-
PDF
- Split View
-
Views
-
Cite
Cite
Troels Yndigegn, Bertil Lindahl, Joakim Alfredsson, Jocelyne Benatar, Lisa Brandin, David Erlinge, Urban Haaga, Claes Held, Pelle Johansson, Patric Karlström, Thomas Kellerth, Toomas Marandi, Katarina Mars, Annica Ravn-Fischer, Johan Sundström, Ollie Östlund, Robin Hofmann, Tomas Jernberg, Design and rationale of randomized evaluation of decreased usage of beta-blockers after acute myocardial infarction (REDUCE-AMI), European Heart Journal - Cardiovascular Pharmacotherapy, Volume 9, Issue 2, March 2023, Pages 192–197, https://doi.org/10.1093/ehjcvp/pvac070
Close - Share Icon Share
Abstract
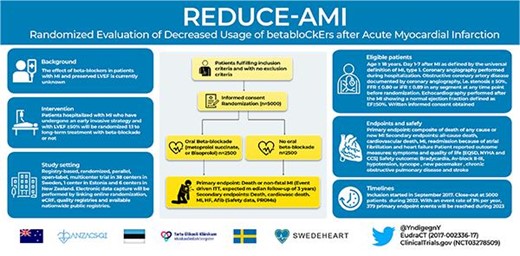
Most trials showing benefit of beta-blocker treatment after myocardial infarction (MI) included patients with large MIs and are from an era before modern biomarker-based MI diagnosis and reperfusion treatment. The aim of the randomized evaluation of decreased usage of beta-blockers after acute myocardial infarction (REDUCE-AMI) trial is to determine whether long-term oral beta-blockade in patients with an acute MI and preserved left ventricular ejection fraction (EF) reduces the composite endpoint of death of any cause or recurrent MI.
It is a registry-based, randomized, parallel, open-label, multicentre trial performed at 38 centres in Sweden, 1 centre in Estonia, and 6 centres in New Zealand. About 5000 patients with an acute MI who have undergone coronary angiography and with EF ≥ 50% will be randomized to long-term treatment with beta-blockade or not. The primary endpoint is the composite endpoint of death of any cause or new non-fatal MI. There are several secondary endpoints, including all-cause death, cardiovascular death, new MI, readmission because of heart failure and atrial fibrillation, symptoms, functional status, and health-related quality of life after 6–10 weeks and after 1 year of treatment. Safety endpoints are bradycardia, AV-block II-III, hypotension, syncope or need for pacemaker, asthma or chronic obstructive pulmonary disease, and stroke.
The results from REDUCE-AMI will add important evidence regarding the effect of beta-blockers in patients with MI and preserved EF and may change guidelines and clinical practice.
Introduction
There is solid evidence for the benefit of beta-blockers in patients with heart failure and reduced ejection fraction (EF). Prospective, randomized trials have also shown that long-term treatment with beta-blockers improves outcome and lowers mortality by ∼20% in post myocardial infarction (MI) patients.1–3 However, these trials included mainly patients with large MIs in whom left ventricular (LV) systolic dysfunction was common. In addition, most of these trials are from the 1980s, an era before the widespread use of high-sensitive troponins, percutaneous coronary intervention, antithrombotic agents, high-intensity statins, and renin-angiotensin-aldosterone system antagonists. In a meta-analysis, stratifying trials into pre-reperfusion and reperfusion era, beta-blockers did not reduce mortality in the reperfusion era.4
Long-term beta-blocker therapy has not been investigated in contemporary, adequately powered, randomized clinical trials (RCTs) in patients with acute MI with preserved systolic LV-function. Large observational studies and meta-analyses of observational studies have come to somewhat different conclusions. Dondo et al. reported no association between beta-blockers and mortality among 179 810 survivors of acute MI without heart failure or LV systolic dysfunction,5 whereas others have demonstrated a lower mortality in those receiving beta-blockers.6 Joo et al. reported no association between beta-blockers and major adverse cardiovascular events in patients with left ventricular ejection fraction (EF) ≥50% but a better outcome in those with beta-blockers if EF was mildly reduced (40–49%).7 Meta-analyses of observational studies have yielded opposing results, with some reporting association between the use of beta-blockers and survival in patients with MI and preserved EF8 whereas other meta-analyses did not find long-term beta-blockade to be associated with a lower mortality in patients with MI and EF > 40%.9,10 Finally, a recent Cochrane report concluded that new trials are needed in contemporary MI patients without heart failure to properly assess the benefits and harms of beta-blockers.11
