-
PDF
- Split View
-
Views
-
Cite
Cite
Victor Razuk, Mauro Chiarito, Davide Cao, Johny Nicolas, Carlo A Pivato, Anton Camaj, David Power, Frans Beerkens, Davis Jones, Aviv Alter, Alvin Mathew, Alessandro Spirito, Johanna P Contreras, George D Dangas, Roxana Mehran, SGLT-2 inhibitors and cardiovascular outcomes in patients with and without a history of heart failure: a systematic review and meta-analysis, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 8, Issue 6, October 2022, Pages 557–567, https://doi.org/10.1093/ehjcvp/pvac001
Close - Share Icon Share
Abstract
Sodium–glucose cotransporter 2 (SGLT-2) inhibitors have cardiovascular (CV) benefits in patients with heart failure with reduced ejection fraction (HFrEF). Whether these medications improve CV outcomes irrespective of heart failure history or left ventricular ejection fraction (LVEF) in HFrEF remains unknown.
All randomized, placebo-controlled trials of SGLT-2 inhibitors reporting similar CV outcomes were searched in PubMed from 1 January 2010 to 1 October 2021. The primary outcome was the composite of hospitalization for heart failure or CV death. Secondary outcomes included all-cause mortality. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were used as effect estimates and calculated with a random-effects model. Data from 11 trials and a total of 66 957 patients (n = 36 758 SGLT-2 group, n = 30 199 placebo group) were included. SGLT-2 inhibitors reduced the risk of hospitalization for heart failure or CV death in patients with (HR 0.76, 95% CI 0.71–0.80) and without (HR 0.76, 95% CI 0.68–0.86; Pinteraction = 0.69) heart failure. Patients with (HR 0.87, 95% CI 0.80–0.95) and without (HR 0.84, 95% CI 0.73–0.95; Pinteraction = 0.67) heart failure treated with SGLT-2 inhibitors had a reduction in all-cause mortality. Reduction in the primary outcome was consistently observed in HFrEF patients with (HR 0.68, 95% CI 0.59–0.78) and without (HR 0.84, 95% CI 0.71–0.99; Pinteraction = 0.13) severely reduced LVEF, and in heart failure with preserved ejection fraction patients (HR 0.80, 95% CI 0.70–0.92; Pinteraction = 0.65).
SGLT-2 inhibitors improved CV outcomes irrespective of heart failure history or type, and severity of LVEF reduction.
Introduction
Sodium–glucose cotransporter 2 (SGLT-2) inhibitors are a groundbreaking therapy for the treatment of cardiovascular (CV) disease, regardless of diabetic status. Several randomized studies of SGLT-2 inhibitors in patients with type 2 diabetes mellitus have shown favourable CV outcomes and reduced progression of renal disease.1–6 There has been growing interest in the benefits of SGLT-2 inhibitors in patients with congestive heart failure and atherosclerotic CV disease. A number of major randomized controlled trials have evaluated SGLT-2 inhibitors in patients with heart failure with reduced ejection fraction (HFrEF) showing a reduction in the primary composite outcome of hospitalization for heart failure or CV death.7–9 Secondary outcomes from these trials, albeit not individually powered to study these endpoints, have shown conflicting results in regard to improving overall survival.7–9 Current societal guidelines recommend the addition of a glucagon-like peptide 1 receptor agonist (GLP-1RA) or SGLT-2 inhibitor to metformin in patients with type 2 diabetes mellitus and known CV disease.10,11
Previous meta-analyses of SGLT-2 inhibitors have pooled study data from either the CV outcome trials of type 2 diabetic patients or the trials of patients with HFrEF independent of diabetic status.12–15 However, there is limited evidence on the safety and efficacy of SGLT-2 inhibitors according to the history of heart failure and previous studies were only powered to study the overall patient population.16 Second, it is unclear whether SGLT-2 inhibitors have benefits in patients with heart failure with preserved ejection fraction (HFpEF) or HFrEF with severely reduced left ventricular ejection fraction (LVEF). Herein, we aim to present an updated and comprehensive systematic review and meta-analysis of SGLT-2 inhibitors in patients with and without a history of heart failure, stratified by type of heart failure (HFpEF vs. HFrEF) and severity of LVEF reduction in HFrEF.
