-
PDF
- Split View
-
Views
-
Cite
Cite
Peter Moritz Becher, Gianluigi Savarese, Lina Benson, Ulf Dahlström, Patric Karlström, Peter G M Mol, Marco Metra, Deepak L Bhatt, Bertram Pitt, Lars H Lund, Eligibility for sotagliflozin in a real-world heart failure population based on the SOLOIST-WHF trial enrolment criteria: data from the Swedish heart failure registry, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 9, Issue 4, June 2023, Pages 343–352, https://doi.org/10.1093/ehjcvp/pvad012
Close - Share Icon Share
Abstract
The SOLOIST‐WHF trial demonstrated efficacy of sotagliflozin in patients with type 2 diabetes mellitus (T2DM) and recent worsening heart failure (HF) regardless of ejection fraction (EF). Selection criteria in trials may limit their generalizability. Therefore, we aimed to investigate eligibility for sotagliflozin based on the SOLOIST-WHF criteria in a real-world HF population.
SOLOIST-WHF criteria were applied to patients stabilized after HF hospitalization in the Swedish HF Registry according to (i) literal scenario (all inclusion/exclusion criteria) or (ii) pragmatic scenario (only criteria likely to influence treatment decisions). Of 5453 inpatients with T2DM and recent worsening HF, 51.4% had reduced EF (HFrEF), 19.1% mildly reduced (HFmrEF), and 29.5% preserved EF (HFpEF). Eligibility (literal) was: 27.2% (32.4% in HFrEF, 24.7% in HFmrEF, 19.7% in HFpEF) and eligibility (pragmatic) was 62.8% (69.1%, 60.3%, 53.4%, respectively). In the literal scenario, criteria limiting eligibility were HF duration <3 months, eGFR <30 ml/min/1.73 m2, age >85 years, acute coronary syndrome <3 months, and insufficiently high N-terminal pro-B-type natriuretic peptide levels. Eligible vs. non-eligible patients had more severe HF, higher cardiovascular (CV) comorbidity burden, higher use of HF treatments, and higher event rates (all-cause death 30.8 vs. 27.2 per 100 patient-years, CV death 19.1 vs. 16.6, and HF hospitalization 36.7 vs. 24.0).
In this large, real-world HF cohort with T2DM, ∼1/3 of patients were eligible for sotagliflozin in the literal and ∼2/3 of patients in the pragmatic scenario. Eligible patients had more severe HF and higher event rates, in particular CV and HF events.
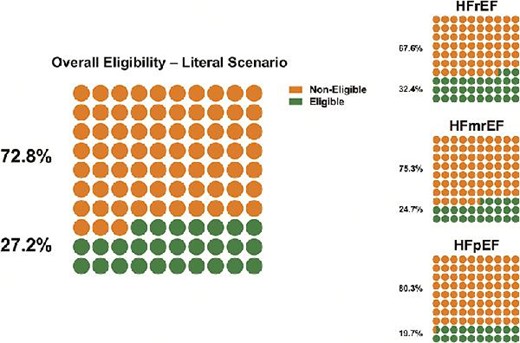
Overall eligibility and eligibility rates across the EF spectrum in patients eligible vs. non-eligible based on the literal scenario (all enrolment criteria).
Introduction
Heart failure (HF) is a major and growing public health problem affecting >60 million people globally.1 Its incidence is higher and outcomes worse in patients who also have diabetes,2 even after adjusting for other cardiovascular (CV) risk factors.3
In several CV outcome trials (CVOTs) in type 2 diabetes mellitus (T2DM) sodium-glucose co-transporter-2 inhibitors (SGLT2i) improved CV and kidney outcomes, including the risk of hospitalization for HF.4–8 The SGLT2i dapagliflozin and empagliflozin showed significant reductions of CV death/HF hospitalization in patients with HF across the EF spectrum and regardless of diabetes status.9–11
The SOLOIST-WHF (Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure) trial tested the efficacy and safety of sotagliflozin (which provides combined SGLT1/2 inhibition, rather than SGLT2 inhibition alone) in patients with T2DM and a recent episode of acute decompensated HF, regardless of EF.12 Sotagliflozin reduced CV death/HF hospitalizations or urgent visits by 33%, which was consistent across multiple subgroups including timing of treatment initiation (before or after discharge) and EF category.12 These results support the use of sotagliflozin in patients with T2DM and recent worsening HF.13–15
Randomized controlled trials (RCTs) apply selection criteria to ensure presence of a diagnosis of HF and to enrich for events and potential treatment effect, which leads to limited enrolment of older patients, women, and those with multimorbidity. This selection also limits the generalizability of trial findings, which might affect regulatory approval and labelling, guideline recommendations, reimbursement, and clinical acceptance and implementation of novel interventions in daily clinical practice.
Therefore, the aim of this study was to investigate (i) the proportion of; (ii) clinical characteristics of; and (iii) outcomes across the whole EF spectrum in patients eligible for sotagliflozin based on the SOLOIST-WHF trial selection criteria in a real-world setting.
Methods
Study population and setting
The Swedish Heart Failure Registry (SwedeHF) has been previously described.16,17 For the current analysis, SwedeHF was linked with the National Patient Registry, Statistics Sweden and the Cause of Death Registry. More details on the data sources are reported in the Supplementary Material.
The current analysis included the SwedeHF inpatient population with T2DM discharged alive and registered between 30 June 2010 (after which data on T2DM and use of loop diuretics was recorded in the registry) and 31 December 2018. Although sotagliflozin was not tested in patients without T2DM, other SGLT2i trials in HF allowed patients without T2DM, demonstrating similar benefit regardless of diabetes status. To provide a hypothetical but analogous number, we also carried out a sensitivity analysis in the SwedeHF inpatient population without T2DM.
2.2 Eligibility for Sotagliflozin
Two scenarios were considered. In the literal scenario, the selection criteria were defined based on the published enrolment criteria of the SOLOIST-WHF trial (Supplementary material online, Table S1).12 As the SOLOIST-WHF trial addressed a population with recent worsening HF, criteria for the pragmatic scenario were selected to reflect disease severity [N-terminal pro-B type natriuretic peptide (NT-proBNP levels), use of loop diuretics] and estimated glomerular filtration rate (eGFR) ≥30 mL min−1/1.73 m² to reflect chronic kidney disease as a highly relevant comorbidity. In the pragmatic scenario, eligibility was defined according to selected SOLOIST-WHF trial inclusion and exclusion criteria which may be more likely to influence the likelihood of receiving sotagliflozin in clinical practice NT-proBNP criteria, eGFR ≥30 mL min−1/1.73 m2, use of loop diuretics, but not e.g. HF duration (pre-existing HF duration ≥ 3 months) or age (age >85 years).
Not all data required for defining inclusion and exclusion criteria were available in the data sources. Thus, a few selection criteria could not be applied or were applied only by using slightly different definitions or surrogates. The inclusion and exclusion criteria from SOLOIST-WHF and the corresponding definitions in SwedeHF are reported in Supplementary material online, Table S1.12 In particular, SOLOIST-WHF required T2DM and hospitalization for recent worsening HF but allowed enrolment in the first few days after discharge. In this study only inpatients discharged alive were included. Intravenous diuretic usage was not captured in SwedeHF, so we assumed that all patients hospitalized for acute HF met this criterion.