This lack of clear evidence of benefit in the contemporary setting is reflected in the inconsistent recommendations in guidelines. Guidelines from the European Society of Cardiology (ESC) for management of ST-segment elevation myocardial infarction suggest that beta-blockers should be considered during hospital stay and continued thereafter (class 2a),12 while guidelines from the American College of Cardiology/American Heart Association (ACC/AHA) endorse a class Ia recommendation.13 For patients with acute coronary syndromes without persistent ST-segment elevation, the ESC recommends beta-blockers only to patients with reduced systolic LV function (EF ≤ 40%) (Class IA),14 whereas AHA/ACC also find it reasonable to continue beta-blocker therapy in patients with normal systolic LV function (Class IIa), despite the lack of clear evidence.15
In summary, contemporary clinical trials addressing the role of beta-blockers in patients with MI and preserved EF are lacking. Currently, results from observational studies, with their inherent limitations to address causality, point in different directions. New prospective randomized trials addressing this important issue are therefore warranted.
Methods/Design
Study objectives and hypothesis
The primary objective of the REDUCE-AMI trial is to determine whether long-term treatment with oral beta-blockade initiated early after the acute event in patients with MI and preserved LV systolic EF reduces the composite of death of any cause or new MI. We hypothesize that beta-blockers will reduce the risk of all-cause death or myocardial infarction in this population. Secondary objectives are to examine whether beta-blockers reduce the risk of all-cause death and MI, separately, and the risk of cardiovascular death, readmission because of atrial fibrillation and heart failure. We will also determine whether long-term treatment with oral beta-blockade influences the risk of readmission to hospital because of bradycardia, AV-block II-III, hypotension, syncope or need for pacemaker, asthma or chronic obstructive pulmonary disease, and stroke. In a subset of at least 1000 patients, we will also assess whether long-term treatment with oral beta-blockade influences symptoms, functional status, and health-related quality of life after 6–10 weeks and after 1 year of treatment.
Study population and inclusion procedures
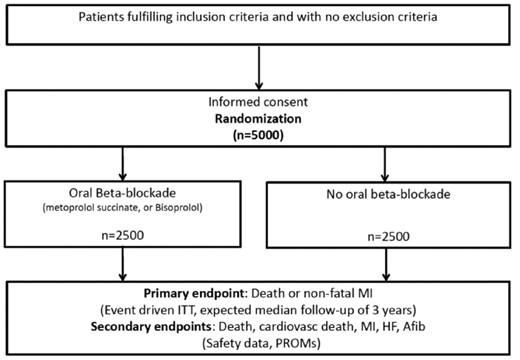
This is a registry-based, prospective, randomized, open-label, parallel trial conducted in Sweden (38 centres, The Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) registry), Estonia (1 centre), and New Zealand (6 centres, ANZAQS-QI registry) (Figure1). Consenting patients, day 1–7 after MI, who have undergone a coronary angiography and with preserved LV systolic EF are eligible for participation in the study. Major exclusion criteria are indication or contraindication for beta-blocker treatment according to the treating physician (See Table1 for details). Non-residents cannot be randomized since follow is performed in registries. In Sweden, randomization is performed via an online web-based randomization module which is linked to the electronic data capture system (EDC). Prescribed treatment, start dose, and target dose are recorded at the randomization. Patients included in New Zealand and Estonia are randomized using a separate web-based randomization application (randomization.net). The randomization is stratified by centre for administrative reason and performed in the modules using permuted block randomization in a 1:1 ratio.
