-
PDF
- Split View
-
Views
-
Cite
Cite
Samuel Seitler, Mohamed Zuhair, Aamir Shamsi, Jonathan J H Bray, Alexandra Wojtaszewska, Atif Siddiqui, Mahmood Ahmad, Jonathan Fairley, Rui Providencia, Abid Akhtar, Cardiac imaging in rheumatic heart disease and future developments, European Heart Journal Open, Volume 3, Issue 2, March 2023, oeac060, https://doi.org/10.1093/ehjopen/oeac060
Close - Share Icon Share
Abstract
Rheumatic heart disease (RHD) is the most common cause of valvular heart disease worldwide, affecting millions, especially in low- and middle-income countries. Multiple imaging modalities such as cardiac CT, cardiac MRI, and three-dimensional echocardiography may be utilized in diagnosing, screening, and managing RHD. However, two-dimensional transthoracic echocardiography remains the cornerstone of imaging in RHD. Criteria developed by the World Heart Foundation in 2012 sought to unify the diagnostic imaging criteria for RHD, but concerns remain regarding their complexity and reproducibility. In the intervening years, further measures have been developed to find a balance between simplicity and accuracy. Nonetheless, there remain significant unresolved problems within imaging in RHD, including the development of a practical and sensitive screening tool to identify patients with RHD. The emergence of handheld echocardiography has the potential to revolutionize RHD management in resource-poor settings, but its role as a screening or diagnostic tool is yet to be fully established. The dramatic evolution of imaging modalities over the last few decades has not addressed RHD compared to other forms of structural heart disease. In this review, we examine the current and latest developments concerning cardiac imaging and RHD.
Introduction and background
Rheumatic heart disease (RHD) is a chronic valvular heart disease with a significant burden in low- and middle-income countries. It is believed to affect up to 40 million people worldwide and contribute to half a million deaths per year.1 RHD is a long-term consequence of untreated acute rheumatic fever (ARF), an autoimmune response to infection with Group A Streptococcus (Strep A), with ARF-associated carditis leading to lasting damage to heart valves. The exact pathophysiology is still being clarified through further research, but RHD is thought to develop due to an abnormal immune response to Strep A infection which cross-reacts with host antigens because of molecular mimicry.2 This abnormal immune response leads to inflammation of the valves and myocardium due to immune complex formation. While myocardial inflammation is transient, valvular regurgitation and/or stenosis may persist, resulting in myocardial dilatation, fibrosis, and left atrial dilatation. The chronic cardiac damage resulting from ARF is termed RHD.3 Morphologically, RHD is typified by valvular fibrosis and stenosis, with the presence of inflammatory Aschoff bodies, pathognomonic of RHD.4 RHD presents clinically with valvular heart failure, arrhythmias, and pulmonary hypertension, with the mitral valve (MV) being the most commonly affected valve. Patients may also present with complications of progressive or advanced RHD such as atrial fibrillation, ischaemic stroke, native valve endocarditis, as well as maternal and neonatal morbidity and mortality.5
2D echocardiography is still the gold standard diagnostic test for RHD. Echocardiography can identify and measure the severity of acute valvular disease, and cardiac function status, monitor disease progression, decide suitability, and plan for intervention where indicated. In 2012, the World Heart Federation (WHF) developed the first evidence-based criteria for the echocardiographic diagnosis of RHD in patients without a history of ARF.6 This included latent RHD, where asymptomatic RHD is diagnosed through echocardiographic screening with or without clinical signs such as a pathological murmur (Figures 1 and 2). Alternative diagnostic tools are beneficial in detecting RHD but remain inferior to echocardiography but can still provide valuable supplementary information. Clinical features suggestive of RHD may include signs of heart failure, most notably progressive exertional dyspnoea. Electrocardiographic findings in RHD may demonstrate left atrial or left ventricular enlargement and ventricular strain, but these are not specific to RHD. Atrial fibrillation may occur due to severe MV disease.7 Transoesophageal and three-dimensional (3D) echo are valuable tools in assessing RHD, but their utility is limited by the availability of technology and required procedural expertise. Transoesophageal echocardiography (TOE) plays a key role in planning surgical intervention and post-operative assessment of procedural success (Figures 3 and 4). 3D echo is advantageous in cases where 2D echo images are suboptimal or inconclusive. There is some emerging evidence that it may be more accurate in assessing suitability for valve surgery.8 Finally, cardiac MRI can identify the impact of valvular disease on the myocardium and thus predict post-repair outcomes. However, it is limited in its utility as a screening or diagnostic tool due to cost, scanner availability, and technical expertise.9
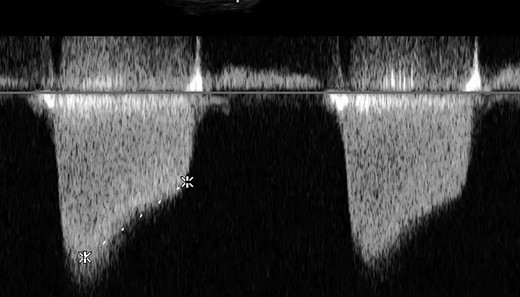
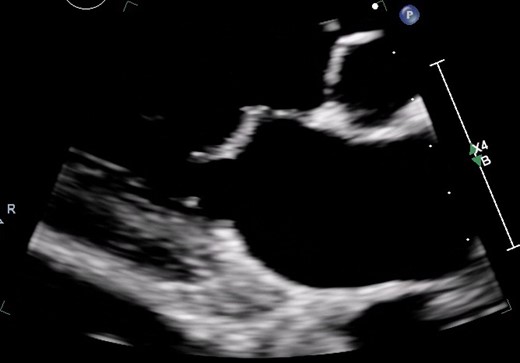
Increased velocity time integral indicating mitral stenosis on transoesophageal echocardiography (TOE).
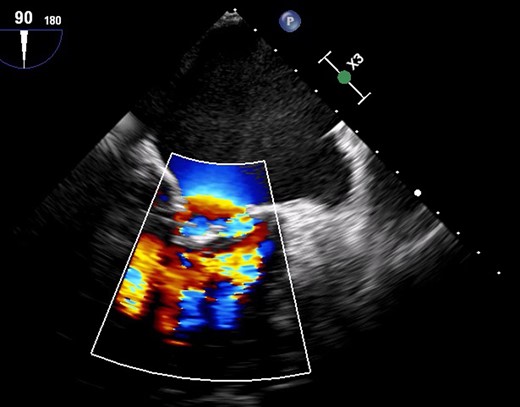
Colour Doppler due to severe mitral regurgitation and stenosis.
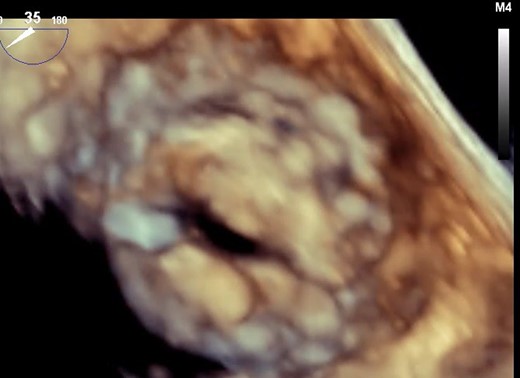
3D TOE for rheumatic heart disease-related mitral stenosis. TOE, transoesophageal echocardiography.
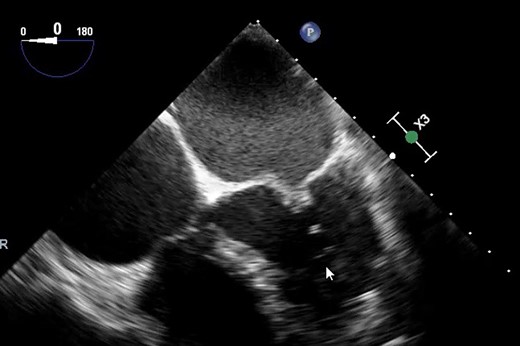
Classical hockey stick appearance on transoesophageal echocardiography (TOE).