Statistical analyses
Eligibility estimates were reported as frequencies (percentages) and represent the remaining cohort after applying the respective inclusion and exclusion criteria.
Baseline characteristics were reported as frequencies (percentages) for categorical and as median [interquartile range (IQR)] for continuous variables and compared in eligible vs. non-eligible patients by χ2 and Mann–Whitney tests, respectively.
In the main analysis, missing data were handled by single imputation (since one single value was needed for eligibility calculation) using R Software Package multiple imputation by chained equations (MICE). Imputed variables are shown in Table 1. Incidence rates (per 100 patient-years) for the composite endpoint of HF hospitalization or CV death, first HF hospitalization, all-cause death, CV death, and non-CV death were calculated and compared in eligible vs. non-eligible patients by exact Poisson test. Baseline characteristics affect eligibility and when assessing outcomes, we wanted all these factors to be reflected. Therefore, when assessing the association between eligibility and outcomes, no multivariable adjustment was performed.
Baseline characteristics in patients eligible vs. non-eligible based on the SOLOIST WHF enrolment criteria
| Number of Patients . | Missing % . | Non-eligible . | Eligible . | P-value . |
|---|---|---|---|---|
| . | . | 3970 (72.8%) . | 1483 (27.2%) . | . |
| Demographics | ||||
| Sex—female | 0 | 1558 (39.2) | 447 (30.1) | <0.001 |
| Age—median (IQR) | 0 | 78 (70, 85) | 75 (69, 80) | <0.001 |
| Follow-up location* | 4 | 0.339 | ||
| Hospital | 2206 (55.6) | 838 (56.5) | ||
| Primary care | 1632 (41.1) | 586 (39.5) | ||
| Other | 132 (3.3) | 59 (4.0) | ||
| Follow-up HF nurse-led clinic* | 7 | 1746 (44.0) | 684 (46.1) | 0.116 |
| Year of registration | 0 | 0.001 | ||
| 2010–14 | 2121 (53.4) | 871 (58.7) | ||
| 2015–18 | 1849 (46.6) | 612 (41.3) | ||
| Family type* | <1 | 0.007 | ||
| Cohabitating | 1781 (44.9) | 727 (49.0) | ||
| Living alone | 2189 (55.1) | 756 (51.0) | ||
| Education level* | 3 | 0.140 | ||
| University | 502 (12.6) | 165 (11.1) | ||
| Secondary school or less | 3468 (87.4) | 1318 (88.9) | ||
| Income* | <1 | 0.081 | ||
| Low | 1642 (41.4) | 625 (42.1) | ||
| Medium | 1552 (39.1) | 607 (40.9) | ||
| High | 776 (19.5) | 251 (16.9) | ||
| Clinical characteristics and laboratory measurements | ||||
| Duration HF ≥ 6 months* | 4 | 2193 (57.8) | 1204 (83.0) | <0.001 |
| NYHA class at discharge* | 57 | <0.001 | ||
| I | 237 (6.0) | 51 (3.4) | ||
| II | 1370 (34.5) | 451 (30.4) | ||
| III | 1900 (47.9) | 772 (52.1) | ||
| IV | 463 (11.7) | 209 (14.1) | ||
| HF phenotype | 0 | <0.001 | ||
| HFrEF | 1893 (47.7) | 909 (61.3) | ||
| HFmrEF | 783 (19.7) | 257 (17.3) | ||
| HFpEF | 1294 (32.6) | 317 (21.4) | ||
| BMI* | 23 | 0.458 | ||
| <18.5 kg/m2 | 50 (1.3) | 18 (1.2) | ||
| ≥18.5–24 kg/m2 | 976 (24.6) | 345 (23.3) | ||
| ≥25–29 kg/m2 | 1354 (34.1) | 491 (33.1) | ||
| ≥30 kg/m2 | 1590 (40.1) | 629 (42.4) | ||
| Systolic blood pressure (mmHg)—median (IQR)* | <1 | 130 (114, 143) | 122 (110, 140) | <0.001 |
| Diastolic blood pressure (mmHg)—median (IQR)* | <1 | 70 (63, 80) | 70 (60, 80) | 0.003 |
| Heart rate bpm* median (IQR) | <1 | 74 (65, 85) | 74 (65, 85) | 0.689 |
| ECG rhythm* | 14 | 0.146 | ||
| Sinus | 2092 (52.7) | 748 (50.4) | ||
| Atrial fibrillation | 1878 (47.3) | 735 (49.6) | ||
| eGFR* | <1 | <0.001 | ||
| <30 ml/min/1.73 m2 | 987 (24.9) | 0 (0.0) | ||
| 30-59 ml/min/1.73 m2 | 1600 (40.4) | 986 (66.8) | ||
| ≥60 ml/min/1.73 m2 | 1369 (34.6) | 491 (33.2) | ||
| Haemoglobin (g/L) median (IQR) | 1 | 123 (111, 136) | 123 (112, 138) | 0.041 |
| Anaemia* | 1 | 2148 (54.1) | 799 (53.9) | 0.904 |
| NT-proBNP (ng/L) median (IQR)* | 46 | 4002 (1561, 9301) | 4698 (2760, 8975) | <0.001 |
| Treatments | ||||
| RASi/ARNI* | 1 | 3015 (75.9) | 1243 (83.8) | <0.001 |
| Betablocker* | <1 | 3497 (88.1) | 1346 (90.8) | 0.006 |
| MRA* | <1 | 1463 (36.9) | 719 (48.5) | <0.001 |
| Digoxin* | <1 | 448 (11.3) | 240 (16.2) | <0.001 |
| Loop diuretics* | 6 | 3554 (89.5) | 1483 (100.0) | <0.001 |
| Nitrates* | <1 | 783 (19.7) | 317 (21.4) | 0.188 |
| Antiplatelet therapy* | <1 | 1787 (45.0) | 629 (42.4) | 0.091 |
| Anticoagulant therapy* | <1 | 1802 (45.4) | 793 (53.5) | <0.001 |
| Statins* | <1 | 2411 (60.7) | 1016 (68.5) | <0.001 |
| HF Device* | <1 | <0.001 | ||
| None | 3222 (81.2) | 1120 (75.5) | ||
| Pacemaker | 467 (11.8) | 167 (11.3) | ||
| CRT-P | 72 (1.8) | 42 (2.8) | ||
| CRT-D | 115 (2.9) | 89 (6.0) | ||
| ICD | 94 (2.4) | 65 (4.4) | ||
| Comorbidities | ||||
| Smoking* | 25 | <0.001 | ||
| Current | 414 (10.4) | 184 (12.4) | ||
| Former | 1754 (44.2) | 756 (51.0) | ||
| Never | 1802 (45.4) | 543 (36.6) | ||