| INCLUSION CRITERIA |
| 1. Age ≥ 18 years. |
| 2. Day 1–7 after MI as defined by the universal definition of MI, type 1. |
| 3. Coronary angiography performed during hospitalization. |
| 4. Obstructive coronary artery disease documented by coronary angiography, i.e. stenosis ≥ 50%, FFR ≤ 0.80, or iFR ≤ 0.89 in any segment at any time point before randomization. |
| 5. Echocardiography performed after the MI showing a normal ejection fraction defined as EF ≥ 50%. |
| 6. Written informed consent obtained. |
| EXCLUSION CRITERIA |
| 1. Any condition that may influence the patient's ability to comply with study protocol. |
| 2. Contraindications for beta-blockade. |
| 3. Indication for beta-blockade other than as secondary prevention according to the treating physician. |
| INCLUSION CRITERIA |
| 1. Age ≥ 18 years. |
| 2. Day 1–7 after MI as defined by the universal definition of MI, type 1. |
| 3. Coronary angiography performed during hospitalization. |
| 4. Obstructive coronary artery disease documented by coronary angiography, i.e. stenosis ≥ 50%, FFR ≤ 0.80, or iFR ≤ 0.89 in any segment at any time point before randomization. |
| 5. Echocardiography performed after the MI showing a normal ejection fraction defined as EF ≥ 50%. |
| 6. Written informed consent obtained. |
| EXCLUSION CRITERIA |
| 1. Any condition that may influence the patient's ability to comply with study protocol. |
| 2. Contraindications for beta-blockade. |
| 3. Indication for beta-blockade other than as secondary prevention according to the treating physician. |
| INCLUSION CRITERIA |
| 1. Age ≥ 18 years. |
| 2. Day 1–7 after MI as defined by the universal definition of MI, type 1. |
| 3. Coronary angiography performed during hospitalization. |
| 4. Obstructive coronary artery disease documented by coronary angiography, i.e. stenosis ≥ 50%, FFR ≤ 0.80, or iFR ≤ 0.89 in any segment at any time point before randomization. |
| 5. Echocardiography performed after the MI showing a normal ejection fraction defined as EF ≥ 50%. |
| 6. Written informed consent obtained. |
| EXCLUSION CRITERIA |
| 1. Any condition that may influence the patient's ability to comply with study protocol. |
| 2. Contraindications for beta-blockade. |
| 3. Indication for beta-blockade other than as secondary prevention according to the treating physician. |
| INCLUSION CRITERIA |
| 1. Age ≥ 18 years. |
| 2. Day 1–7 after MI as defined by the universal definition of MI, type 1. |
| 3. Coronary angiography performed during hospitalization. |
| 4. Obstructive coronary artery disease documented by coronary angiography, i.e. stenosis ≥ 50%, FFR ≤ 0.80, or iFR ≤ 0.89 in any segment at any time point before randomization. |
| 5. Echocardiography performed after the MI showing a normal ejection fraction defined as EF ≥ 50%. |
| 6. Written informed consent obtained. |
| EXCLUSION CRITERIA |
| 1. Any condition that may influence the patient's ability to comply with study protocol. |
| 2. Contraindications for beta-blockade. |
| 3. Indication for beta-blockade other than as secondary prevention according to the treating physician. |
In Sweden, applications to the Ethical Review Board in Stockholm and the Swedish Medical Product Agency (MPA) have been approved, and all data linkages will be approved by the National Board of Health and Welfare. In New Zealand, the study has been approved by the National Health and Disability Ethics Committee and by hospital research review committees. In Estonia, the study has been approved by Research Ethics Committee of the National Institute for Health Development. The study is registered in EudraCT (2017-002336-17) and ClinicalTrials.gov (NCT03278509).
Intervention and control group
Patients randomized to beta-blockade are administered the assigned treatment (metoprolol or bisoprolol) during the rest of the hospital stay and receive a prescription for continued use after discharge. The treating physician is encouraged to aim for a dose of at least 100 mg daily for metoprolol and at least 5 mg daily for bisoprolol. Patients will be encouraged to continue the use of beta-blockade following discharge until contraindications. Sustained-release metoprolol succinate, which is the most commonly used beta-blockade drug, is recommended as first choice. Bisoprolol, which is used to a small extent and with evidence of effect in patients with heart failure, is allowed as an alternative. Atenolol is not allowed due to weak therapeutic efficacy after MI in long-term trials.