With timely therapy, advanced RHD may be preventable, and over the past 30 years, the global health burden of RHD has lessened. Nonetheless, it remains endemic in developing areas and low-income nations. According to a 2015 Global Burden of Diseases study, prevalence is highest in south Asia and sub-Saharan Africa, with rates of >1000 cases per 100 000 population and rates of RHD are closely interlinked with health inequality. Improvements in national socioeconomic and health conditions result in an apparent reduction in RHD mortality.10 RHD is a chronic disease, a consequence of recurrent episodes of ARF, it is viewed as preventable and treatable. As such, significant efforts have been dedicated to the early detection and eradication of RHD. In this review article, we will discuss the latest developments and guidelines in cardiac imaging in RHD with a focus on the emerging role of handheld echocardiography. Several echocardiographic-based criteria have been developed, each with its limitations which we will review and compare.11 Finally, we will discuss the potential benefits and disadvantages of handheld echocardiography in RHD, considering its use as a screening tool in endemic areas.
Structural abnormalities caused by RHD and findings by imaging modality
Two-dimensional echocardiography
Initial attempts at diagnosing and screening for RHD primarily consisted of cardiac auscultation. The advent of echocardiography has changed this, notably as many studies have demonstrated the superiority of echocardiography in diagnosing and screening for RHD compared to cardiac auscultation alone.12,13 Two-dimensional echocardiography is the cornerstone of screening for RHD. In 2012, the WHF created evidence-based criteria for diagnosing RHD.6 They aimed to make echocardiography reporting simple, reproducible, and consistent worldwide to facilitate screening for RHD. The WHF guidelines specify that echocardiography should be interpreted in light of an individual’s clinical findings and the risk or likelihood of having RHD to determine their diagnosis.
The key echocardiographic findings in RHD are morphological and pathological abnormalities of the mitral or aortic valve. The mitral valves in RHD are thickened with doming and restricted mobility, producing the characteristic ‘elbow’ or ‘dog leg’ deformity of the anterior mitral valve leaflet. Mitral regurgitation (MR) is the most common abnormality in RHD, with a posteriorly directed jet visible in at least two views. There is also usually left atrial enlargement in severe MR. Mitral stenosis (MS) may develop due to fibrosis, and valvular thickening, secondary to chronic rheumatic inflammation and is considered RHD if the mean gradient is ≥4 mmHg.6 Mitral stenosis severity can be graded by mitral valve area (MVA), measured by planimetry or pressure half-time.14 M-mode echo may demonstrate a reduced E-F slope or absent A-wave of the anterior mitral leaflet. There may also be evidence of right atrial or ventricular enlargement due to pulmonary hypertension.15 Aortic valve disease is less common than mitral valve disease and may also be regurgitant or stenotic. Aortic valve leaflets are thickened with commissural fusion, restricted leaflet motion, and a central jet of aortic regurgitation (AR) from incomplete coaptation. Aortic stenosis may occur because of AR and valvular thickening.
Two-dimensional (2D) echocardiography has long been the mainstay imaging modality for monitoring RHD patients, with MVA measurement traditionally used to assess MS severity and guide the timing of surgical intervention. A new 2D echocardiography measurement has been developed: the ‘mitral leaf separation index’, defined as the average distance between mitral valve leaflet tips in parasternal long-axis and four-chamber 2D echocardiography views. Raafat et al. demonstrated that this new measurement correlated strongly with MVA by 2D planimetry when assessing the MV both pre- and post-percutaneous balloon mitral valvuloplasty and with MVA using pressure half-time following percutaneous balloon mitral valvuloplasty (r = 0.768, P = 0.0001).16 This novel measurement provides an alternative and potentially more accurate way of assessing the severity of MS in RHD patients.
In addition to screening and diagnosis, 2D echocardiography can aid in prognostic evaluation in RHD patients undergoing surgery. In the retrospective observational study, Borde et al. demonstrated that RHD patients with MS and severe pulmonary arterial hypertension (PAH) had much higher mortality on longer-term follow-up than patients with mild or no PAH.17 Furthermore, echocardiographic measurements can also help to predict the onset and assess the severity of tricuspid regurgitation progression following valvular surgery in RHD patients (a common complication). Kim et al.'s retrospective analysis showed that patients with a substantial reduction in post-operative pulmonary arterial systolic pressure (PASP) and right ventricular systolic pressure—defined as a greater than 7.0 mmHg reduction in PASP or 8.0 mmHg reduction in right ventricular systolic pressure—were protected against TR progression at 10 years14 (hazard ratio [95% confidence interval], 0.966 [0.942–0.991], P = 0.008 and 0.973 [0.960–0.986], P < 0.001, respectively).18
Colour Doppler is an essential component in the echocardiographic assessment of RHD for identifying and quantifying pathological regurgitation. The WHF criteria state that for MR, the regurgitant jets must be pan-systolic, high velocity—defined as greater than 3 m/s and visible from two views in order to be considered pathological. The regurgitant jet must also be at least 2 cm in length in one view. The regurgitant jet in AR is a pan-diastolic, high-velocity jet, visible in two views, and measuring at least 1 cm in length in one view. The WHF recommend that for both AR and MR, all four diagnostic criteria must be met to distinguish physiological from pathological regurgitation.6 Alternative, simplified criteria for screening echocardiography utilizing colour Doppler have been proposed, assessing jet length in a single view with varying degrees of sensitivity and specificity.11
Transoesophageal echocardiography
While less widely available than 2D transthoracic echocardiography (TTE), TOE can help clarify disease mechanisms or severity where this is unclear. TOE can also help aid intervention planning, including excluding left atrial appendage thrombus prior to percutaneous balloon mitral valvuloplasty in patients with rheumatic mitral stenosis, and clarifying suitability for valve repair or replacement. TOE has also been used to look at post-operative results, with Verdonk et al. showing that 9% of patients post-TOE have a paravalvular leak.19 TOE is also useful for evaluation during surgery and is especially useful for minimally invasive surgery.20 It is also useful for looking at left atrial appendage and left atrial sizes, which Jain et al. found were enlarged in RHD patients, with the mean left atrium diameter being significantly higher in RHD patients than in non-RHD patients (52.08 ± 10.13 vs. 46.67 ± 6.78 mm, P = 0.001).21
Handheld echocardiography
Handheld 2D echocardiography may have an emerging role in screening for RHD. At the time, the WHF criteria were developed, handheld technology had limited functionality and therefore the WHF criteria were not for application with these machines. However, the capacity of handheld devices has progressed significantly, leading to their use in screening studies. Portable echo is less expensive, allows increased portability, and can be largely reliant on the battery instead of needing reliable electricity. At the same time, handheld devices lack functionality (e.g. spectral Doppler, which is needed for WHF criteria), and most of the research on handheld devices has been performed on a single system (GE Vscan TM), although other systems are increasingly available. Some studies demonstrate 79% sensitivity and 87% specificity for all latent RHD, improving to 98% sensitivity for definite RHD. However, up to one-third of borderline RHD cases may be missed, even by experts.22
Three-dimensional echocardiography
More recently, clinicians have used 3D echocardiography in the valve assessment for RHD patients, with evidence that it may be more accurate than conventional 2D imaging at assessing suitability for valve surgery. Mahia et al.'s prospective study of 109 RHD patients showed that measuring 3D valve areas (adjusted for body surface area) using TTE to measure tricuspid annulus dilatation helped to reclassify patients in favour of surgical intervention in 14% of cases with mild tricuspid regurgitation (95% CI 1–15%; P = 0.03) and 37% with moderate tricuspid regurgitation (95% CI 22–37%; P < 0.0001) when compared with conventional 2D TTE dimensional criteria.23 However, the number of patients from this study who underwent surgery is not established.
