-
PDF
- Split View
-
Views
-
Cite
Cite
Felice Gragnano, Vincenzo De Sio, Paolo Calabrò, The year in cardiovascular pharmacotherapy 2022: landmark evidence at a glance, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 9, Issue 6, September 2023, Pages 499–501, https://doi.org/10.1093/ehjcvp/pvad043
Close - Share Icon Share
There have been major advances in cardiovascular pharmacotherapy in recent decades, but many evidence gaps remain. Our patients have high expectations of cardiology research and pharmacological innovation to improve and prolong their lives, and these expectations should not be betrayed. In this regard, 2022 was successful in delivering important, practice-changing novelties in cardiology (Figure 1).1 Scientific discoveries broke new ground with the introduction of first-in-class drugs, and old drugs were refreshed with updated evidence.1
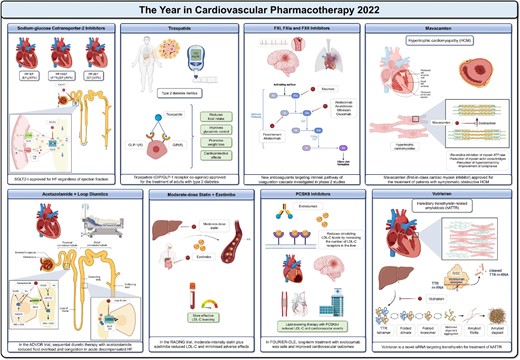
The year in cardiovascular pharmacotherapy 2022. EF = ejection fraction; GIP(R) = GIP receptor; GLP-1(R) = GLP-1 receptor. Other abbreviations as in the text. Created with BioRender.com.
The ESC Working Group on Cardiovascular Pharmacotherapy is to be congratulated on its efforts to bring together all this evidence in a comprehensive review to provide the cardiology community with an up-to-date snapshot of the literature from the past year.1
Heart failure (HF)
Sodium-glucose cotransporter-2 inhibitors (SGLT2-I) are well-established therapeutic pillars in HF with reduced ejection fraction (HFrEF),2 but their benefit in patients with preserved (HFpEF) or mildly reduced (HFmrEF) ejection fraction was less clear.3 The DELIVER trial showed that dapagliflozin significantly reduced the primary outcome of worsening HF and cardiovascular death compared with placebo and improved symptoms.1 These findings add to the existing evidence for empagliflozin and support SGLT2-I as a first-line therapy for HF, regardless of ejection fraction.1–3 Moving from chronic to acute settings, new evidence has also emerged. Acute decompensated HF (ADHF) is a leading cause of mortality and rehospitalisation in the HF population and is hallmarked by fluid overload.1 Loop diuretics are the drug of choice in this setting, but their use can be challenging due to renal function decline, electrolyte disturbances, and diuretic resistance. The ADVOR trial1 evaluated sequential diuretic therapy with acetazolamide (a carbonic anhydrase inhibitor) to improve the efficiency of loop diuretics in relieving fluid overload in ADHF. At 3 days post-randomisation, successful decongestion was more frequent with acetazolamide than with placebo, along with greater diuresis and shorter hospital stays.1 More evidence is now needed to prove that this effect translates into a reduction in clinical events, which was not shown in the ADVOR trial.1
Diabetes mellitus and obesity
Diabetes and obesity are the 21st century pandemics in cardiovascular medicine.1 Because many pharmacotherapies have previously failed to treat overweight/obese diabetic patients, the discovery of incretins was a turning point. In addition to their hypoglycaemic effect, these drugs can improve weight loss and cardiovascular health.1,4 Recent approval of glucagon-like peptide-1 (GLP-1) receptor agonists as anti-obesity drugs, regardless of hyperglycaemia, paved the way for the new pharmacological class of twincretins, of which tirzepatide is the progenitor.1 Tirzepatide is a glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist that improves glycaemic control, promotes weight loss, and has several protective cardio-metabolic effects. Based on growing evidence, the drug was approved in 2022 for use in adults with type 2 diabetes, with greater benefits expected in the presence of obesity/overweight.1
Dyslipidaemias