Patients randomized to no beta-blockade are discouraged to use beta-blockade as long as there is no other indication than secondary prevention after MI. For blood pressure control, other drugs than beta-blockers are recommended as first-line treatment according to guidelines. If a patient is already on treatment with a beta-blocker when enlisted in the study and randomized to no treatment, a tapering of the beta-blocker must be carried out during a minimum time period of 2 weeks and a maximum time-period of 4 weeks. It is recommended that tapering of beta-blockade must end with at least 4 days of lowest possible dose, corresponding to 12.5 mg metoprolol or 1.25 mg bisoprolol.
The inclusion in the study and the randomization to treatment are documented in the patient health records. The importance of continuing the randomized treatment is documented. Patients randomized to either beta-blockade or no beta-blockade receive written information about the study explaining the importance of continuing randomized treatment unless contraindications or indication for beta-blockers other than secondary prevention arises after randomization. The patients also receive a summary of this letter in ID-card size format to wear in case of medical contact.
Prescribed treatment and dosing are registered at randomization and initiation (whether the prescribed drug is dispensed), adherence (defined as proportion of prescribed tablets that are dispensed), and persistence (time on treatment) will be followed in the Drug prescription registries in all three countries. All other prescribed cardiovascular drugs will also be followed through the Drug prescription registries.
Outcomes
Primary endpoint is time to the composite of death of any cause or new MI. In Sweden, information on death will be obtained from the Swedish population registry, including the vital status of all Swedish residents. This registry also includes data regarding emigration. New MI during the initial hospital stay and readmission due to a non-fatal MI will be collected from the SWEDEHEART registry16 with a high degree of completeness in the present population. In Sweden, reliability of clinical events (MI) in the SWEDEHEART registry is secured via the internal monitoring of the SWEDHEART registry. To further ensure correctness of follow-up data and exclude the possibility of administrative error, the PI will validate all MIs sampled from the SWEDEHEART registry, according to a checklist. Cardiovascular death, defined as ICD codes I00-I99 or unclassified, will be obtained from the cause-of-death registry. Atrial fibrillation (I48), heart failure (I50) will be obtained from the national patient registry, a mandatory registry including all ICD codes for all admission to hospital in Sweden. Linkage with the national cause of death registry and the patient registry will be performed at the end of follow-up. Regarding patient reported outcome measures (PROMs), symptoms and functional status are measured as Canadian Cardiovascular Society (CCS) grading of angina and New York Heart Association (NYHA) functional classification of heart failure. Health-related quality of life is measured with European Quality of Life Five Dimensions (EQ-5D) questionnaire EQ5D, NYHA, and CCS are registered in SWEDEHEART in the clinical routine for patients with myocardial infarction and included in the secondary prevention part (most of these patients are below the age of 80). Safety outcome (Bradycardia [R00.1, I49.5], AV-block II-III [I44.1–3], hypotension [I95], syncope [R55.9, T67.1] or need for pacemaker [FPE00-26, FPF00-20, TFP00], asthma [J45–46] or chronic obstructive pulmonary disease [J44] and stroke [I60-64]) will be obtained from the national patient registry.
In New Zealand, data collection and management will be performed through an EDC that is embedded in the research module in the All of New Zealand, Acute Coronary Syndrome—Quality Improvement Registry (ANZACS-QI). All individuals in New Zealand have a unique national health index number (NHI) that is linked to all encounters with health providers, prescriptions, and investigations. Clinically important outcomes, including hospital admissions and deaths, will be obtained by linkage to the National Institute of Health Innovation (NIHI). Data linkage is by encryption of the NHIs using systems established within ANZACS-QI. No individual identifying information can be identified following this encryption. Data on emigration will be available. New Zealand data will be included in the final statistical analysis after a data download at study end. In New Zealand, reliability of events is secured by internal monitoring by the ANZACS-QI committee and by the Ministry Of Health. In Estonia, data on outcomes will be obtained from e-health records, the national MI-registry, and Estonian Causes of Death Registry. These registries are 100% complete for Estonia.