3D TTE measurements have also been used to assess TR severity and progression pre- and post-mitral valve replacement. Zhong et al.'s retrospective study of 170 RHD patients demonstrated that measuring the septal-lateral tricuspid valve tenting area was correlated with pre-operative functional TR severity (P < 0.001), while septal-lateral tricuspid valve annular diameter independently determined residual tricuspid regurgitation at one-year follow-up post-mitral valve replacement +/− tricuspid valve annuloplasty (P < 0.001).24
The 3D TOE can help clarify the MVA by planimetry where transthoracic images are suboptimal. 3D TOE also offers accurate and valuable MV measurements that help classify MS severity and guide management. Traditionally, MVA is used in 3D echocardiography; however, in Gok et al.'s 2018 study of 40 RHD patients, 3D TOE measurement of the vena contracta area (measured in end-diastole during its largest dimensions) was highly correlated with the 3D TOE MVA (r = 0.82, P < 0.001).25 This measurement may, therefore, be practical and could be combined with MVA to assess the severity of MS in RHD patients more accurately.
3D TTE and TOE were used in Shojaeifard et al.'s prospective study to compare pre- and post-mitral valve surgery outcomes in RHD patients with severe MR. Using measurements of right ventricular free wall longitudinal strain pre- and post-mitral valve commissurotomy (PMC), they demonstrated significant and rapid improvement in right ventricular function following surgery. Pre-surgery, the mean longitudinal strain was −19.00 ± 5.14% and this rose significantly to −20.97 ± 3.81 (P < 0.05) after PMC.26
Cardiac CT
Cardiac Computed Tomography (CT) utilizes X-ray beams and contrast injection to acquire cross-sectional images of a structure or region of interest. CT can provide comprehensive additional information on the integrity and mobility of valves and aid in the identification of paravalvular pathology.27 CT also facilitates a detailed assessment of valve thickness, calcification, and annular fibrosis, in addition to information on the subvalvular structures and left ventricle.28 Beaudoin et al. demonstrated that CT reconstruction and assessment of mitral valve leaflets correlate closely with 3D echocardiography in standard or regurgitant valves.29 CT is utilized in the pre-operative evaluation before MV repair and can help predict clinical outcomes following surgery. CT may be preferable to echo for follow-up imaging after MV repair due to enhanced special resolution and reduced interference from metal artefact.30,31 Within RHD, CT has been demonstrated to assess MV calcification more reliably than echo or CMRI and may be employed to aid quantification of MS severity through assessment of MVA. Studies have shown that MVA measurements acquired by dual-source CT were closely correlated with TTE MVA measurements by both planimetry and pressure half-time and may be beneficial in patients with limited acoustic windows.28,29 Finally, cardiac CT can identify insufficient coaptation of aortic valve leaflets and pathognomonic central regurgitation. Despite the potential benefits of cardiac CT, the potential risk from ionizing radiation and iodine-based contrast precludes its utility and widespread use.32 Nonetheless, as CT technology develops and radiation doses are reduced, cardiac CT remains a propitious tool for evaluating RHD, especially in patients with poor echocardiographic windows.
Cardiac MRI
Cardiac magnetic resonance imaging (CMRI) is another imaging modality that may prove beneficial in assessing the impact of RHD on cardiac function and can be utilized to determine the aetiology of left ventricular dysfunction and aid quantification of regurgitant volumes. Soesanto et al.'s prospective study of 36 RHD patients awaiting MV surgery looked to assess the correlation between left ventricular myocardial fibrosis using late gadolinium enhancement (LGE) on CMRI with the echocardiographic global longitudinal strain (GLS) and MVA measurements.33 It showed that while there was a moderate correlation between GLS and LGE (r = − 0.432, P = 0.009), there was no correlation between LGE and MVA, the standard parameter for assessment of MS (r = 0.149, P = 0.076). This study suggests that while CMRI may not aid in the classification of valvular disease severity, it may help to assess the impact of the valvular disease on the myocardium. However, Uyger et al. concluded that CMRI may be utilized in the assessment of MS severity, demonstrating that planimetric MVA measurements on CMRI were highly correlated with 3D TOE measures (P < 0.0001, r = 0.744).34
Similarly, CMRI has demonstrated favourable cardiac remodelling following balloon mitral valvuloplasty. Samaanet et al. performed interval CMRI scans on 30 patients with rheumatic mitral stenosis who underwent balloon mitral valvotomy (BMV) and showed improvements in Left Ventricle (LV) global longitudinal and circumferential strain, LV end-systolic volume, and ejection fraction at 6 months and 12 months post-valvuloplasty.35 CMRI assessment supports increased availability of BMV for patients with rheumatic MS by demonstrating post-procedural favourable remodelling of the LV.
The ability of CMRI to accurately estimate LV myocardial fibrosis may also be used in the prognostic assessment of morbidity in RHD patients following surgery. Putra et al.'s prospective observational study performed pre-operative CMRI on 47 RHD patients with significant MS.36 It found that there was a significant mean difference in myocardial fibrosis volume, assessed using LGE, between patients with and without morbidity after MV surgery (5.97 ± 4.16 and 3.12 ± 2.62%, P = 0.04) where morbidity was defined as stroke, renal failure, or prolonged mechanical ventilation. This study indicates that pre-operative myocardial fibrosis is associated with poorer post-operative outcomes in RHD patients undergoing MV surgery and hence could help determine patients’ suitability for surgical intervention.
CMRI allows for detailed assessment and evaluation of regurgitant valves. Velocity-encoded phase contrast CMR provides a precise visual evaluation of the signal void produced by the regurgitant jet, affording measurements of pressure gradients and regurgitant volumes across the affected valve.37 Quantifying MR severity on CMRI correlates well with 2D echocardiography without systematic overestimating.38 MR severity may be calculated by assessing the regurgitant fraction—the ratio of regurgitant volume to the LV stroke volume or as the comparison between LV stroke volume and aortic outflow stroke volume. Alternative methods include a comparison of ventricular stroke volumes, where the difference between right and left ventricle stroke volumes represents the MR volume, or by direct MR volume measurement using phase contrast aligned to the plane of the MR jet.39 One advantage of CMRI over echocardiography is the capability to calculate MR severity regardless of the regurgitant jet shape or direction. Still, concurrent cardiac arrhythmias may impact image quality and MR assessment on CMRI.40
RHD imaging guidelines
In 2010, the World Health Organisation (WHO) and National Institutes of Health (NIH) published consensus case definitions for RHD, focusing on the use of echocardiography, which was not the case in previous guidelines. These definitions were criticized for lack of a solid evidence base, insufficient inclusion of normal echocardiographic variants, and inadequate consideration of all possible morphological features of RHD.6 Therefore, in 2012, the WHF sought to improve the WHO/NIH definitions and created updated evidence-based criteria for diagnosing RHD from echocardiographic, surgical, and pathological information that considered normal variants in adults and children. These WHF criteria were the first to include both morphological and functional aspects of mitral and aortic valve disease in RHD, but had the disadvantage of being resource intensive.
The WHF criteria have been further adapted into the significant available international RHD guidelines—namely the Australian41 and New Zealand42 RHD guidelines, as well as the American College of Cardiology Consensus statement on RHD.22
WHF criteria 2012
The WHF criteria for RHD divide patients into three groups: definite RHD, borderline RHD, and normal. The full diagnostic criteria are summarized in Table 1. A diagnosis of definite RHD requires (a) pathological mitral or aortic regurgitation (MR/AR) alongside two morphological features of valvular RHD, or (b) MS with a mean gradient of >4 mmHg, or (c) borderline disease of both the aortic and mitral valve (Figures 5 and 6). The borderline RHD category was established to improve the sensitivity of echocardiography for individuals from areas with a high prevalence of RHD. Due to their age, they may not have sufficient time to develop the full echocardiographic features of definite RHD.36 Conversely, to avoid overdiagnosing RHD, the WHF criteria state that the borderline category is not applicable to those >20 years old as these individuals are more likely to have minor age-related changes that can be mistaken for early RHD in someone younger. Depending on their categorization, patients either undergo routine echocardiographic surveillance are offered secondary prophylactic penicillin therapy, are assessed for invasive intervention, medically managed for any RHD complications, or are reassured that they do not have RHD (Table 2).
Severe rheumatic mitral valve disease on transthoracic echocardiography (TTE).