Methods
Search strategy and selection criteria
The present meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.17 A systematic search for all randomized, placebo-controlled trials of SGLT-2 inhibitors reporting similar CV outcomes was evaluated for inclusion. PubMed was searched from 1 January 2010 to 1 October 2021 with no language restrictions set. The detailed search strategy for PubMed is available in the Supplementary material online, Table S1. Articles retrieved from the systematic search were screened for eligibility based on the title, abstract, study design, and study quality. Studies were included if they met all of the following inclusion criteria: (1) SGLT-2 inhibitors were compared with placebo; (2) they reported at least the predefined primary outcome of interest; and (3) they provided demographic information for a history of heart failure. We excluded studies that were not randomized controlled trials, studies comparing SGLT-2 inhibitors with other drugs for type 2 diabetes mellitus, studies with less than 3 months of active comparison, and studies not reporting clinical outcomes. Articles were independently reviewed and selected by two authors (V.R. and M.C.) using a standardized approach for eligibility. Conflicts in selection of articles were discussed and resolved with the senior principal investigator (R.M.).
Outcomes and study population
The primary outcome of interest was the composite of hospitalization for heart failure or CV death. Secondary outcomes included the single components of the primary outcome and all-cause mortality. The study population was analysed based on the following subgroups: (1) patients with or without a history of heart failure; (2) patients with HFrEF with or without severely reduced LVEF (defined as LVEF ≤30% or 35% depending on the original study definition); and (3) patients with HFpEF (defined as LVEF ≥50%) vs. HFrEF (defined as LVEF ≤40%). The LVEF was quantified using multiple modalities and details on which modalities were used for each individual trial in the LVEF subanalysis can be found in the Supplementary material online, Table S2.
The definitions of each pre-specified outcome according to the original study protocols were assessed individually and are summarized in the Supplementary material online, Table S3. The risk of bias was assessed using the revised Cochrane risk-of-bias tool (Supplementary material online, Table S4). The five domains of bias were independently assessed by three investigators (J.N., C.A.P., and D.C.) for each outcome, which included (1) randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported results.
Statistical analysis
Study characteristics, baseline demographics, and outcome data were extracted into a pre-designed data collection form. The hazard ratio (HR) and 95% confidence interval (CI) were extracted from each of the selected trials and used as effect estimates. For this meta-analysis, we used a random-effects model to account for heterogeneity in study design and patient features, with inverse variance weighting. The presence of heterogeneity among studies was measured with Cochran's Q χ² test, with a P-value of up to 0.10 considered significant, and to measure consistency we used the I² test. An I² value of 0% indicates no observed heterogeneity; I² values of up to 25% indicate low heterogeneity; I² values of up to 50% indicate moderate heterogeneity; and I² values of above 50% indicate high heterogeneity. Publication bias and small study effects were assessed for the primary endpoint by visual estimation with a funnel plot (Supplementary material online, Figure S1). To assess for interaction between treatment effect and (1) history of heart failure, (2) severity of LVEF reduction in HFrEF, or (3) type of heart failure (HFpEF vs. HFrEF), a random effects metaregression analysis with the empirical Bayes (Paule–Mandel) method was utilized. Interaction testing was considered significant if the P-value <0.05. To estimate the between-study variance, tau-squared and Hartung–Knapp–Sidik–Jonkman adjustments were performed. All statistical analyses were performed according to the intention-to-treat principle and were conducted using Stata (version 16; StataCorp, College Station, TX, USA). This study was registered with PROSPERO (CRD42020219082).