Data collection and monitoring
In Sweden, study specific data will be collected through an EDC, which is linked, to the randomization module managed by Uppsala Clinical Research Center (UCR). Data entered into the online randomization module at the participating centres will be automatically transferred to the EDC. All other baseline data, as well as follow-up data will be collected from the SWEDEHEART registry. To ensure traceability and prevent loss of data, SWEDEHEART data will be extracted every third month and transferred to the EDC. The extracted data will also be utilized for monitoring purposes.
In New Zealand, all data are entered into ANZACS-QI that is later merged with data from Sweden and Estonia. Study specific data are registered to a study-specific module. In Estonia, data manually entered into eCRF, with the same structure as the SWEDEHEART registry, will be transferred for central analysis.
In accordance with the principles of Good Clinical Practice (ICH-GCP), the Sponsor and SWEDEHEART arrange monitoring of the study. During the study, the monitors have regular contacts with the study site to ensure that the study is conducted and documented properly in compliance with the protocol, GCP, and applicable regulatory requirements. In Sweden, the monitoring is performed by UCR and SWEDEHEART monitor organization. Monitoring performed by the UCR consist of both on-site visits (including Source Data Verification) and centralized monitoring activities and is described in detail in the monitoring plan. In New Zealand and Estonia, monitoring is performed by independent partners according to a monitoring plan similar to the one in Sweden. The study centres may also be subject to quality assurance audit by the Sponsor as well as inspection by the MPA.
Statistical analyses
All patients included in the study will be included in the ITT (intention-to-treat population) analyses. These analyses will be based on events of all follow-up time of each patient from randomization to end of follow-up. All endpoints (except for PROMs) will be presented as Kaplan–Meier plots and frequency tables, by randomized treatment, and analysed using Cox proportional hazards regression with treatment contrast presented as a hazard ratio with 95% confidence interval and associated P-value. Adjustment will be performed for country. We expect the proportional hazard assumption to be fulfilled, an issue that will be described in more detail in the statistical analysis plan. In the primary analysis, all patients without an event will be censored on the last day of follow-up. Patients that withdraw from follow-up will be considered censored on the day of withdrawal. For endpoints that do not include all-cause death, patients that die before the last day of follow-up without reaching an endpoint will be considered censored on the day of death. Sensitivity analyses dealing with competing risk and multiple testing will be added in the statistical analysis plan.
The above analyses will be performed after at least 379 primary endpoints have been observed. All accessible safety data and secondary endpoints at this time point will be included in the primary publication. Since an intention-to-treat approach will be applied, treatment compliance will not be included in the primary analyses. However, dispensed drugs during follow-up will be reported and ‘on-treatment analyses’ will be included as sensitivity analyses for patients with such data available (not in the primary report).
Before study initiation, based on historical data from SWEDEHEART, we assumed an event-rate of 7.2% per year in the no-betablockade arm. A 16.7% relative risk reduction corresponding to a 1.2% absolute risk reduction was considered a minimal important difference to detect. To detect 944 events, a sample size of 7000 patients were originally planned. During the study following considerations from the steering committee, the sponsor together with the steering committee and patient representatives came to the conclusion that a 25% relative risk reduction would be a more clinically relevant effect to detect and made this change in the protocol. To detect a hazard ratio of 0.75 using the log-rank test, with 80% power at a two-sided 5% significance level, 379 events are required. As the actual event-rate in the study is 3% per year, we plan to include ∼5000 patients, at a uniform rate of ∼1000 patients per year for 5 years. After inclusion is complete, follow-up will continue until 379 events have been observed.
Study organization and data safety monitoring board
Karolinska Institutet, Stockholm, Sweden, is the sponsor of this investigator-initiated trial. The study is funded by the Swedish Research Council (Clinical therapy research, grant 2016-00493) and the Swedish heart- and lung foundation (grant 20210423). The steering group consists of investigators from 10 centres and a representative from the Swedish Heart- and Lung disease patient association. Uppsala Clinical Research centre provides project management, monitoring of the trial, data management, and statistical analyses. An independent data safety monitoring board (DSMB) will ensure the safety of the intervention as well as the general execution of the trial on behalf of the trial participants. The responsibilities of the DSMB have been defined in a separate charter agreed upon by the steering committee and the DSMB members. Outcome analyses for the DSMB have been performed after 2 and 4 years of recruitment.