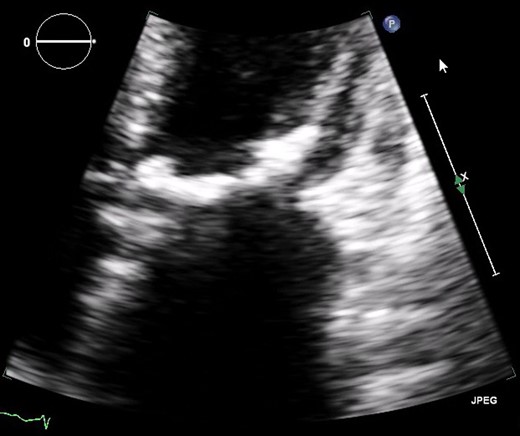
Severe mitral valve calcification on transthoracic echocardiography (TTE).
| Echocardiographic criteria for individuals aged ≧20 years |
| Definite RHD (either A, B, C, or D): |
| (A) Pathological MR and at least two morphological features of RHD of the MV |
| (B) MS mean gradient ≥4 mmHga |
| (C) Pathological AR and at least two morphological features of RHD of the AVb |
| (D) Borderline disease of both the AV and MVc |
| Borderline RHD (either A, B, or C): |
| (A) At least two morphological features of RHD of the MV without pathological MR or MS |
| (B) Pathological MR |
| (C) Pathological AR |
| Normal echocardiographic findings (all of A, B, C, and D): |
| (A) MR that does not meet all four Doppler echocardiographic criteria (physiological MR) |
| (B) AR that does not meet all four Doppler echocardiographic criteria (physiological AR) |
| (C) An isolated morphological feature of RHD of the MV (e.g. valvular thickening) without any associated pathological stenosis or regurgitation |
| (D) Morphological features of RHD of the AV (e.g. valvular thickening) |
| Echocardiographic criteria for individuals aged <20 years |
| Definite RHD (either A, B, C, or D): |
| (A) Pathological MR and at least two morphological features of RHD of the MV |
| (B) MS mean gradient ≥4 mmHga |
| (C) Pathological AR and at least two morphological features of RHD of the AV, only in individuals aged <35 yearsb |
| (D) Pathological AR and at least two morphological features of RHD of the MV |
| Echocardiographic criteria for individuals aged ≧20 years |
| Definite RHD (either A, B, C, or D): |
| (A) Pathological MR and at least two morphological features of RHD of the MV |
| (B) MS mean gradient ≥4 mmHga |
| (C) Pathological AR and at least two morphological features of RHD of the AVb |
| (D) Borderline disease of both the AV and MVc |
| Borderline RHD (either A, B, or C): |
| (A) At least two morphological features of RHD of the MV without pathological MR or MS |
| (B) Pathological MR |
| (C) Pathological AR |
| Normal echocardiographic findings (all of A, B, C, and D): |
| (A) MR that does not meet all four Doppler echocardiographic criteria (physiological MR) |
| (B) AR that does not meet all four Doppler echocardiographic criteria (physiological AR) |
| (C) An isolated morphological feature of RHD of the MV (e.g. valvular thickening) without any associated pathological stenosis or regurgitation |
| (D) Morphological features of RHD of the AV (e.g. valvular thickening) |
| Echocardiographic criteria for individuals aged <20 years |
| Definite RHD (either A, B, C, or D): |
| (A) Pathological MR and at least two morphological features of RHD of the MV |
| (B) MS mean gradient ≥4 mmHga |
| (C) Pathological AR and at least two morphological features of RHD of the AV, only in individuals aged <35 yearsb |
| (D) Pathological AR and at least two morphological features of RHD of the MV |
AR, aortic regurgitation; AV, aortic valve; MR, mitral regurgitation; MS, mitral stenosis; MV, mitral valve; RHD, rheumatic heart disease; WHF, World Heart Federation.
Congenital anomalies must be excluded. Inflow obstruction due to non-rheumatic mitral annular calcification must be excluded in adults.
Bicuspid AV, dilated aortic root, and hypertension must be excluded.
Combined AR and MR in high prevalence regions and in the absence of congenital heart disease is regarded as rheumatic.
| Echocardiographic criteria for individuals aged ≧20 years |
| Definite RHD (either A, B, C, or D): |
| (A) Pathological MR and at least two morphological features of RHD of the MV |
| (B) MS mean gradient ≥4 mmHga |
| (C) Pathological AR and at least two morphological features of RHD of the AVb |
| (D) Borderline disease of both the AV and MVc |
| Borderline RHD (either A, B, or C): |
| (A) At least two morphological features of RHD of the MV without pathological MR or MS |
| (B) Pathological MR |
| (C) Pathological AR |
| Normal echocardiographic findings (all of A, B, C, and D): |
| (A) MR that does not meet all four Doppler echocardiographic criteria (physiological MR) |
| (B) AR that does not meet all four Doppler echocardiographic criteria (physiological AR) |
| (C) An isolated morphological feature of RHD of the MV (e.g. valvular thickening) without any associated pathological stenosis or regurgitation |
| (D) Morphological features of RHD of the AV (e.g. valvular thickening) |
| Echocardiographic criteria for individuals aged <20 years |
| Definite RHD (either A, B, C, or D): |
| (A) Pathological MR and at least two morphological features of RHD of the MV |
| (B) MS mean gradient ≥4 mmHga |
| (C) Pathological AR and at least two morphological features of RHD of the AV, only in individuals aged <35 yearsb |
| (D) Pathological AR and at least two morphological features of RHD of the MV |
| Echocardiographic criteria for individuals aged ≧20 years |
| Definite RHD (either A, B, C, or D): |
| (A) Pathological MR and at least two morphological features of RHD of the MV |
| (B) MS mean gradient ≥4 mmHga |
| (C) Pathological AR and at least two morphological features of RHD of the AVb |
| (D) Borderline disease of both the AV and MVc |
| Borderline RHD (either A, B, or C): |
| (A) At least two morphological features of RHD of the MV without pathological MR or MS |
| (B) Pathological MR |
| (C) Pathological AR |
| Normal echocardiographic findings (all of A, B, C, and D): |
| (A) MR that does not meet all four Doppler echocardiographic criteria (physiological MR) |
| (B) AR that does not meet all four Doppler echocardiographic criteria (physiological AR) |
| (C) An isolated morphological feature of RHD of the MV (e.g. valvular thickening) without any associated pathological stenosis or regurgitation |
| (D) Morphological features of RHD of the AV (e.g. valvular thickening) |
| Echocardiographic criteria for individuals aged <20 years |
| Definite RHD (either A, B, C, or D): |
| (A) Pathological MR and at least two morphological features of RHD of the MV |
| (B) MS mean gradient ≥4 mmHga |
| (C) Pathological AR and at least two morphological features of RHD of the AV, only in individuals aged <35 yearsb |
| (D) Pathological AR and at least two morphological features of RHD of the MV |
AR, aortic regurgitation; AV, aortic valve; MR, mitral regurgitation; MS, mitral stenosis; MV, mitral valve; RHD, rheumatic heart disease; WHF, World Heart Federation.
Congenital anomalies must be excluded. Inflow obstruction due to non-rheumatic mitral annular calcification must be excluded in adults.
Bicuspid AV, dilated aortic root, and hypertension must be excluded.