Results
Baseline study and patient characteristics
A total of 11 randomized placebo-controlled trials were included in the present meta-analysis (Figure1).1–9,18,19 Five studies enrolled patients with type 2 diabetes mellitus and high CV risk.1–5 DAPA-CKD and SCORED enrolled patients with chronic kidney disease irrespective of their diabetes mellitus status.6,19 Four studies enrolled patients with symptomatic congestive heart failure and included both diabetic and non-diabetic patients.7–9,18 DAPA-HF and EMPEROR-REDUCED included only HFrEF patients with an LVEF ≤40%. Only three trials (SOLOIST-WHF, EMPEROR-PRESERVED, and SCORED) reported outcomes on patients with HFpEF.7,18,19 VERTIS and DECLARE-TIMI were excluded from the HFpEF analysis as the diagnostic criterion for HFpEF was an LVEF >45%.3,20 SOLOIST-WHF and SCORED classified HFpEF as an LVEF ≥50% with known symptomatic heart failure.7,19 The recently published EMPEROR-PRESERVED trial defined HFpEF as an LVEF ≥40%, but only data from the additional subanalysis reporting outcomes for an LVEF ≥50% were included.18 Only three trials (DECLARE-TIMI, EMPEROR-REDUCED, and DAPA-HF) reported outcomes stratified by the presence of severely reduced LVEF. DAPA-HF and EMPEROR-REDUCED stratified LVEF by ≤30% and >30%, while DECLARE-TIMI stratified LVEF by ≤35% and >35%.4,8,9,20
Data from a total of 66 957 patients (n = 36 758 in SGLT-2 inhibitor group; n = 30 199 in placebo group) were included. Table1 summarizes the baseline study and patient characteristics of each randomized trial. Among all 11 trials, the median follow-up period ranged from 0.8 to 4.2 years. The majority of patients were male (55–76%) and white (53–93%). The prevalence of established CV disease and diabetes mellitus ranged from 37% to 100% and 41% to 100%, respectively. Previous angiotensin converting enzyme inhibitor and angiotensin receptor blocker use was high, ranging from 70% to 99.9%, while beta-blocker usage was less prevalent, especially in the studies including only type 2 diabetic patients.
| Characteristics . | CREDENCE . | CANVAS . | DECLARE-TIMI . | EMPA-REG . | VERTIS . | DAPA-CKD . |
|---|---|---|---|---|---|---|
| SGLT-2 inhibitor | Canagliflozin | Canagliflozin | Dapagliflozin | Empagliflozin | Ertugliflozin | Dapagliflozin |
| Year of publication | 2019 | 2017 | 2019 | 2015 | 2020 | 2020 |
| Median follow-up period (years) | 2.6 | 2.4 | 4.2 | 3.1 | 3.0 | 2.4 |
| Patient characteristics | ||||||
| Age, mean (SD) (years) | 63.0 (9.2) | 63.3 (8.3) | 63.9 (6.8) | 63.1 (8.6) | 64.4 (8.1) | 61.8 (12.1) |
| Men, n (%) | 2907 (66.1) | 6509 (64.2) | 10 738 (62.6) | 5016 (71.5) | 5769 (70.0) | 2879 (66.9) |
| Race, n (%) | ||||||
| White | 2931 (66.6) | 7944 (78.3) | 13 653 (79.6) | 5081 (72.4) | 7240 (87.8) | 2290 (53.2) |