| Hypertension* | <1 | 3456 (87.1) | 1268 (85.5) | 0.146 |
| Diabetes | 0 | 1 | ||
| None | 0 (0.0) | 0 (0.0) | ||
| Type I | 0 (0.0) | 0 (0.0) | ||
| Type II | 3970 (100.0) | 1483 (100.0) | ||
| Myocardial infarction* | <1 | 1868 (47.1) | 843 (56.8) | <0.001 |
| Coronary Revascularization* | <1 | 1359 (34.2) | 681 (45.9) | <0.001 |
| Stroke/TIA* | <1 | 912 (23.0) | 332 (22.4) | 0.673 |
| Valvular disease* | <1 | 873 (22.0) | 407 (27.4) | <0.001 |
| Malignancies within 7 months* | <1 | 131 (3.3) | 0 (0.0) | <0.001 |
| COPD* | <1 | 648 (16.3) | 380 (25.6) | <0.001 |
| Liver disease within 1 year* | <1 | 214 (5.4) | 0 (0.0) | <0.001 |
| Number of Patients . | Missing % . | Non-eligible . | Eligible . | P-value . |
|---|---|---|---|---|
| . | . | 3970 (72.8%) . | 1483 (27.2%) . | . |
| Demographics | ||||
| Sex—female | 0 | 1558 (39.2) | 447 (30.1) | <0.001 |
| Age—median (IQR) | 0 | 78 (70, 85) | 75 (69, 80) | <0.001 |
| Follow-up location* | 4 | 0.339 | ||
| Hospital | 2206 (55.6) | 838 (56.5) | ||
| Primary care | 1632 (41.1) | 586 (39.5) | ||
| Other | 132 (3.3) | 59 (4.0) | ||
| Follow-up HF nurse-led clinic* | 7 | 1746 (44.0) | 684 (46.1) | 0.116 |
| Year of registration | 0 | 0.001 | ||
| 2010–14 | 2121 (53.4) | 871 (58.7) | ||
| 2015–18 | 1849 (46.6) | 612 (41.3) | ||
| Family type* | <1 | 0.007 | ||
| Cohabitating | 1781 (44.9) | 727 (49.0) | ||
| Living alone | 2189 (55.1) | 756 (51.0) | ||
| Education level* | 3 | 0.140 | ||
| University | 502 (12.6) | 165 (11.1) | ||
| Secondary school or less | 3468 (87.4) | 1318 (88.9) | ||
| Income* | <1 | 0.081 | ||
| Low | 1642 (41.4) | 625 (42.1) | ||
| Medium | 1552 (39.1) | 607 (40.9) | ||
| High | 776 (19.5) | 251 (16.9) | ||
| Clinical characteristics and laboratory measurements | ||||
| Duration HF ≥ 6 months* | 4 | 2193 (57.8) | 1204 (83.0) | <0.001 |
| NYHA class at discharge* | 57 | <0.001 | ||
| I | 237 (6.0) | 51 (3.4) | ||
| II | 1370 (34.5) | 451 (30.4) | ||
| III | 1900 (47.9) | 772 (52.1) | ||
| IV | 463 (11.7) | 209 (14.1) | ||
| HF phenotype | 0 | <0.001 | ||
| HFrEF | 1893 (47.7) | 909 (61.3) | ||
| HFmrEF | 783 (19.7) | 257 (17.3) | ||
| HFpEF | 1294 (32.6) | 317 (21.4) | ||
| BMI* | 23 | 0.458 | ||
| <18.5 kg/m2 | 50 (1.3) | 18 (1.2) | ||
| ≥18.5–24 kg/m2 | 976 (24.6) | 345 (23.3) | ||
| ≥25–29 kg/m2 | 1354 (34.1) | 491 (33.1) | ||
| ≥30 kg/m2 | 1590 (40.1) | 629 (42.4) | ||
| Systolic blood pressure (mmHg)—median (IQR)* | <1 | 130 (114, 143) | 122 (110, 140) | <0.001 |
| Diastolic blood pressure (mmHg)—median (IQR)* | <1 | 70 (63, 80) | 70 (60, 80) | 0.003 |
| Heart rate bpm* median (IQR) | <1 | 74 (65, 85) | 74 (65, 85) | 0.689 |
| ECG rhythm* | 14 | 0.146 | ||
| Sinus | 2092 (52.7) | 748 (50.4) | ||
| Atrial fibrillation | 1878 (47.3) | 735 (49.6) | ||
| eGFR* | <1 | <0.001 | ||
| <30 ml/min/1.73 m2 | 987 (24.9) | 0 (0.0) | ||
| 30-59 ml/min/1.73 m2 | 1600 (40.4) | 986 (66.8) | ||
| ≥60 ml/min/1.73 m2 | 1369 (34.6) | 491 (33.2) | ||
| Haemoglobin (g/L) median (IQR) | 1 | 123 (111, 136) | 123 (112, 138) | 0.041 |
| Anaemia* | 1 | 2148 (54.1) | 799 (53.9) | 0.904 |
| NT-proBNP (ng/L) median (IQR)* | 46 | 4002 (1561, 9301) | 4698 (2760, 8975) | <0.001 |
| Treatments | ||||
| RASi/ARNI* | 1 | 3015 (75.9) | 1243 (83.8) | <0.001 |
| Betablocker* | <1 | 3497 (88.1) | 1346 (90.8) | 0.006 |
| MRA* | <1 | 1463 (36.9) | 719 (48.5) | <0.001 |
| Digoxin* | <1 | 448 (11.3) | 240 (16.2) | <0.001 |
| Loop diuretics* | 6 | 3554 (89.5) | 1483 (100.0) | <0.001 |
| Nitrates* | <1 | 783 (19.7) | 317 (21.4) | 0.188 |
| Antiplatelet therapy* | <1 | 1787 (45.0) | 629 (42.4) | 0.091 |
| Anticoagulant therapy* | <1 | 1802 (45.4) | 793 (53.5) | <0.001 |
| Statins* | <1 | 2411 (60.7) | 1016 (68.5) | <0.001 |
| HF Device* | <1 | <0.001 | ||
| None | 3222 (81.2) | 1120 (75.5) | ||
| Pacemaker | 467 (11.8) | 167 (11.3) | ||
| CRT-P | 72 (1.8) | 42 (2.8) | ||
| CRT-D | 115 (2.9) | 89 (6.0) | ||
| ICD | 94 (2.4) | 65 (4.4) | ||
| Comorbidities | ||||
| Smoking* | 25 | <0.001 | ||
| Current | 414 (10.4) | 184 (12.4) | ||
| Former | 1754 (44.2) | 756 (51.0) | ||
| Never | 1802 (45.4) | 543 (36.6) | ||
| Hypertension* | <1 | 3456 (87.1) | 1268 (85.5) | 0.146 |
| Diabetes | 0 | 1 | ||
| None | 0 (0.0) | 0 (0.0) | ||
| Type I | 0 (0.0) | 0 (0.0) | ||
| Type II | 3970 (100.0) | 1483 (100.0) | ||
| Myocardial infarction* | <1 | 1868 (47.1) | 843 (56.8) | <0.001 |
| Coronary Revascularization* | <1 | 1359 (34.2) | 681 (45.9) | <0.001 |
| Stroke/TIA* | <1 | 912 (23.0) | 332 (22.4) | 0.673 |
| Valvular disease* | <1 | 873 (22.0) | 407 (27.4) | <0.001 |
| Malignancies within 7 months* | <1 | 131 (3.3) | 0 (0.0) | <0.001 |
| COPD* | <1 | 648 (16.3) | 380 (25.6) | <0.001 |
| Liver disease within 1 year* | <1 | 214 (5.4) | 0 (0.0) | <0.001 |
Variables used in the imputation model are marked with *.