Combined AR and MR in high prevalence regions and in the absence of congenital heart disease is regarded as rheumatic.
| Modality . | Advantages . | Disadvantages . |
|---|---|---|
| CT | 1. Aid identification of paravalvular pathology | 1. Radiation exposure and not usable in patients with iodine allergy |
| 2. Detailed assessment of valve thickness, calcification, and annular fibrosis | ||
| 3. For pre-operative evaluation before mitral valve repair and can help predict clinical outcomes | ||
| 4. CT may be preferable to echo for follow-up imaging after mitral valve repair due to enhanced special resolution and reduced interference from metal artefact | ||
| 5. Useful in patients with poor echo windows | ||
| 6. Can identify insufficient coaptation of aortic valve leaflets and pathognomonic central regurgitation | ||
| CMR | 1. Beneficial in assessing the impact of RHD on cardiac function and can be utilized to determine the aetiology of left ventricular dysfunction and aid quantification of regurgitant volumes | 1. Concurrent cardiac arrhythmias may impact image quality and MR assessment on CMRI |
| 2. CMRI may be utilized in the assessment of MS severity with planimetric MVA measurements | 2. Expensive modality and not widely available | |
| 3. CMRI can demonstrate favourable cardiac remodelling following balloon mitral valvuloplasty | ||
| 4. CMRI to accurately estimate LV myocardial fibrosis may also be used in the prognostic assessment of morbidity in RHD patients following surgery | ||
| 5. CMRI allows for detailed assessment and evaluation of regurgitant valves without systematic overestimating | ||
| 6. Advantage of CMRI over echocardiography is the capability to calculate MR severity regardless of the regurgitant jet shape or direction | ||
| Three-dimensional Echocardiography | 1. More accurate than conventional 2D imaging at assessing suitability for valve surgery | 1. Expensive |
| 2. Used to assess TR severity and progression pre- and post-mitral valve replacement | 2. Requires special training | |
| 3. The 3D TOE can help clarify the MVA by planimetry where transthoracic images are suboptimal | ||
| 4. Post-surgical right ventricle assessment is more accurate | ||
| TOE | 1. Can help clarify disease mechanisms or severity where this is unclear | 1. Uncomfortable for patients not under anaesthetic |
| 2. Helps aid intervention planning including excluding left atrial appendage thrombus prior to percutaneous balloon mitral valvuloplasty in patients with rheumatic mitral stenosis and clarifying suitability for valve repair or replacement | 2. Not repeatable for regular follow-up | |
| 3. TOE has also been used to look at results post for paravalvular leak | 3. Expensive | |
| 4. Useful for evaluation during surgery and is especially useful for minimally invasive surgery |
| Modality . | Advantages . | Disadvantages . |
|---|---|---|
| CT | 1. Aid identification of paravalvular pathology | 1. Radiation exposure and not usable in patients with iodine allergy |
| 2. Detailed assessment of valve thickness, calcification, and annular fibrosis | ||
| 3. For pre-operative evaluation before mitral valve repair and can help predict clinical outcomes | ||
| 4. CT may be preferable to echo for follow-up imaging after mitral valve repair due to enhanced special resolution and reduced interference from metal artefact | ||
| 5. Useful in patients with poor echo windows | ||
| 6. Can identify insufficient coaptation of aortic valve leaflets and pathognomonic central regurgitation | ||
| CMR | 1. Beneficial in assessing the impact of RHD on cardiac function and can be utilized to determine the aetiology of left ventricular dysfunction and aid quantification of regurgitant volumes | 1. Concurrent cardiac arrhythmias may impact image quality and MR assessment on CMRI |
| 2. CMRI may be utilized in the assessment of MS severity with planimetric MVA measurements | 2. Expensive modality and not widely available | |
| 3. CMRI can demonstrate favourable cardiac remodelling following balloon mitral valvuloplasty | ||
| 4. CMRI to accurately estimate LV myocardial fibrosis may also be used in the prognostic assessment of morbidity in RHD patients following surgery | ||
| 5. CMRI allows for detailed assessment and evaluation of regurgitant valves without systematic overestimating | ||
| 6. Advantage of CMRI over echocardiography is the capability to calculate MR severity regardless of the regurgitant jet shape or direction | ||
| Three-dimensional Echocardiography | 1. More accurate than conventional 2D imaging at assessing suitability for valve surgery | 1. Expensive |
| 2. Used to assess TR severity and progression pre- and post-mitral valve replacement | 2. Requires special training | |
| 3. The 3D TOE can help clarify the MVA by planimetry where transthoracic images are suboptimal | ||
| 4. Post-surgical right ventricle assessment is more accurate | ||
| TOE | 1. Can help clarify disease mechanisms or severity where this is unclear | 1. Uncomfortable for patients not under anaesthetic |
| 2. Helps aid intervention planning including excluding left atrial appendage thrombus prior to percutaneous balloon mitral valvuloplasty in patients with rheumatic mitral stenosis and clarifying suitability for valve repair or replacement | 2. Not repeatable for regular follow-up | |
| 3. TOE has also been used to look at results post for paravalvular leak | 3. Expensive | |
| 4. Useful for evaluation during surgery and is especially useful for minimally invasive surgery |
CMR, cardiac magnetic resonance; CMRI, cardiac magnetic resonance imaging; CT, computed tomography; MR, mitral regurgitation; MS, mitral stenosis; MVA, mitral valve area; RHD, rheumatic heart disease; TOE, transoesophageal echocardiography; TR, tricuspid regurgitation.
| Modality . | Advantages . | Disadvantages . |
|---|---|---|
| CT | 1. Aid identification of paravalvular pathology | 1. Radiation exposure and not usable in patients with iodine allergy |
| 2. Detailed assessment of valve thickness, calcification, and annular fibrosis | ||
| 3. For pre-operative evaluation before mitral valve repair and can help predict clinical outcomes | ||
| 4. CT may be preferable to echo for follow-up imaging after mitral valve repair due to enhanced special resolution and reduced interference from metal artefact | ||
| 5. Useful in patients with poor echo windows | ||
| 6. Can identify insufficient coaptation of aortic valve leaflets and pathognomonic central regurgitation | ||
| CMR | 1. Beneficial in assessing the impact of RHD on cardiac function and can be utilized to determine the aetiology of left ventricular dysfunction and aid quantification of regurgitant volumes | 1. Concurrent cardiac arrhythmias may impact image quality and MR assessment on CMRI |
| 2. CMRI may be utilized in the assessment of MS severity with planimetric MVA measurements | 2. Expensive modality and not widely available | |
| 3. CMRI can demonstrate favourable cardiac remodelling following balloon mitral valvuloplasty | ||
| 4. CMRI to accurately estimate LV myocardial fibrosis may also be used in the prognostic assessment of morbidity in RHD patients following surgery | ||
| 5. CMRI allows for detailed assessment and evaluation of regurgitant valves without systematic overestimating | ||
| 6. Advantage of CMRI over echocardiography is the capability to calculate MR severity regardless of the regurgitant jet shape or direction | ||
| Three-dimensional Echocardiography | 1. More accurate than conventional 2D imaging at assessing suitability for valve surgery | 1. Expensive |
| 2. Used to assess TR severity and progression pre- and post-mitral valve replacement | 2. Requires special training | |
| 3. The 3D TOE can help clarify the MVA by planimetry where transthoracic images are suboptimal | ||
| 4. Post-surgical right ventricle assessment is more accurate | ||
| TOE | 1. Can help clarify disease mechanisms or severity where this is unclear | 1. Uncomfortable for patients not under anaesthetic |
| 2. Helps aid intervention planning including excluding left atrial appendage thrombus prior to percutaneous balloon mitral valvuloplasty in patients with rheumatic mitral stenosis and clarifying suitability for valve repair or replacement | 2. Not repeatable for regular follow-up | |
| 3. TOE has also been used to look at results post for paravalvular leak | 3. Expensive | |
| 4. Useful for evaluation during surgery and is especially useful for minimally invasive surgery |
| Modality . | Advantages . | Disadvantages . |
|---|---|---|
| CT | 1. Aid identification of paravalvular pathology | 1. Radiation exposure and not usable in patients with iodine allergy |
| 2. Detailed assessment of valve thickness, calcification, and annular fibrosis | ||
| 3. For pre-operative evaluation before mitral valve repair and can help predict clinical outcomes | ||
| 4. CT may be preferable to echo for follow-up imaging after mitral valve repair due to enhanced special resolution and reduced interference from metal artefact | ||
| 5. Useful in patients with poor echo windows | ||
| 6. Can identify insufficient coaptation of aortic valve leaflets and pathognomonic central regurgitation | ||
| CMR | 1. Beneficial in assessing the impact of RHD on cardiac function and can be utilized to determine the aetiology of left ventricular dysfunction and aid quantification of regurgitant volumes | 1. Concurrent cardiac arrhythmias may impact image quality and MR assessment on CMRI |
| 2. CMRI may be utilized in the assessment of MS severity with planimetric MVA measurements | 2. Expensive modality and not widely available | |
| 3. CMRI can demonstrate favourable cardiac remodelling following balloon mitral valvuloplasty | ||
| 4. CMRI to accurately estimate LV myocardial fibrosis may also be used in the prognostic assessment of morbidity in RHD patients following surgery | ||
| 5. CMRI allows for detailed assessment and evaluation of regurgitant valves without systematic overestimating | ||
| 6. Advantage of CMRI over echocardiography is the capability to calculate MR severity regardless of the regurgitant jet shape or direction | ||
| Three-dimensional Echocardiography | 1. More accurate than conventional 2D imaging at assessing suitability for valve surgery | 1. Expensive |
| 2. Used to assess TR severity and progression pre- and post-mitral valve replacement | 2. Requires special training | |
| 3. The 3D TOE can help clarify the MVA by planimetry where transthoracic images are suboptimal | ||
| 4. Post-surgical right ventricle assessment is more accurate | ||
| TOE | 1. Can help clarify disease mechanisms or severity where this is unclear | 1. Uncomfortable for patients not under anaesthetic |
| 2. Helps aid intervention planning including excluding left atrial appendage thrombus prior to percutaneous balloon mitral valvuloplasty in patients with rheumatic mitral stenosis and clarifying suitability for valve repair or replacement | 2. Not repeatable for regular follow-up | |
| 3. TOE has also been used to look at results post for paravalvular leak | 3. Expensive | |
| 4. Useful for evaluation during surgery and is especially useful for minimally invasive surgery |
CMR, cardiac magnetic resonance; CMRI, cardiac magnetic resonance imaging; CT, computed tomography; MR, mitral regurgitation; MS, mitral stenosis; MVA, mitral valve area; RHD, rheumatic heart disease; TOE, transoesophageal echocardiography; TR, tricuspid regurgitation.