| Asian | 877 (19.9) | 1284 (12.7) | 2303 (13.4) | 1517 (21.6) | 498 (6.0) | 1467 (34.1) |
| Black | 224 (5.1) | 336 (3.3) | 603 (3.5) | 357 (5.1) | 235 (2.8) | 191 (4.4) |
| Other/missing | 369 (8.4) | 578 (5.7) | 601 (3.5) | 65 (0.9) | 273 (3.3) | 356 (8.3) |
| Medical history | ||||||
| CVD, n (%) | 2220 (50.4) | 6656 (65.6) | 6974 (40.6) | 7020 (100) | 8246 (100) | 1610 (37.4) |
| CHF, n (%) | 652 (14.8) | 1461 (14.4) | 1724 (10.0) | 706 (10.1) | 1958 (23.7) | 468 (10.9) |
| CKD, n (%) | 2631 (59.8) | 2039 (20.1) | 1265 (7.4) | 1819 (25.9) | 1807 (21.9) | 4304 (100) |
| DM, n (%) | 4401 (100) | 10 142 (100) | 17 160 (100) | 7020 (100) | 8246 (100) | 2906 (67.5) |
| Cardiovascular medications | ||||||
| ACEI or ARB, n (%) | 4395 (99.9) | 8116 (80.0) | 13 950 (81.3) | 5666 (80.7) | 6686 (81.1) | 4224 (98.1) |
| Beta blocker, n (%) | 1770 (40.2) | 5421 (53.5) | 9030 (52.6) | 4554 (64.9) | 5692 (69.0) | — |
| Statin/ezetimibe, n (%) | 3036 (69.0) | 7599 (74.9) | 12868 (75.0) | 5403 (77.0) | 6790 (82.3) | 2794 (64.9) |
| Characteristics | EMPEROR REDUCED | SOLOIST-WHF | EMPEROR-PRESERVED | SCORED | DAPA-HF | |
| SGLT-2 inhibitor | Empagliflozin | Sotagliflozin | Empagliflozin | Sotagliflozin | Dapagliflozin | |
| Year of publication | 2020 | 2021 | 2021 | 2021 | 2019 | |
| Median follow-up period, (years) | 1.3 | 0.8 | 2.2 | 1.3 | 1.5 | |
| Patient characteristics | ||||||
| Age, mean (SD) (years) | 66.9 (11) | 69.5 | 71.8 (9.3) | 69 | 66.4 (10.9) | |
| Men, n (%) | 2837 (76.1) | 810 (66.2) | 3312 (55.3) | 5830 (55.1) | 3635 (76.6) | |
| Race, n (%) | ||||||
| White | 2629 (70.5) | 1139 (93.2) | 4542 (75.6) | 8749 (82.7) | 3333 (70.3) | |
| Asian | 672 (18.0) | 15 (1.2) | 824 (13.8) | 682 (6.4) | 1116 (23.5) | |
| Black | 257 (6.9) | 50 (4.1) | 258 (4.3) | 364 (3.4) | 226 (4.8) | |
| Other/missing | 172 (4.6) | 18 (1.5) | 364 (6.1) | 789 (7.5) | 69 (1.5) | |
| Medical history | ||||||
| CVD, n (%) | 3730 (100) | 1222 (100) | 5988 (100) | 9381 (88.6) | 4744 (100) | |
| CHF, n (%) | 3730 (100) | 1222 (100) | 5988 (100) | 3283 (31.0) | 4744 (100) | |
| CKD, n (%) | 1799 (48.2) | — | 2988 (49.9) | 10 584 (100) | 1926 (40.6) | |
| DM, n (%) | 1856 (49.8) | 1222 (100) | 2938 (49.1) | 10 584 (100) | 1983 (41.8) | |
| Cardiovascular medications | ||||||
| ACEI or ARB, n (%) | 2600 (69.7) | 1010 (82.7) | 4832 (80.7) | 9365 (88.5) | 3968 (83.6) | |
| Beta blocker, n (%) | 3533 (94.7) | 1125 (92.1) | 5167 (86.3) | 6616 (62.5) | 4558 (96.1) | |
| Statin/ezetimibe, n (%) | — | — | 4131 (70.0) | — | — |
| Characteristics . | CREDENCE . | CANVAS . | DECLARE-TIMI . | EMPA-REG . | VERTIS . | DAPA-CKD . |
|---|---|---|---|---|---|---|
| SGLT-2 inhibitor | Canagliflozin | Canagliflozin | Dapagliflozin | Empagliflozin | Ertugliflozin | Dapagliflozin |