Timelines
Inclusion started in September 2017. In November 2021, 4000 patients had been included and we expect to reach 5000 included patients by the end of 2022. With an event rate of 3% per year, 379 primary endpoints will be reached during 2023.
Baseline characteristics
Baseline characteristics for the first 2123 patients were extracted in November 2019 when the first outcome analyses were performed for DSMB and are listed in Table2. The median age was 65 and 77% were men. About 9% had a prior MI and 34% had a STEMI. Percutaneous coronary intervention had been performed in 95% of the patients and coronary artery by-pass grafting in 4%. At discharge, 95% received aspirin, 94% P2Y12-receptor blocker, and 97% statins.
| Demography | ||
| Age, Median (IQR) | 65 | (57–72) |
| Men, n (%) | 1629 | (77) |
| Risk factors | ||
| Current smoker, n (%) | 465 | (22) |
| Hypertension, n (%) | 959 | (45) |
| Diabetes, n (%) | 298 | (14) |
| Prior cardiovascular disease | ||
| Prior myocardial infarctions, n (%) | 190 | (9) |
| Prior PCI | 172 | (8) |
| Prior CABG | 33 | (22) |
| Prior Stroke, n (%) | 52 | (2) |
| Prior Heart failure, n (%) | 19 | (1) |
| Presentation characteristics | ||
| Chest pain as main symptoms, n (%) | 2024 | (95) |
| CPR before hospital, n (%) | 8 | (0) |
| Pulmonary rales | 26 | (1) |
| Heart rate, median (IQR) | 75 | (65–85) |
| Systolic blood pressure, median (IQR) | 152 | (137–170) |
| Atrial fibrillation | 20 | (1) |
| ST-elevation MI | 726 | (34) |
| In-hospital course | ||
| Coronary angiography | ||
| 1-vessel disease | 1152 | (55) |
| 2-vessel disease | 559 | (27) |
| LM or 3-vessel disease | 344 | (16) |
| Percutaneous coronary intervention | 2014 | (95) |
| Coronary artery by-pass grafting | 77 | (4) |
| Medication at discharge | ||
| Aspirin, n (%) | 2046 | (95) |
| P2Y12-rec blockade, n (%) | 2001 | (94) |
| Beta-blockade, n (%) | 1095 | (52) |
| ACEI, n (%) | 1116 | (53) |
| ARB, n (%) | 576 | (27) |
| Statins, n (%) | 2050 | (97) |
| Diuretics, n (%) | 172 | (8) |
| Demography | ||
| Age, Median (IQR) | 65 | (57–72) |
| Men, n (%) | 1629 | (77) |
| Risk factors | ||
| Current smoker, n (%) | 465 | (22) |
| Hypertension, n (%) | 959 | (45) |
| Diabetes, n (%) | 298 | (14) |
| Prior cardiovascular disease | ||
| Prior myocardial infarctions, n (%) | 190 | (9) |
| Prior PCI | 172 | (8) |
| Prior CABG | 33 | (22) |
| Prior Stroke, n (%) | 52 | (2) |
| Prior Heart failure, n (%) | 19 | (1) |
| Presentation characteristics | ||
| Chest pain as main symptoms, n (%) | 2024 | (95) |
| CPR before hospital, n (%) | 8 | (0) |
| Pulmonary rales | 26 | (1) |
| Heart rate, median (IQR) | 75 | (65–85) |
| Systolic blood pressure, median (IQR) | 152 | (137–170) |
| Atrial fibrillation | 20 | (1) |
| ST-elevation MI | 726 | (34) |
| In-hospital course | ||
| Coronary angiography | ||
| 1-vessel disease | 1152 | (55) |
| 2-vessel disease | 559 | (27) |
| LM or 3-vessel disease | 344 | (16) |
| Percutaneous coronary intervention | 2014 | (95) |
| Coronary artery by-pass grafting | 77 | (4) |
| Medication at discharge | ||
| Aspirin, n (%) | 2046 | (95) |
| P2Y12-rec blockade, n (%) | 2001 | (94) |
| Beta-blockade, n (%) | 1095 | (52) |
| ACEI, n (%) | 1116 | (53) |
| ARB, n (%) | 576 | (27) |
| Statins, n (%) | 2050 | (97) |
| Diuretics, n (%) | 172 | (8) |
| Demography | ||
| Age, Median (IQR) | 65 | (57–72) |
| Men, n (%) | 1629 | (77) |
| Risk factors | ||
| Current smoker, n (%) | 465 | (22) |
| Hypertension, n (%) | 959 | (45) |
| Diabetes, n (%) | 298 | (14) |
| Prior cardiovascular disease | ||
| Prior myocardial infarctions, n (%) | 190 | (9) |
| Prior PCI | 172 | (8) |