Large-scale studies using the WHF criteria have since been carried out. Two population-based screening studies in India concluded that the prevalence of RHD obtained using WHF criteria was higher than clinical criteria, which consisted of the presence of a murmur and associated echocardiographic standards. Saxena et al. applied the WHF criteria to 16 294 children. Using clinical criteria, the prevalence of RHD was 0.36 per 1000 (95% CI 0.1–0.7), whereas using WHF criteria, the prevalence of borderline and definite RHD was 7.7 per 1000 (95% CI 6.3–9.0).43 Similarly, Nair et al. applied the WHF criteria to 2060 randomly selected children and found a prevalence of 2.4/2000 RHD cases according to clinical criteria, compared to a WHF criteria prevalence of 5.83/1000.44
There remains controversy regarding reproducibility in some studies, such as Remenyi et al. showing substantial inter-rater and intra-rater reliability in diagnosing definite RHD (kappa of 0.65 and 0.69, respectively) in 200 studies using the WHF criteria.45 Each study was reported by 15 international cardiologists, resulting in a comprehensive data set of 3000. This study shows that the WHF criteria enable reproducible categorization of echocardiograms. In contrast, Culliford-Semmens et al. looked at two reviewers reporting on 144 cases using the WHF criteria. They found only moderate agreement for any RHD with a kappa of 0.57 (CI 0.44–0.70) and 11% of cases requiring an open-label discussion.46 This study shows a higher inter-reporter variability than Remenyi et al.'s sample, though only two reviewers were involved compared to the former’s fifteen. No available studies examine inter-rater and intra-rater reliability in developing countries where access to expert interpretation is likely to be limited, creating the issue that the WHF criteria may be too complicated for operators with varying experience in performing and interpreting echocardiography. Additionally, transthoracic echocardiogram machines are expensive and may be less accessible in the remote African villages. Furthermore, the above-named studies have several limitations, including short duration of follow-up (from 4 to 60 months), small cohorts, variable implementation of secondary prophylaxis, and variable definitions of progression/regression of valve changes.
A key advantage of the WHF criteria is that patients do not require a history of ARF, as the majority of patients with RHD do not have a documented history of ARF.47 In individuals without a documented history of ARF, alternative causes (congenital, acquired, or degenerative) for the patient’s valvular findings must be first excluded by echocardiography and by the clinical context. An echocardiographic diagnosis of RHD in these patients would, therefore, be a diagnosis of exclusion. The WHF criteria have the further advantage of facilitating disease progression monitoring. In the largest cohort of latent RHD to date, describing 227 children from Uganda, 10% of children with borderline RHD showed progression. In contrast, 25% of children with mild definite RHD showed progression over a median of 2.3 years.48 Comparatively, 45% of children with mild definite RHD demonstrated improvement in echocardiographic findings in that period. Children with moderate/severe RHD at time of screening who were treated in accordance with local recommendations for clinically detected RHD did substantially worse; an observation consistent with data from Fiji that showed >80% of this group demonstrated persistence of progression of RHD, including death.49 The authors propose that children with screening-detected moderate/severe RHD can be considered ‘missed clinical RHD’.
Among the mild RHD cases, it is not currently possible to predict which individuals are more likely to progress or regress. The Ugandan study suggested that younger age at diagnosis and the presence of pathological AR or morphological MV features at diagnosis were independent risk factors for unfavourable outcomes, but these criteria for progression have not been replicated in all cohorts.47,48 Mild definite RHD should receive secondary prophylaxis according to their age and local guidelines (as per the Australian 2020 Guidelines, for example).40 The most common current practice is to monitor patients with borderline RHD without prophylaxis and for medical review with a repeat echocardiogram 1, 3, and 5 years after diagnosis. Recent data from the ‘Echo in Africa’ project identified inter-scallop separations of the posterior mitral valve leaflet as a common underlying mechanism of isolated ‘pathological’ MR without other morphological features of RHD. This normal variant in MV anatomy was responsible for 70.5% of cases of isolated MR and resulted in children being mislabelled as borderline RHD. This misclassification significantly impacts the efficiency and feasibility of screening programmes due to the subsequent requirement for detailed scans and long-term follow-up. The authors propose that screening should focus on the assessment of morphological abnormalities rather than MR severity, due to the high prevalence of physiological, non-rheumatic causes of isolated MR.50
The future of RHD diagnosis imaging: echocardiography screening in RHD and the role of handheld echo
Echocardiographic screening for RHD meets some, but not all, of the requirements of a screening programme. RHD has a significant burden of disease in endemic areas and has a clear latent stage that is detectable by a simple, accessible, and sensitive test, namely echocardiography. The main benefit of screening is proposed to be for subclinical RHD cases, as secondary penicillin prophylaxis may halt their progression to clinical or advanced RHD and prevent their associated complications.51
Evidence for screening in RHD was previously limited to longitudinal studies, often without including effective secondary prophylaxis delivery. There are also barriers to the implementation of screening programmes, most significantly limited resources—both financially and with respect to competent clinicians to undertake them. Studies that assessed the cost-effectiveness of screening had broad assumptions leading to hypothetical conclusions, and the impact of secondary prophylaxis on latent RHD was not fully understood. As such, the guidelines did not advocate a nationwide, routine screening programme for RHD with echocardiography.
There was scientific consensus that asymptomatic people with definite RHD on screening echo are presumed to be at risk of valve disease progression and should receive secondary prophylaxism22,40,41 but the benefit of secondary prophylaxis in latent RHD was unclear. GOAL sought to clarify this uncertainty in latent RHD. 916 Ugandan children with latent RHD were randomized to receive injections of penicillin G benzathine every 4 weeks for 2 years or no prophylaxis. This landmark study demonstrated that regular penicillin prophylaxis reduces the risk of progression of latent RHD by 90%.50 The results of the GOAL trial should lead to a reassessment of the value of screening in RHD, as they provide evidence in favour of a nationwide routine screening programme. There is a clear, asymptomatic latent stage of RHD which can be routinely detected by a safe screening test, and a cost-effective intervention which can prevent disease progression. Despite these promising results, further research on a population level is required before the widespread implementation of national screening can be recommended. Studies and economic models assessing the cost-effectiveness of RHD screening are also required in light of the encouraging findings from the GOAL trial.