In patients with atherosclerotic disease, the linear relation between low-density lipoprotein cholesterol (LDL-C) lowering and cardiovascular risk reduction is an evidence-based dogma.1,5 Yet, the best approach to improve clinical outcomes, maximise adherence, and reduce drug-related adverse effects remains to be defined.1,5–7 Drug combinations may be more effective and safer than increasing doses of a single drug. Under this rationale, the RACING trial1 was designed to test whether a moderate-intensity statin plus ezetimibe could effectively lower LDL-C while reducing adverse effects compared with high-intensity statin monotherapy. In this study, published in The Lancet in July 2022, a strategy of rosuvastatin 10 mg plus ezetimibe was non-inferior to rosuvastatin 20 mg for the 3-year composite of cardiovascular death, major cardiovascular events, or stroke, with fewer intolerance-related drug discontinuation.1 Just 1 month after the publication of these data, the FOURIER open-label extension (OLE) study was presented at ESC Congress 2022, reinforcing the evidence for the long-term benefit of combination therapy.1 Patients who were initially randomised to evolocumab in the parent trial (median follow-up: 2.2 years) and continued treatment in the OLE study (median follow-up: 5.0 years) showed a 15% lower risk of cardiovascular events compared with patients who were initially randomised to placebo and crossed-over to evolocumab after completion of the parent trial.1 FOURIER-OLE included patients with the longest study exposure to a proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9-I) to date and provided additional support for guidelines recommending PCSK9-I and targeting very-low LDL-C levels.5
Antithrombotic therapy
The year 2022 has also set the stage for pioneering anticoagulants that inhibit FXI/FXIa, potentially uncoupling the pharmacological effects and adverse events of antithrombotics.1,8–10 FXI inhibitors are expected to maximise both safety and efficacy by hitting the «sweet spot» of preserving physiological haemostasis while preventing pathological thrombosis, and may soon complement (or even replace) current direct oral anticoagulants.1 Antisense oligonucleotides (fesomersen), monoclonal antibodies (abelacimab, osocimab, xisomab), and small molecules (asundexian, milvexian) have been investigated with encouraging preliminary results.1 Yet, initial phase 2 studies were generally underpowered for clinical outcomes, and ongoing phase 3 studies will determine whether the risk-benefit profile of these novel drugs is similar (or superior) to that of current anticoagulation strategies for the prevention of arterial, venous, and cardiac thromboembolism.1
Rare diseases
Awareness of rare diseases has also been boosted by 2022. For decades, medical therapy for hypertrophic cardiomyopathy has been elusive and not disease-specific.1 The cardiac myosin inhibitor mavacamten now offers, for the first time, precision pharmacotherapy by targeting sarcomere hypercontractility.1 In phase 2 and 3 studies, treatment with mavacamten improved exercise capacity, left ventricular outflow tract obstruction, and health status in symptomatic patients with obstructive hypertrophic cardiomyopathy,1 data that supported the US Food and Drug Administration's decision to approve the drug in April 2022.
Hereditary transthyretin-related amyloidosis (hATTR) is another rare but important disease that can lead to HF.1 Transthyretin mutations cause protein misfolding and aggregation into amyloid fibrils, which can accumulate in the heart. Without treatment, the survival of patients with amyloid cardiomyopathy is approximately 3 years.1 Molecular biology is increasingly developing small interfering RNA (siRNA) technology.11 Vutrisiran is a shining example of how siRNA technology can target dysfunctional/pathogenic molecules before they are biosynthesised to antagonise disease progression.1 Treatment with vutrisiran (subcutaneous every 3 months) was effective in improving signs and symptoms of amyloidotic polyneuropathy in the HELIOS-A study and is currently being investigated in the HELIOS-B study in patients with transthyretin amyloid cardiomyopathy.1
Conclusions
The year 2022 has brought us solid evidence and exciting perspectives in cardiovascular pharmacotherapy that have already influenced current clinical practice. Our hope is to have a similarly rich review article from the Working Group next year.
References
Author notes
Relationships with Industry and Other Entities: Drs. Gragnano, De Sio, and Calabrò have no relationships relevant to the contents of this paper to disclose.