IQR, interquartile range; HF, heart failure; HFpEF, HF with preserved ejection fraction; HFmrEF, HF with mildly reduced ejection fraction; HFrEF; HF with reduced ejection fraction; NYHA, New York Heart Association; BMI, body mass index, SD, standard deviation; bpm, beats per minute; ECG, electrocardiogram; NT-proBNP, N-terminal pro-brain natriuretic peptide; RASi, renin angiotensin system inhibitor; ARNI, angiotensin receptor neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist; CRT-P/D; cardiac resynchronization therapy pacemaker/defibrillator; ICD, implantable cardioverter defibrillator; TIA, transient ischaemic attack; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate.
Baseline characteristics in patients eligible vs. non-eligible based on the SOLOIST WHF enrolment criteria
| Number of Patients . | Missing % . | Non-eligible . | Eligible . | P-value . |
|---|---|---|---|---|
| . | . | 3970 (72.8%) . | 1483 (27.2%) . | . |
| Demographics | ||||
| Sex—female | 0 | 1558 (39.2) | 447 (30.1) | <0.001 |
| Age—median (IQR) | 0 | 78 (70, 85) | 75 (69, 80) | <0.001 |
| Follow-up location* | 4 | 0.339 | ||
| Hospital | 2206 (55.6) | 838 (56.5) | ||
| Primary care | 1632 (41.1) | 586 (39.5) | ||
| Other | 132 (3.3) | 59 (4.0) | ||
| Follow-up HF nurse-led clinic* | 7 | 1746 (44.0) | 684 (46.1) | 0.116 |
| Year of registration | 0 | 0.001 | ||
| 2010–14 | 2121 (53.4) | 871 (58.7) | ||
| 2015–18 | 1849 (46.6) | 612 (41.3) | ||
| Family type* | <1 | 0.007 | ||
| Cohabitating | 1781 (44.9) | 727 (49.0) | ||
| Living alone | 2189 (55.1) | 756 (51.0) | ||
| Education level* | 3 | 0.140 | ||
| University | 502 (12.6) | 165 (11.1) | ||
| Secondary school or less | 3468 (87.4) | 1318 (88.9) | ||
| Income* | <1 | 0.081 | ||
| Low | 1642 (41.4) | 625 (42.1) | ||
| Medium | 1552 (39.1) | 607 (40.9) | ||
| High | 776 (19.5) | 251 (16.9) | ||
| Clinical characteristics and laboratory measurements | ||||
| Duration HF ≥ 6 months* | 4 | 2193 (57.8) | 1204 (83.0) | <0.001 |
| NYHA class at discharge* | 57 | <0.001 | ||
| I | 237 (6.0) | 51 (3.4) | ||
| II | 1370 (34.5) | 451 (30.4) | ||
| III | 1900 (47.9) | 772 (52.1) | ||
| IV | 463 (11.7) | 209 (14.1) | ||
| HF phenotype | 0 | <0.001 | ||
| HFrEF | 1893 (47.7) | 909 (61.3) | ||
| HFmrEF | 783 (19.7) | 257 (17.3) | ||
| HFpEF | 1294 (32.6) | 317 (21.4) | ||
| BMI* | 23 | 0.458 | ||
| <18.5 kg/m2 | 50 (1.3) | 18 (1.2) | ||
| ≥18.5–24 kg/m2 | 976 (24.6) | 345 (23.3) | ||
| ≥25–29 kg/m2 | 1354 (34.1) | 491 (33.1) | ||
| ≥30 kg/m2 | 1590 (40.1) | 629 (42.4) | ||
| Systolic blood pressure (mmHg)—median (IQR)* | <1 | 130 (114, 143) | 122 (110, 140) | <0.001 |
| Diastolic blood pressure (mmHg)—median (IQR)* | <1 | 70 (63, 80) | 70 (60, 80) | 0.003 |
| Heart rate bpm* median (IQR) | <1 | 74 (65, 85) | 74 (65, 85) | 0.689 |
| ECG rhythm* | 14 | 0.146 | ||
| Sinus | 2092 (52.7) | 748 (50.4) | ||
| Atrial fibrillation | 1878 (47.3) | 735 (49.6) | ||
| eGFR* | <1 | <0.001 | ||
| <30 ml/min/1.73 m2 | 987 (24.9) | 0 (0.0) | ||
| 30-59 ml/min/1.73 m2 | 1600 (40.4) | 986 (66.8) | ||
| ≥60 ml/min/1.73 m2 | 1369 (34.6) | 491 (33.2) | ||
| Haemoglobin (g/L) median (IQR) | 1 | 123 (111, 136) | 123 (112, 138) | 0.041 |
| Anaemia* | 1 | 2148 (54.1) | 799 (53.9) | 0.904 |
| NT-proBNP (ng/L) median (IQR)* | 46 | 4002 (1561, 9301) | 4698 (2760, 8975) | <0.001 |
| Treatments | ||||
| RASi/ARNI* | 1 | 3015 (75.9) | 1243 (83.8) | <0.001 |
| Betablocker* | <1 | 3497 (88.1) | 1346 (90.8) | 0.006 |
| MRA* | <1 | 1463 (36.9) | 719 (48.5) | <0.001 |
| Digoxin* | <1 | 448 (11.3) | 240 (16.2) | <0.001 |
| Loop diuretics* | 6 | 3554 (89.5) | 1483 (100.0) | <0.001 |
| Nitrates* | <1 | 783 (19.7) | 317 (21.4) | 0.188 |
| Antiplatelet therapy* | <1 | 1787 (45.0) | 629 (42.4) | 0.091 |
| Anticoagulant therapy* | <1 | 1802 (45.4) | 793 (53.5) | <0.001 |
| Statins* | <1 | 2411 (60.7) | 1016 (68.5) | <0.001 |
| HF Device* | <1 | <0.001 | ||
| None | 3222 (81.2) | 1120 (75.5) | ||
| Pacemaker | 467 (11.8) | 167 (11.3) | ||
| CRT-P | 72 (1.8) | 42 (2.8) | ||
| CRT-D | 115 (2.9) | 89 (6.0) | ||
| ICD | 94 (2.4) | 65 (4.4) | ||
| Comorbidities | ||||
| Smoking* | 25 | <0.001 | ||
| Current | 414 (10.4) | 184 (12.4) | ||
| Former | 1754 (44.2) | 756 (51.0) | ||
| Never | 1802 (45.4) | 543 (36.6) | ||
| Hypertension* | <1 | 3456 (87.1) | 1268 (85.5) | 0.146 |
| Diabetes | 0 | 1 | ||
| None | 0 (0.0) | 0 (0.0) | ||
| Type I | 0 (0.0) | 0 (0.0) | ||
| Type II | 3970 (100.0) | 1483 (100.0) | ||
| Myocardial infarction* | <1 | 1868 (47.1) | 843 (56.8) | <0.001 |
| Coronary Revascularization* | <1 | 1359 (34.2) | 681 (45.9) | <0.001 |
| Stroke/TIA* | <1 | 912 (23.0) | 332 (22.4) | 0.673 |
| Valvular disease* | <1 | 873 (22.0) | 407 (27.4) | <0.001 |
| Malignancies within 7 months* | <1 | 131 (3.3) | 0 (0.0) | <0.001 |
| COPD* | <1 | 648 (16.3) | 380 (25.6) | <0.001 |
| Liver disease within 1 year* | <1 | 214 (5.4) | 0 (0.0) | <0.001 |