Echocardiographic screening performed using the WHF criteria also has the added benefit of increasing the likelihood of detecting previously undiagnosed congenital heart defects, which may also benefit from cardiology review and intervention. The prevalence of incidental congenital heart disease detected during echo screening for RHD is consistently around 1%, and a management pathway for these cases needs to be available.52–54
Targeted screening in high-risk groups may be more appropriate, helping clarify disease prevalence as well as raise community awareness and improve engagement with the healthcare profession. Some studies indicate strong support for screening from parents of screened children in New Zealand and Uganda. Peer support groups and community-engaged research may minimize the negative effects of RHD screening on children and communities. Whichever protocol is used, appropriate sensitivity and specificity thresholds need to be defined and may vary between settings. High sensitivity is usually a priority in a screening context to ensure cases are not missed and to maximize the potential benefit of early intervention. High specificity can also be advantageous in an environment where resources are scarce, to reduce the number of false positive cases and to ensure resources for diagnostic confirmation and ongoing treatment are effectively targeted.55,56
The emerging role of handheld and portable echocardiography as a screening tool
In response to advances in portable echo technology, a refined and simpler set of echocardiographic criteria for the diagnosis of RHD to be used with handheld and portable devices was developed by Nunes et al. based on components of the 2012 WHF criteria.11 The Nunes Criteria can be used in RHD screening as well as predicting disease progression for latent RHD. This study included echocardiograms from school children: 9501 patients formed the derivation cohort, and 7312 patients composed the validation cohort. The final criteria consisted of five variables: anterior leaflet thickening, excessive leaflet tip motion, regurgitation jet length ≥2 cm for the mitral valve, and focal thickening and any regurgitation of the aortic valve. These simplified criteria demonstrated near-perfect discrimination between normal and definite RHD (c-statistic of 0.99). The Nunes Criteria also increases the likelihood of adoption by non-expert echocardiographers—the primary workforce in many RHD endemic areas. The components of the Nunes Criteria can be fully assessed by handheld echocardiography, unlike the WHF criteria. While the Nunes Criteria are more practical and possibly more cost-effective than the WHF criteria, they can miss isolated morphological abnormalities that can be present in the absence of pathological regurgitation. Table 3 compares the specifications and functionality of the different types of echocardiography available in screening and diagnosing echocardiography.
General specifications and functionality of different categories of echocardiogram machines used in RHD screening and diagnosis34
| Specification . | High-end machine . | Portable machine . | Handheld machine . |
|---|---|---|---|
| Technical capabilities | |||
| 2D image quality | +++ | +++ | +++ |
| Colour Doppler | +++ | +++ | ++ |
| Pulsed wave/continuous wave Doppler | +++ | +++ | − |
| Measurements (linear) | +++ | +++ | + |
| Measurements (volume) | +++ | +++ | − |
| Affordability | + | ++ | +++ |
| Console size and portability | − | ++ | +++ |
| Screen size/resolution | +++ | ++ | ++ |
| Battery capacity | − | ++ | ++ |
| Additional probes | +++ | ++ | −/+ |
| Storage and transfer of images | +++ | ++ | ++ |
| Specification . | High-end machine . | Portable machine . | Handheld machine . |
|---|---|---|---|
| Technical capabilities | |||
| 2D image quality | +++ | +++ | +++ |
| Colour Doppler | +++ | +++ | ++ |
| Pulsed wave/continuous wave Doppler | +++ | +++ | − |
| Measurements (linear) | +++ | +++ | + |
| Measurements (volume) | +++ | +++ | − |
| Affordability | + | ++ | +++ |
| Console size and portability | − | ++ | +++ |
| Screen size/resolution | +++ | ++ | ++ |
| Battery capacity | − | ++ | ++ |
| Additional probes | +++ | ++ | −/+ |
| Storage and transfer of images | +++ | ++ | ++ |
RHD, rheumatic heart disease.
General specifications and functionality of different categories of echocardiogram machines used in RHD screening and diagnosis34
| Specification . | High-end machine . | Portable machine . | Handheld machine . |
|---|---|---|---|
| Technical capabilities | |||
| 2D image quality | +++ | +++ | +++ |
| Colour Doppler | +++ | +++ | ++ |
| Pulsed wave/continuous wave Doppler | +++ | +++ | − |
| Measurements (linear) | +++ | +++ | + |
| Measurements (volume) | +++ | +++ | − |
| Affordability | + | ++ | +++ |
| Console size and portability | − | ++ | +++ |
| Screen size/resolution | +++ | ++ | ++ |
| Battery capacity | − | ++ | ++ |
| Additional probes | +++ | ++ | −/+ |
| Storage and transfer of images | +++ | ++ | ++ |
| Specification . | High-end machine . | Portable machine . | Handheld machine . |
|---|---|---|---|
| Technical capabilities | |||
| 2D image quality | +++ | +++ | +++ |
| Colour Doppler | +++ | +++ | ++ |
| Pulsed wave/continuous wave Doppler | +++ | +++ | − |
| Measurements (linear) | +++ | +++ | + |
| Measurements (volume) | +++ | +++ | − |
| Affordability | + | ++ | +++ |
| Console size and portability | − | ++ | +++ |
| Screen size/resolution | +++ | ++ | ++ |
| Battery capacity | − | ++ | ++ |
| Additional probes | +++ | ++ | −/+ |
| Storage and transfer of images | +++ | ++ | ++ |
RHD, rheumatic heart disease.
There is varying consensus on the real-world application of handheld echocardiography. Two extensive studies were carried out by Marijon et al. in Cambodia (n = 3677 patients) and Mozambique (n = 2170 patients) among school children.13 Handheld echocardiography was used, followed by echocardiograms with spectral Doppler if handheld scans were consistent with RHD. Their criteria for definite RHD were mitral or aortic valve regurgitation seen in two planes by echocardiography with Doppler, accompanied by at least two of the following three abnormalities: restricted leaflet mobility, focal or generalized valvular thickening, and abnormal subvalvular thickening. They found 21.5 definite RHD cases per 1000 (95% CI 16.8–26.2) for Cambodia and 30.4 cases per 1000 (95% CI 23.2–37.6) for Mozambique, of which 90% were undetected by clinical examination alone. Their study was the first in a growing body of data that suggests handheld echocardiography may be best utilized as a part of a two-stage screening approach where a brief, abbreviated screening test is conducted with either a portable or handheld machine, followed by a more detailed study if the results are concerning for RHD. However, the false positive rate from the Mozambique arm remained a significant concern, with 58 of the 124 children positive for suspected RHD on screening echo having normal results on the definitive echo.
Beaton et al. initially investigated the use of handheld echocardiography for screening for RHD in 125 Ugandan school children.57 Their study used a modified version of the WHF criteria (similar to the Nunes Criteria) that did not require spectral Doppler and achieved 90.2% sensitivity and 92.9% specificity. However, their results were based on analysis of RHD of the MV only, excluding aortic valve disease. Mitral valve disease was the predominant form of RHD in their cohort, but the generalizability of their study is limited. Furthermore, while they report promising sensitivity and specificity in definite RHD, the confidence intervals in borderline RHD were much wider. As such, they recommended that at this stage in its development, handheld echocardiography functions best as a screening tool, with positive results confirmed by fully functional echocardiographic machines with spectral Doppler capabilities. The initial work of Beaton et al. was followed up by performing a large-scale screening programme in Uganda for 1420 schoolchildren, where they evaluated the efficacy of handheld echocardiography against echocardiograms with spectral Doppler.58 The study demonstrated a handheld echocardiography sensitivity of 78.9% and specificity of 87.2% for all forms of RHD and 97.9% sensitivity for definite RHD. The high rate of false positives in handheld echo was attributed to limitations of measurement software resulting in overestimations of anterior MV length. As handheld echo technology develops, such errors will likely be resolved, improving the specificity and utility as a screening tool, but the personal and societal burden of a high false positive rate must be addressed, and screening criteria refined before widespread application.
