as such, assessment of the risk and benefit balance for anticoagulation implementation should be performed early in all cancer patients with NOAF who have indications for anticoagulant therapy. Whether risk stratification through application of the CHA2DS2-Vasc score is sufficient in the cancer population remains undetermined and is a topic of ongoing research.15 In terms of antiarrhythmic therapy, as with anticoagulation, in the present study we showed that it was rather sparsely utilized and cardiac rhythm monitoring was often not performed for screening for recurrent occult AF. Given recent evidence from the general population favoring early initiation of a rhythm control strategy for prevention of adverse CV outcomes, mainly CV readmissions,16 whether a more rigorous approach to cardiac rhythm monitoring and early institution of antiarrhythmic therapies would benefit the cancer population should be investigated in dedicated studies. Last, results from the present study suggest that even within a relatively short follow-up period, rates of adverse CV events in hospitalized cancer patients with NOAF are frequent and contribute to morbidity and mortality in this complex patient population; as such, occurrence of NOAF in patients with cancer in the acute hospitalization setting should be managed rigorously and following standard guidelines for medical therapy as in patients with established AF.
Study strengths and limitations
The present cohort study was based on a single centre clinical registry, was not randomized, and had varied clinical follow-up periods; the results should thus only be considered hypotheses generating. Recurrent AF and therapy escalation during follow-up was recorded only if clinically manifest or documented by electrocardiography or cardiac rhythm monitoring and as such, asymptomatic or transient AF episodes may not have been captured. We cannot exclude some overlap between NOAF and preexistent AF as history of AF was ascertained through medical record review. In addition, the present study assessed only cancer patients acutely hospitalized largely for a non-AF-related reason; as such, our results may not apply to patients seen in routine outpatient practice. Hospitalized patients with a prior AF history who did not have a documented AF episode on electrocardiography or cardiac telemetry were not included in the study. Overall rates of certain individual endpoints, including stroke and AF-related rehospitalization, were low and larger studies are needed to confirm results reported in this analysis. Finally, the present study was not designed to determine the safety, efficacy, or the optimal monitoring, pharmacologic or invasive approaches to NOAF; as such, we cannot exclude medication non-adherence and subtherapeutic anticoagulation as potential confounders in the present analyses.
Conclusions
In the MSKCC AF registry, hospitalized patients with cancer and clinically manifest NOAF had a higher adjusted risk for the composite outcomes of death, CV hospitalization or CVE, as well as the secondary CV composite outcome of clinical AF recurrence, CV death, CV hospitalization or CVE.
Funding
This work was supported by National Cancer Institute grant P01 CA23766 and National Cancer Institute Cancer Center Support Grant P30 CA008748.
Data availability statement
The data underlying this article are available in the article and in its online supplementary material. Additional data not presented in this article will be shared on reasonable request to the corresponding author.
Disclosures
Dr Shaffer reports consulting honoraria from Gamida Cell and Hansa BioPharma Inc and research support from Merck, Inc. Dr Liu reports speaker honoraria from GE Healthcare and Philips Medical, research grant support from Johnson and Johnson and DSMB membership honoraria from Caelum Biosciences. Dr Kosmidou has received research support from Amgen Inc and consulting honoraria from Sanofi Inc and Pfizer Inc. The other authors report no relevant conflicts of interest.