| Number of Patients . | Missing % . | Non-eligible . | Eligible . | P-value . |
|---|---|---|---|---|
| . | . | 3970 (72.8%) . | 1483 (27.2%) . | . |
| Demographics | ||||
| Sex—female | 0 | 1558 (39.2) | 447 (30.1) | <0.001 |
| Age—median (IQR) | 0 | 78 (70, 85) | 75 (69, 80) | <0.001 |
| Follow-up location* | 4 | 0.339 | ||
| Hospital | 2206 (55.6) | 838 (56.5) | ||
| Primary care | 1632 (41.1) | 586 (39.5) | ||
| Other | 132 (3.3) | 59 (4.0) | ||
| Follow-up HF nurse-led clinic* | 7 | 1746 (44.0) | 684 (46.1) | 0.116 |
| Year of registration | 0 | 0.001 | ||
| 2010–14 | 2121 (53.4) | 871 (58.7) | ||
| 2015–18 | 1849 (46.6) | 612 (41.3) | ||
| Family type* | <1 | 0.007 | ||
| Cohabitating | 1781 (44.9) | 727 (49.0) | ||
| Living alone | 2189 (55.1) | 756 (51.0) | ||
| Education level* | 3 | 0.140 | ||
| University | 502 (12.6) | 165 (11.1) | ||
| Secondary school or less | 3468 (87.4) | 1318 (88.9) | ||
| Income* | <1 | 0.081 | ||
| Low | 1642 (41.4) | 625 (42.1) | ||
| Medium | 1552 (39.1) | 607 (40.9) | ||
| High | 776 (19.5) | 251 (16.9) | ||
| Clinical characteristics and laboratory measurements | ||||
| Duration HF ≥ 6 months* | 4 | 2193 (57.8) | 1204 (83.0) | <0.001 |
| NYHA class at discharge* | 57 | <0.001 | ||
| I | 237 (6.0) | 51 (3.4) | ||
| II | 1370 (34.5) | 451 (30.4) | ||
| III | 1900 (47.9) | 772 (52.1) | ||
| IV | 463 (11.7) | 209 (14.1) | ||
| HF phenotype | 0 | <0.001 | ||
| HFrEF | 1893 (47.7) | 909 (61.3) | ||
| HFmrEF | 783 (19.7) | 257 (17.3) | ||
| HFpEF | 1294 (32.6) | 317 (21.4) | ||
| BMI* | 23 | 0.458 | ||
| <18.5 kg/m2 | 50 (1.3) | 18 (1.2) | ||
| ≥18.5–24 kg/m2 | 976 (24.6) | 345 (23.3) | ||
| ≥25–29 kg/m2 | 1354 (34.1) | 491 (33.1) | ||
| ≥30 kg/m2 | 1590 (40.1) | 629 (42.4) | ||
| Systolic blood pressure (mmHg)—median (IQR)* | <1 | 130 (114, 143) | 122 (110, 140) | <0.001 |
| Diastolic blood pressure (mmHg)—median (IQR)* | <1 | 70 (63, 80) | 70 (60, 80) | 0.003 |
| Heart rate bpm* median (IQR) | <1 | 74 (65, 85) | 74 (65, 85) | 0.689 |
| ECG rhythm* | 14 | 0.146 | ||
| Sinus | 2092 (52.7) | 748 (50.4) | ||
| Atrial fibrillation | 1878 (47.3) | 735 (49.6) | ||
| eGFR* | <1 | <0.001 | ||
| <30 ml/min/1.73 m2 | 987 (24.9) | 0 (0.0) | ||
| 30-59 ml/min/1.73 m2 | 1600 (40.4) | 986 (66.8) | ||
| ≥60 ml/min/1.73 m2 | 1369 (34.6) | 491 (33.2) | ||
| Haemoglobin (g/L) median (IQR) | 1 | 123 (111, 136) | 123 (112, 138) | 0.041 |
| Anaemia* | 1 | 2148 (54.1) | 799 (53.9) | 0.904 |
| NT-proBNP (ng/L) median (IQR)* | 46 | 4002 (1561, 9301) | 4698 (2760, 8975) | <0.001 |
| Treatments | ||||
| RASi/ARNI* | 1 | 3015 (75.9) | 1243 (83.8) | <0.001 |
| Betablocker* | <1 | 3497 (88.1) | 1346 (90.8) | 0.006 |
| MRA* | <1 | 1463 (36.9) | 719 (48.5) | <0.001 |
| Digoxin* | <1 | 448 (11.3) | 240 (16.2) | <0.001 |
| Loop diuretics* | 6 | 3554 (89.5) | 1483 (100.0) | <0.001 |
| Nitrates* | <1 | 783 (19.7) | 317 (21.4) | 0.188 |
| Antiplatelet therapy* | <1 | 1787 (45.0) | 629 (42.4) | 0.091 |
| Anticoagulant therapy* | <1 | 1802 (45.4) | 793 (53.5) | <0.001 |
| Statins* | <1 | 2411 (60.7) | 1016 (68.5) | <0.001 |
| HF Device* | <1 | <0.001 | ||
| None | 3222 (81.2) | 1120 (75.5) | ||
| Pacemaker | 467 (11.8) | 167 (11.3) | ||
| CRT-P | 72 (1.8) | 42 (2.8) | ||
| CRT-D | 115 (2.9) | 89 (6.0) | ||
| ICD | 94 (2.4) | 65 (4.4) | ||
| Comorbidities | ||||
| Smoking* | 25 | <0.001 | ||
| Current | 414 (10.4) | 184 (12.4) | ||
| Former | 1754 (44.2) | 756 (51.0) | ||
| Never | 1802 (45.4) | 543 (36.6) | ||
| Hypertension* | <1 | 3456 (87.1) | 1268 (85.5) | 0.146 |
| Diabetes | 0 | 1 | ||
| None | 0 (0.0) | 0 (0.0) | ||
| Type I | 0 (0.0) | 0 (0.0) | ||
| Type II | 3970 (100.0) | 1483 (100.0) | ||
| Myocardial infarction* | <1 | 1868 (47.1) | 843 (56.8) | <0.001 |
| Coronary Revascularization* | <1 | 1359 (34.2) | 681 (45.9) | <0.001 |
| Stroke/TIA* | <1 | 912 (23.0) | 332 (22.4) | 0.673 |
| Valvular disease* | <1 | 873 (22.0) | 407 (27.4) | <0.001 |
| Malignancies within 7 months* | <1 | 131 (3.3) | 0 (0.0) | <0.001 |
| COPD* | <1 | 648 (16.3) | 380 (25.6) | <0.001 |
| Liver disease within 1 year* | <1 | 214 (5.4) | 0 (0.0) | <0.001 |
Variables used in the imputation model are marked with *.
IQR, interquartile range; HF, heart failure; HFpEF, HF with preserved ejection fraction; HFmrEF, HF with mildly reduced ejection fraction; HFrEF; HF with reduced ejection fraction; NYHA, New York Heart Association; BMI, body mass index, SD, standard deviation; bpm, beats per minute; ECG, electrocardiogram; NT-proBNP, N-terminal pro-brain natriuretic peptide; RASi, renin angiotensin system inhibitor; ARNI, angiotensin receptor neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist; CRT-P/D; cardiac resynchronization therapy pacemaker/defibrillator; ICD, implantable cardioverter defibrillator; TIA, transient ischaemic attack; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate.