These studies confirmed that the potential utility of handheld echo as a screening test cannot be overlooked. Nonetheless, there remains a trade-off between simplified positive criteria and the sensitivity/specificity of scoring systems. In New Zealand, Webb et al. demonstrated the efficacy of handheld screening for RHD in their study, where 1142 predominantly Maori and Pacific children were screened.52 Their findings highlighted the possibility of over-diagnosis in handheld echocardiography as the 8.3% prevalence detected by portable echocardiography was reduced to 5.2% following echocardiographic assessment with spectral Doppler. Allen et al. carried out a screening programme on 11 434 Samoan children using a 60 s focused echocardiogram with a comprehensive echocardiogram as a follow-up for children with suspicious findings.59 Their rapid screening protocol entailed a parasternal long-axis view, focused on the mitral and aortic valves with and without colour Doppler for each student. Definite RHD was found in 115 students (10.0 per 1000), of which 92 students were classified as borderline (8.0 per 1000), similar to the previously reported prevalence of RHD in Samoa. Their methodology was remarkable for the efficiency and scale of the screening test, performing 60 rapid echocardiograms in an hour. However, the authors did not attempt to assess the sensitivity or specificity of their rapid screening method, sacrificing accuracy in favour of wider availability and access to a greater number of patients.
In an attempt to find a balance between applicability and reliability, simplified criteria for handheld echocardiography screening have been proposed and investigated. A study by Lu et al. is based on the presence of MR jet length ≥ 1.5 cm and aortic insufficiency.60 From a sample of 1439 schoolchildren from Uganda, using the WHF criteria as the gold standard, these new criteria demonstrated an excellent sensitivity of 97.9% for definite RHD while also showing a sensitivity of 73.3% and specificity of 82.4% for borderline or definite RHD. Similarly, Mirabel et al. investigated the efficacy of even more simplified criteria that are based simply on the MR jet length against a referenced set of criteria that were made up of WHO’s Doppler criteria and morphological echocardiographic features of RHD.61 Despite its simplicity, when evaluated in a retrospective study of echocardiograms of 2170 schoolchildren from Mozambique, it produced encouraging results of 73% sensitivity and a positive predictive value of 92%.
A more recent study performed in East Timor of 1365 children utilizing a modified parasternal long-axis view involving a whole sweep through the heart with 2D and colour images focusing on aortic and mitral valves showed 100% sensitivity (95% CI 0.93–1.0) and 95% specificity (95% CI 0.94–0.96) for all RHD in a paediatric population when performed by a cardiologist on a portable standard echocardiogram machine.62 All children in this study underwent follow-up full screening echo, which confirmed no cases of RHD had been missed. This modified technique has demonstrated high diagnostic accuracy and is a promising screening protocol for RHD, which may be appropriate for portable echo. However, these screening scans were performed by experienced paediatric cardiologists, and significant further training is required for other healthcare workers to emulate their rates of specificity and sensitivity.
Discussion
There are increasing efforts to incorporate imaging into the diagnosis and management of RHD, particularly as imaging modalities can detect more individuals with early RHD and provide more information than traditional screening methods such as auscultation. ‘Subclinical’ or latent RHD appears to provide the highest return on screening as patients could potentially avoid progression to clinical RHD, particularly advanced forms, by receiving secondary preventative penicillin therapy. This hope is supported by recent findings from the GOAL trial indicating a delay in disease progression of 2 years. Further research is needed to evaluate the longer-term benefits of secondary penicillin therapy.
The WHF criteria are more sensitive and specific than auscultation alone in diagnosing RHD. Data provided by these echocardiographic-based criteria may cause a re-evaluation of prevalence rates in these countries, which are often based on data derived by auscultation alone. The disadvantages of WHF criteria are its relative complexity, particularly for non-expert echocardiographers (the primary workforce in endemic areas) as well as the potential considerable financial burden in the purchase of standard echocardiography machines as well as their maintenance and storage. The Nunes Criteria improve on both these disadvantages but sacrifice the detection of early RHD due to the lack of a spectral Doppler facility. Some longitudinal data exists to support the use of the Nunes Criteria, and both criteria emphasise the importance of adapting criteria to a patient's age and geographical location.
A concern of the WHF criteria is the danger of labelling normal variants in children as ‘rheumatic’ echocardiographic features. Mislabeling healthy children as having RHD may lead to an increased psychological and financial burden on individuals and families that may already be significantly disadvantaged socio-economically.63,64 The cost-efficacy of echocardiographic-based screening programmes remains uncertain. It is not known whether healthcare systems in endemic areas, which are often socio-economically disadvantaged, can cope with further downstream testing and monitoring that may occur from identifying more cases of RHD.
Newer imaging techniques such as 3D echocardiography or CMRI may not be relevant in screening programmes. However, they can help facilitate the optimal management of patients being considered for invasive intervention for their RHD as well as post-intervention monitoring, particularly for complications. Handheld echocardiography has demonstrated substantial promise on its own as a diagnostic tool or, more likely, as part of a two-step screening pathway. Initial results suggest a rapid, focused echocardiogram can be performed with reliable accuracy and reproducibility for it to be utilized as a screening test. Concerns remain regarding the high false positive rates, which have a significant impact on patients as individuals due to the cost and strain of long-term penicillin use, in addition to the potential stigma of chronic disease. Furthermore, there are societal implications as high false positive rates may overwhelm healthcare services in resource-poor settings with the need for further investigation and management. Portable echo is likely to play a significant role in the future of RHD screening, but further research is needed to develop and refine screening criteria that find an equipoise between simplicity, efficiency, and safety.
There are promising developments in the earlier detection of subclinical RHD and the administration of secondary therapy. Secondary preventative penicillin has been shown to reduce episodes of ARF and some cases have demonstrated regression of ‘rheumatic’ lesions with a prolonged course of penicillin. The findings from the GOAL trial support the use of regular penicillin prophylaxis in latent RHD and represent a milestone in the prevention of clinical RHD. Further investigation is needed, but these results are a promising step toward the development of a robust international screening protocol and effective intervention, resulting in a significant reduction in clinical RHD cases and mortality worldwide.
Conclusion
A significant amount of research has been conducted into RHD, especially looking at the role of imaging in its diagnosis and management. The WHF criteria for RHD remain the gold standard echocardiographic-based criteria for diagnosis and several subsequent criteria have been developed, each with its advantages and limitations. Each of the criteria aims to find a balance between specificity and applicability, particularly when being used in resource-poor, non-expert settings. The benefits of current screening programmes appear to be supported by some longitudinal studies, and there is emerging potential for secondary prevention of disease progression with penicillin, following the results of the GOAL trial. Portable echocardiography has the potential to revolutionize RHD screening and diagnosis, especially in combination with simplified but precise criteria. However, it remains in the early stages of development as a screening and diagnostic tool, and further research is needed to confirm its safety and real-world utility, alongside the development of accurate and high-quality diagnostic criteria specific to portable echocardiography. As imaging modalities become more advanced, we will be able to screen for RHD more affordably and practically, but also be able to manage RHD cases that require intervention in a more precise and accurate manner. This includes identifying only the right patients for intervention and ensuring that complications from procedures are anticipated and avoided where possible. The global burden of RHD remains dismally high and international expertise and cooperation is required to tackle this disease and improve the lives and health of millions worldwide.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Funding
None declared.
References
Author notes
Conflict of interest: None declared.
- rheumatic heart disease
- echocardiography
- echocardiography, three-dimensional
- cardiac ct
- heart valve diseases
- two-dimensional echocardiography
- developing countries
- diagnosis
- diagnostic imaging
- heart
- echocardiography, transthoracic
- scanning systems, ultrasonic, portable
- cardiac mri
- clinical diagnostic instrument
- cardiac imaging procedures
- world heart federation
- lack of medical resources




Comments