| Prior CABG | 33 | (22) |
| Prior Stroke, n (%) | 52 | (2) |
| Prior Heart failure, n (%) | 19 | (1) |
| Presentation characteristics | ||
| Chest pain as main symptoms, n (%) | 2024 | (95) |
| CPR before hospital, n (%) | 8 | (0) |
| Pulmonary rales | 26 | (1) |
| Heart rate, median (IQR) | 75 | (65–85) |
| Systolic blood pressure, median (IQR) | 152 | (137–170) |
| Atrial fibrillation | 20 | (1) |
| ST-elevation MI | 726 | (34) |
| In-hospital course | ||
| Coronary angiography | ||
| 1-vessel disease | 1152 | (55) |
| 2-vessel disease | 559 | (27) |
| LM or 3-vessel disease | 344 | (16) |
| Percutaneous coronary intervention | 2014 | (95) |
| Coronary artery by-pass grafting | 77 | (4) |
| Medication at discharge | ||
| Aspirin, n (%) | 2046 | (95) |
| P2Y12-rec blockade, n (%) | 2001 | (94) |
| Beta-blockade, n (%) | 1095 | (52) |
| ACEI, n (%) | 1116 | (53) |
| ARB, n (%) | 576 | (27) |
| Statins, n (%) | 2050 | (97) |
| Diuretics, n (%) | 172 | (8) |
| Demography | ||
| Age, Median (IQR) | 65 | (57–72) |
| Men, n (%) | 1629 | (77) |
| Risk factors | ||
| Current smoker, n (%) | 465 | (22) |
| Hypertension, n (%) | 959 | (45) |
| Diabetes, n (%) | 298 | (14) |
| Prior cardiovascular disease | ||
| Prior myocardial infarctions, n (%) | 190 | (9) |
| Prior PCI | 172 | (8) |
| Prior CABG | 33 | (22) |
| Prior Stroke, n (%) | 52 | (2) |
| Prior Heart failure, n (%) | 19 | (1) |
| Presentation characteristics | ||
| Chest pain as main symptoms, n (%) | 2024 | (95) |
| CPR before hospital, n (%) | 8 | (0) |
| Pulmonary rales | 26 | (1) |
| Heart rate, median (IQR) | 75 | (65–85) |
| Systolic blood pressure, median (IQR) | 152 | (137–170) |
| Atrial fibrillation | 20 | (1) |
| ST-elevation MI | 726 | (34) |
| In-hospital course | ||
| Coronary angiography | ||
| 1-vessel disease | 1152 | (55) |
| 2-vessel disease | 559 | (27) |
| LM or 3-vessel disease | 344 | (16) |
| Percutaneous coronary intervention | 2014 | (95) |
| Coronary artery by-pass grafting | 77 | (4) |
| Medication at discharge | ||
| Aspirin, n (%) | 2046 | (95) |
| P2Y12-rec blockade, n (%) | 2001 | (94) |
| Beta-blockade, n (%) | 1095 | (52) |
| ACEI, n (%) | 1116 | (53) |
| ARB, n (%) | 576 | (27) |
| Statins, n (%) | 2050 | (97) |
| Diuretics, n (%) | 172 | (8) |
Discussion
The REDUCE-AMI trial will provide new and crucial evidence on the effect of long-term treatment with beta-blockers in patients with MI and preserved EF, and may thereby influence current guidelines and practice. If beta-blockers are proven effective in this population, this will strengthen the recommendation for use of beta-blockers in patients with MI and preserved EF. If the study does not demonstrate a reduction in future cardiac events, routine use of beta-blockers in patients with MI and preserved EF should be discouraged. Despite the widespread use and tolerability of beta-blockers, these drugs have several well-known side effects, such as fatigue, depression, nightmares, sexual dysfunction, weight gain, hypotension, and bradycardia.17 These side effects may be less tolerable for individuals in a low-risk population. Substudies in the REDUCE-AMI trial will evaluate possible side effects. The presented baseline characteristics confirmed that patients included in the trial are of overall low risk, with a median age of 65, 14% with previous diabetes mellitus, 9% with prior MI, 1% with atrial fibrillation on admission, 1% with previous HF, and in general very well treated with revascularization and evidence-based medication at discharge. Indeed, the event rate of 3% for the primary endpoint is lower than expected before the trial initiation.