Two consistency analyses were performed: (i) only patients with available entries for the variable needed for the eligibility assessment were considered (complete-case analysis); (ii) patients with missing entries for variables needed for eligibility assessment were considered as fulfilling eligibility criteria.
A P-value < 0.05, two-sided, was considered as statistically significant. All analyses were performed using R Software version 3.5.3.
Results
Eligibility proportions for sotagliflozin according to the literal and the pragmatic scenarios
A total of 5453 unique inpatients with T2DM and hospitalized with HF were considered for this analysis. Of these, 36.7% were female and the median age was 77 (IQR 70–83) years; 51.4% had HFrEF, 19.1% HFmrEF, and 29.5% HFpEF.
In the literal scenario, overall eligibility for sotagliflozin was 27.2%, 32.4% in HFrEF, 24.7% in HFmrEF, and 19.7% in HFpEF (Graphical Abstract, Supplementary material online, Tables S2–5). Inclusion criteria were fulfilled by 53.6% of patients. After applying all exclusion criteria 54.1% of patients were eligible (Supplementary material online, Table S2).
Common inclusion criterion limiting eligibility were HF duration ≥3 months, 65.8% of patients eligible after applying this criterion; NT-proBNP ≥600 pg/mL or ≥1800 pg/mL with atrial fibrillation, 85.3% eligible after applying this criterion); and continued use of loop diuretics after stabilization in the hospital (92.4% eligible after applying this criterion) (Figure 1, Supplementary material online, Table S2). Major exclusion criteria were eGFR <30 mL/min/1.73 m² (81.8% still eligible), age >85 years (82.9% still eligible), and a history of acute coronary syndrome (ACS) within 3 months (91.3% still eligible) (Figure 1, Supplementary material online, Table S2).
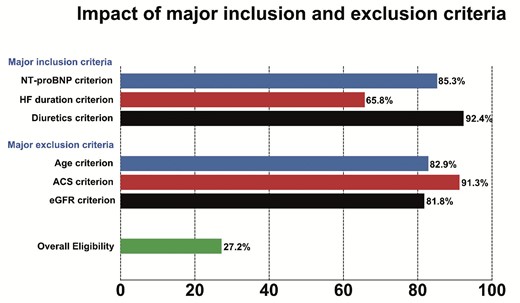
Impact of major inclusion and exclusion criteria based on the SOLOIST-WHF enrolment criteria.
In the pragmatic scenario, eligibility was 62.8% (69.1% in HFrEF, 60.3% in HFmrEF, and 53.4% in HFpEF). In addition, 79.3% of patients fulfilled the inclusion criteria, whereas 81.8% of patients were eligible after considering only the exclusion criteria (Figure 2, Supplementary material online, Tables S3–5).
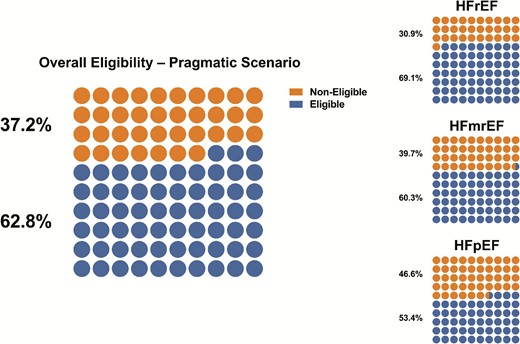
Overall eligibility and eligibility rates across the EF spectrum in patients eligible vs. non-eligible based on the pragmatic scenario (only enrolment criteria more likely to influence treatment decision).
A sensitivity analysis was performed in 12 826 inpatients without T2DM (not eligible in SOLOIST-WHF but relevant for the SGLT2i field) and hospitalized due to worsening HF and revealed that 42.6% were female and the median age was 80 (IQR 70–86) years. Of these, 54.6% had HFrEF, 17.7% HFmrEF, and 27.7% HFpEF. In the literal scenario, eligibility was 19.3%, (21.1% in HFrEF, 18.8% in HFmrEF, and 16.0% in HFpEF, slightly lower than in patients with T2DM) (Supplementary material online, Figure S1, Supplementary material online, Tables S6–9). Reasons for ineligibility were distributed similarly in patients without T2DM as with T2DM (Supplementary material online, Figure S2, Table S6). In the pragmatic scenario, overall eligibility for sotagliflozin was 67.0%, 69.5% were eligible in HFrEF, 65.5% in HFmrEF, and 62.9% in HFpEF in patients without T2DM. In addition, 78.0% of patients fulfilled the inclusion criteria, whereas 87.9% of patients were eligible after considering only the exclusion criteria (Supplementary Figure S3, Supplementary material online, Table S6). Overall, the findings in the non-T2DM pragmatic scenario were similar to those in patients with T2DM.
Clinical characteristics in eligible vs. non-eligible patients
Eligible vs. non-eligible patients were younger, less likely female, and more likely to have HFrEF and more severe/symptomatic HF [e.g. higher NT-proBNP levels, higher NYHA (New York Heart Association) class, use of mineralocorticoid receptor antagonists (MRAs), and longer duration of HF] (Table 1). Eligible patients were also more likely to have better kidney function (in part because eGFR ≥30 ml/min/1.73 m2 was required) and to have a higher CV comorbidity burden, with a more frequent history of myocardial infarction and coronary revascularization. Eligible patients were more likely to be treated with renin-angiotensin-system/angiotensin-receptor-neprilysin inhibitors (RASi/ARNI), betablockers, digoxin, statins, oral anticoagulants, and HF device therapies (implantable cardioverter defibrillator or cardiac resynchronization therapy) (Table 1).
In the pragmatic scenario, eligible vs. non-eligible patients were again younger, less likely female, and had more severe HF (e.g. higher NT-proBNP levels and use of MRAs). History of comorbidities such as atrial fibrillation, hypertension, and anaemia were less likely in eligible compared with non-eligible patients (Supplementary material online, Table S10).
The results observed in the main analysis were overall consistent across the EF spectrum, except for patients with HFpEF (Supplementary material online, Tables S11–13) with no differences in NYHA class and NT-proBNP levels in eligible compared with non-eligible patients in HFpEF (Supplementary material online, Table S10, S13).
In the sensitivity analysis, the characteristics of patients without T2DM according to their eligibility status were overall similar to those observed in patients with T2DM (Supplementary material online, Tables S14–18).
Outcomes in eligible vs. non-eligible patients
Event rates for all measured outcomes in eligible vs. non-eligible patients are shown in Figure 3 and Supplementary material online, Table S19. In the literal scenario, eligible patients had higher event rates for the composite endpoint of HF hospitalization or CV death, HF hospitalization, all-cause death, and CV death, but not non-CV death, compared with non-eligible patients. Similar results were observed in HFrEF (Supplementary material online, Table S20). In contrast, there were no differences for any measured outcomes in eligible vs. non-eligible patients in the literal scenario in HFmrEF and HFpEF (Supplementary material online, Tables S21–22).
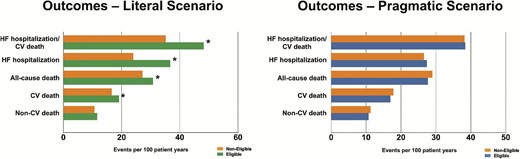
Outcome analysis in patients eligible vs. non-eligible based on the SOLOIST-WHF enrolment criteria. *P-value < 0.05.
In the pragmatic scenario, there were no differences in event rates for any measured outcomes in eligible vs. non-eligible patients (Figure 3, Supplementary material online, Table S19). Only in HFrEF, eligible vs. non-eligible patients had significantly higher rates of all-cause death and CV death (Supplementary material online, Tables S23–26).
In the sensitivity analysis of patients without T2DM, in the literal scenario, overall and in the different EF categories, and for most outcomes, the results were similar to in patients with T2DM. In the pragmatic scenario, with T2DM eligible vs. ineligible patients had similar event rates for most outcomes, whereas without T2DM, eligible patients had higher event rates for CV outcomes (Supplementary material online, Figure S4, Tables S24–26).
Consistency analysis
As compared with the main analysis (where missing values were imputed), eligibility rates were overall consistent in the complete-case analysis but as expected, slightly higher when missing entries were considered as eligible (Supplementary material online, Table S27).
Discussion
In this comprehensive assessment on eligibility for sotagliflozin in a real-world HF population, we found that (i) ∼1/3 of patients with T2DM hospitalized due to recent worsening HF were eligible based on the literal enrolment criteria; (ii) eligibility was considerably higher with pragmatic interpretation of the enrolment criteria (∼2/3); (iii) patients who were eligible vs. non-eligible had more severe/symptomatic HF, higher comorbidity burden, higher use of HF treatments and higher rates of the composite of HF hospitalization or CV death, HF hospitalization, all-cause death, and CV death, but not higher rates of non-CV death; (iv) the proportion of patients eligible vs. non-eligible increased with decreasing EF; (v) the literal vs. the pragmatic enrolment criteria identified higher risk patients; and (vi) in the sensitivity analysis, we observed overall similar results in patients without T2DM.
Proportions of patients with type 2 diabetes mellitus and worsening heart failure eligible for Sotagliflozin
In this study, ∼1/3 of the population met enrolment criteria for sotagliflozin from the SOLOIST-WHF trial. Eligibility for sotagliflozin significantly increases with a more pragmatic interpretation of the enrolment criteria (∼2/3 of the population), e.g. enrolment criteria most likely to be applied in clinical practice. A recent analysis from SwedeHF found that eligibility in chronic HFrEF and HFpEF for the SGLT2i empagliflozin and dapagliflozin) ranged between 30% and 35% when applying all the (literal) inclusion and exclusion criteria.18
In hospitalized HF patients without T2DM, ∼1/5 met SOLOIST-WHF literal enrolment criteria (except T2DM), which is fewer than the ∼1/3 with T2DM, and ∼2/3 met the pragmatic criteria, similar to with T2DM. As with T2DM, eligibility was higher in HFrEF vs. HFmrEF vs. HFpEF. Overall, the eligibility criteria were even more effective for selecting greater CV vs. non-CV risk in patients without T2DM, than they were with T2DM.
Short HF duration was a common exclusion criterion and is frequently used in trials to exclude new-onset HF, where clinical status often improves with the reversal of precipitating causes and treatment. RCTs that included patients with hospitalized de novo HF have also been positive, without an interaction between treatment and de novo vs. pre-existing HF.19,20 Both EMPULSE (The SGLT2 Inhibitor Empagliflozin in Patients Hospitalized for Acute Heart Failure: A Multinational Randomized Trial) and PIONEER-HF (Angiotensin–Neprilysin Inhibition in Acute Decompensated Heart Failure) trial including patients with de novo HF demonstrated efficacy and a favourable safety profile.19,20
A not trivial proportion of patients (∼15%) was non-eligible due to the pre-defined NT-proBNP thresholds (≥600 pg/mL or ≥1800 pg/mL in atrial fibrillation), although the proportion excluded for this reason was lower than in chronic HF trials. Patients with HFrEF were more likely to fulfil the NT-proBNP criterion compared with patients with HFmrEF or HFpEF, consistent with HFrEF being more severe with higher natriuretic peptide levels, both in the inpatient and outpatient setting.21 A recent 515-patient study reported eligibility of 65% for DAPA-HF and 53% for EMPEROR-Reduced.22 The main criterion leading to non-eligibility was not meeting the required NT-proBNP thresholds.9,10 This finding is in line with results from the recent analysis from SwedeHF.18
Another common reason for non-eligibility among SOLOIST-WHF exclusion criteria was eGFR <30 mL min−1/1.73 m2. This is consistent with the higher background prevalence of chronic kidney disease (CKD) in patients hospitalized for worsening HF, and the additional risk of acute kidney injury in the worsening HF setting. However, patients with CKD benefit from SGLT2i, and in SCORED (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk), sotagliflozin vs. placebo resulted in a lower risk of the composite of deaths from CV causes, HF hospitalizations, and urgent visits for HF in patients with T2DM and CKD (eGFR 25–60 mL min−1/1.73 m2).23
Exclusion based on advanced age (>85 years) limited eligibility by 10–20%. Even without formal upper age limits, older patients are less frequently considered, approached and/or accepting of trial participation. Low enrolment of older patients in CVOTs reduces the understanding of efficacy and in particular safety in this population,24 which may contribute to poor implementation of evidence-based therapy in older patients with HF.25
Recent myocardial infarction, cardiac surgery and interventions, stroke, and implantation of cardiac devices represent time-dependent exclusion criteria. Exclusion of patients with ACS within 3 months eliminated ∼10% HFrEF (10.5%) vs. HFmrEF (8.5%) vs. HFpEF (5.8%). Like new-onset HF for any reason, HF after ACS may represent a different phenotype than worsening HF. Medications effective in chronic HFrEF have generally undergone separate trials in post-ACS left ventricular dysfunction, often but not always with similar results, and indeed, trials of SGLT2i in LV dysfunction post-ACS are ongoing. This is also relevant for EF categorization, with a high prevalence ischaemic heart disease including myocardial infarction and revascularization in T2DM and HFrEF and HFmrEF but not HFpEF.26
Clinical characteristics and outcomes according to eligibility status
Eligible vs. non-eligible patients had a higher burden of CV but not non-CV comorbidities which indicates that the SOLOIST-WHF trial design was successful in enriching for potentially modifiable CV events, but also may be less generalizable to ‘real-world’ HF patients with relatively more non-CV comorbidities, especially in HFpEF. Interestingly, the differences in HF severity and CV comorbidity burden in eligible vs. non-eligible patients were more prominent in patients with HFrEF compared with HFmrEF and HFpEF. This suggests that it may be ‘easier’ to design criteria to select suitable patients in HFrEF than in HFmrEF or HFpEF. This may also explain why trials in HFmrEF and HFpEF have, until recently failed.11,12,27
Consistent with eligibility criteria selecting for more CV comorbidities and more severe HF, eligible vs. non-eligible patients also had overall higher risk for all measured outcomes except of non-CV death. It is also noteworthy that highest event rates were observed in HFrEF vs. HFmrEF vs. HFpEF. Compared with other contemporary RCTs in which high-risk patients or patients with multiple comorbidities were often excluded, the applied inclusion and exclusion criteria in the SOLOIST-WHF trial did not lead to the inclusion of a less sick study population, but instead identified HF patients with a high CV risk profile.18 Therefore, our observation of higher CV and all-cause mortality, but not non-CV mortality, in eligible patients compared with non-eligible patients suggests that the design of studies to maximize modifiable CV risk in SOLOIST-WHF can be described as successful.