Three other large trials are also examining long-term treatment with beta-blockers in patients with MI and preserved EF: the Norwegian BETAMI-trial,18 the Danish DANBLOCK-trial,18 and the Spanish-Italian REBOOT trial.20 However, in contrast with this study, these trials define preserved EF as ≥40%. The decision to only include patients with EF ≥ 50% was made after deliberation with potential investigators who were concerned that including those with mid-range EF (EF 40–49%) could be problematic. Later, a meta-analysis21 of clinical trials has suggested a beneficial effect of beta-blockers in this group generally and a large Korean registry7 has suggested benefit specifically post MI. We also wanted to keep the study population as homogeneous as possible since any interaction between treatment and a subgroup makes study results more difficult to interpret and generalize.
The REDUCE-AMI allows only beta-1-receptor selective blockers (metoprolol and bisoprolol) whereas the other trials also include non-selective beta-blockers. Moreover, the REDUCE-AMI and the REBOOT trial mandate an early invasive strategy and revascularization if appropriate, whereas DANBLOCK and BETAMI also include patients treated without an early invasive approach. The rationale to require an early invasive strategy in the REDUCE-AMI trial was chosen as it reflects a contemporary treatment strategy, i.e. the background for re-evaluating beta blockers in a new trial. Altogether, by using somewhat different inclusion and exclusion criteria, these trials will complement each other. Given these trials will include >20 000 patients, planned joint analyses will be able to answer questions that none of the trials can do alone.
Strengths and limitations
Our trial has a pragmatic design which will allow a high proportion of the eligible patients to be included in the study. This will secure its generalizability to a real-world setting. The dose of beta-blockers in the trial will probably be lower than in previous landmark trials.2 This will, however, mirror the current practice of beta-blocker treatment.22 Results from contemporary observational studies comparing different dosing of beta-blockade have been conflicting. In a recent study from the SWEDEHEART registry, comparing 33 126 patients who were discharged with ≥50% of the target beta-blocker dose and 64 449 patients with <50% of the target beta-blocker dose did not show a difference in outcome between the groups.22
A limitation is the open design with no placebo treatment. However, this should only have a limited effect on the hard clinical endpoints of the primary composite outcome, whereas results regarding softer endpoints like symptoms and quality of life need to be interpreted more cautiously. Another limitation of performing a pragmatic trial of a routinely used standard therapy is the potential for crossovers, although measures to prevent that have been performed. Finally, clinical endpoints are obtained from validated registries with a high degree of completeness, but only endpoints resulting in hospital admissions will be captured and the use of different registries in three different countries and the lack of central adjudication are still limitations of the study.
Conclusions
The results from the REDUCE-AMI will add important scientific evidence regarding the effect of beta-blockers in patients with MI and preserved EF and may change both future guidelines and clinical practice.
Funding
The study is funded by the Swedish Research Council (Clinical therapy research, grant 2016-00493), the Swedish heart- and lung foundation (grant 20210423), and Stockholm County Council (ALF project). Work of Toomas Marandi was supported by the Estonian Research Council grant (PRG435).
Conflict of interest: None of the authors have any conflict of interest to declare.
Data availability
Fully anonymized data can be available on request.
References
Dahl Aarvik M,