The pattern of differences in characteristics and outcomes between eligible vs. non-eligible with T2DM vs. between eligible vs. non eligible without T2DM were overall similar, i.e. the eligibility criteria had similar effects regardless of T2DM status. However, extensive previous literature has demonstrated overall higher event rates in HF with vs. without T2DM.28,29 Interestingly, the difference in outcomes between T2DM yes vs. no was considerably lower in patients eligible based on literal than based on pragmatic criteria, suggesting that the detailed eligibility criteria applied in RCTs may ‘standardize’ the population within a narrower clinical and risk profile.
Limitations
Definitions of some inclusion and exclusion criteria slightly differed from the SOLOIST-WHF trial selection criteria due to the definition of variables in SwedeHF. Although more representative than trials populations, SwedeHF is optional and tends to enrol patients that are younger and with less comorbidities.30 Thus, our eligibility proportions may be understated if one considers that new-onset and post-ACS HF will become eligible over time (in SOLOIST-WHF 3 months). For the pragmatic scenario the most likely criteria to affect prescription in clinical practice were selected although these are by necessity arbitrary, and different selection of inclusion and exclusion criteria might yield different results. Ejection fraction remains an important parameter for diagnosis, phenotyping, and treatment of HF.31 Therefore, we only included patients with available data on EF. Importantly, we considered three distinct HF phenotypes, mainly characterized by EF categories. However, recent evidence suggest that EF may also be seen as a continuous spectrum in HF and modelling it as such might have impact on the results of our study. Additionally, intravenous diuretic usage during the index hospitalization was required for SOLOIST-WHF but was not captured in SwedeHF. Our findings are representative of Sweden and may be different in other health care settings.
Conclusions
In a contemporary cohort of patients with HFrEF, HFmrEF or HFpEF, and T2DM, who were stabilized in-hospital after an episode of worsening HF and discharged alive, ∼1/3 of patients were eligible for sotagliflozin applying the SOLOIST-WHF literal enrolment criteria. Eligible patients had more severe HF, a higher CV comorbidity burden, and more potentially modifiable HF and CV events and higher all-cause death but not higher non-CV death. With pragmatic interpretation of the enrolment criteria, e.g. those criteria most likely to be applied in clinical practice, ∼2/3 were eligible. SOLOIST-WHF required T2DM but considering that other SGLT2i are similarly effective in HF regardless of T2DM status, we broadly confirmed our findings in T2DM also in patients without T2DM, although eligibility in T2DM was possibly somewhat lower. These results underscore the wide eligibility of high-risk HF patients for treatment with sotagliflozin.
Acknowledgement
None
Funding
The study was funded through an EU-HORIZON grant with project number 101095479 (More-EUROPA), and a Swedish Heart and Lung Foundation grant with project number 20220680, to Dr. Savarese. Dr Lund is supported by Karolinska Institutet, the Swedish Research Council [grant 523–2014-2336], the Swedish Heart Lung Foundation [grants 20150557, 20190310], and the Stockholm County Council [grants 20170112, 20190525].
Conflict of Interest Dr Savarese reports grants and personal fees from Vifor, grants and personal fees from AstraZeneca, personal fees from Servier, grants and personal fees from Novartis, grants and personal fees from Cytokinetics, grants and personal fees from Pharmacosmos, personal fees from Medtronic, grants from Boston Scientific, grants from Merck, grants from Bayer, personal fees from TEVA outside the submitted work.
Dr Becher received funding from the German Research Foundation. All outside the submitted work.
Dr Dahlström reports grants outside this work from Boehringer Ingelheim, AstraZeneca, Pfizer, Vifor, Boston Scientific, and Roche Diagnostics and consultancies from Amgen and Novartis, and speaker fees from AstraZeneca. All outside the submitted work.
Dr Karlstrom reports personal fees from AstraZeneca and Vifor.
Dr Metra reports the following personal fees of minimal amounts in the last three years: from Actelion, Amgen, Livanova, Servier, and Vifor pharma as member of Executive or Data Monitoring Committees of sponsored clinical trials; from AstraZeneca, Abbott vascular, Bayer, Boheringer Ingelhelm, and Edwards Therapeutics for participation to advisory boards and/or speeches at sponsored meetings.
Dr Bhatt discloses the following relationships—Advisory Board: AngioWave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: AngioWave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Consultant: Broadview Ventures; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; neither I nor Brigham and Women's Hospital receive any income from this patent.) Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Braunwald's Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Takeda.
Dr Pitt served as co-chair of SOLOIST-WHF (Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure) and was on the executive committee of SCORED (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk), and received consulting fees from Sanofi/Lexicon. In addition, Dr Pitt discloses the following: consulting fees from Bayer, Astra Zeneca, Boehringer Ingelheim/Lilly, and Phasebio; consulting fees and stock options from SCPharmaceuticals, SQinnovations, G3pharmaceuticals, Relypsa/Vifor, Cereno scientific, KBP Pharmaceuticals, Sarfez, Tricida, Proton Intel, and Brainstorm Medical. Dr Pitt is chairman of the steering committee for the National Heart, Lung, and Blood Institute's TRANSFORM (Torsemide Comparison With Furosemide For Management of Heart Failure) trial and co-chair of SPIRRIT ([Spironolactone Initiation Registry Randomized Interventional Trial] from the National Heart, Lung, and Blood Institute—Swedish Heart Foundation). He holds US Patent No. 9931412 on site-specific delivery of eplerenone to the myocardium and has a pending US Patent (63/045784) on histone acetylation.
Dr Lund reports personal fees from Merck, grants and personal fees from Vifor-Fresenius, grants and personal fees from AstraZeneca, grants and personal fees from Relypsa, personal fees from Bayer, grants from Boston Scientific, personal fees from Pharmacosmos, personal fees from Abbott, personal fees from Medscape, personal fees from Myokardia, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from Sanofi, personal fees from Lexicon. All outside the submitted work.
Ms Benson has no COI to declare.
Dr Mol has no COI to declare.
Authors´ contributions
P.M.B.: data analysis, data analysis, data interpretation, manuscript drafting; G.S.: data analysis, data interpretation, manuscript drafting; L.B.: data management, data interpretation and critical revision for important intellectual content; U.D.: data interpretation, critical revision for important intellectual content; P.K.: data interpretation, critical revision for important intellectual content; M.M.: data interpretation, critical revision for important intellectual content; P.M.: data interpretation, critical revision for important intellectual content; D.L.B.: data interpretation, critical revision for important intellectual content; B.P.: data interpretation and critical revision for important intellectual content; L.H.L.: conception and design, data interpretation, critical revision for important intellectual content.
Data availability
The data that support the findings of this study are available from the agencies that administrate the registries, provided it is approved by the appropriate ethics committees and administrating agencies and that data sharing are permitted by European Union General Data Protection Regulation.
References
Author notes
These authors equally contributed as first author.