| Year of publication | 2019 | 2017 | 2019 | 2015 | 2020 | 2020 |
| Median follow-up period (years) | 2.6 | 2.4 | 4.2 | 3.1 | 3.0 | 2.4 |
| Patient characteristics | ||||||
| Age, mean (SD) (years) | 63.0 (9.2) | 63.3 (8.3) | 63.9 (6.8) | 63.1 (8.6) | 64.4 (8.1) | 61.8 (12.1) |
| Men, n (%) | 2907 (66.1) | 6509 (64.2) | 10 738 (62.6) | 5016 (71.5) | 5769 (70.0) | 2879 (66.9) |
| Race, n (%) | ||||||
| White | 2931 (66.6) | 7944 (78.3) | 13 653 (79.6) | 5081 (72.4) | 7240 (87.8) | 2290 (53.2) |
| Asian | 877 (19.9) | 1284 (12.7) | 2303 (13.4) | 1517 (21.6) | 498 (6.0) | 1467 (34.1) |
| Black | 224 (5.1) | 336 (3.3) | 603 (3.5) | 357 (5.1) | 235 (2.8) | 191 (4.4) |
| Other/missing | 369 (8.4) | 578 (5.7) | 601 (3.5) | 65 (0.9) | 273 (3.3) | 356 (8.3) |
| Medical history | ||||||
| CVD, n (%) | 2220 (50.4) | 6656 (65.6) | 6974 (40.6) | 7020 (100) | 8246 (100) | 1610 (37.4) |
| CHF, n (%) | 652 (14.8) | 1461 (14.4) | 1724 (10.0) | 706 (10.1) | 1958 (23.7) | 468 (10.9) |
| CKD, n (%) | 2631 (59.8) | 2039 (20.1) | 1265 (7.4) | 1819 (25.9) | 1807 (21.9) | 4304 (100) |
| DM, n (%) | 4401 (100) | 10 142 (100) | 17 160 (100) | 7020 (100) | 8246 (100) | 2906 (67.5) |
| Cardiovascular medications | ||||||
| ACEI or ARB, n (%) | 4395 (99.9) | 8116 (80.0) | 13 950 (81.3) | 5666 (80.7) | 6686 (81.1) | 4224 (98.1) |
| Beta blocker, n (%) | 1770 (40.2) | 5421 (53.5) | 9030 (52.6) | 4554 (64.9) | 5692 (69.0) | — |
| Statin/ezetimibe, n (%) | 3036 (69.0) | 7599 (74.9) | 12868 (75.0) | 5403 (77.0) | 6790 (82.3) | 2794 (64.9) |
| Characteristics | EMPEROR REDUCED | SOLOIST-WHF | EMPEROR-PRESERVED | SCORED | DAPA-HF | |
| SGLT-2 inhibitor | Empagliflozin | Sotagliflozin | Empagliflozin | Sotagliflozin | Dapagliflozin | |
| Year of publication | 2020 | 2021 | 2021 | 2021 | 2019 | |
| Median follow-up period, (years) | 1.3 | 0.8 | 2.2 | 1.3 | 1.5 | |
| Patient characteristics | ||||||
| Age, mean (SD) (years) | 66.9 (11) | 69.5 | 71.8 (9.3) | 69 | 66.4 (10.9) | |
| Men, n (%) | 2837 (76.1) | 810 (66.2) | 3312 (55.3) | 5830 (55.1) | 3635 (76.6) | |
| Race, n (%) | ||||||
| White | 2629 (70.5) | 1139 (93.2) | 4542 (75.6) | 8749 (82.7) | 3333 (70.3) | |
| Asian | 672 (18.0) | 15 (1.2) | 824 (13.8) | 682 (6.4) | 1116 (23.5) | |
| Black | 257 (6.9) | 50 (4.1) | 258 (4.3) | 364 (3.4) | 226 (4.8) | |
| Other/missing | 172 (4.6) | 18 (1.5) | 364 (6.1) | 789 (7.5) | 69 (1.5) | |
| Medical history | ||||||
| CVD, n (%) | 3730 (100) | 1222 (100) | 5988 (100) | 9381 (88.6) | 4744 (100) | |
| CHF, n (%) | 3730 (100) | 1222 (100) | 5988 (100) | 3283 (31.0) | 4744 (100) | |
| CKD, n (%) | 1799 (48.2) | — | 2988 (49.9) | 10 584 (100) | 1926 (40.6) | |
| DM, n (%) | 1856 (49.8) | 1222 (100) | 2938 (49.1) | 10 584 (100) | 1983 (41.8) | |
| Cardiovascular medications | ||||||
| ACEI or ARB, n (%) | 2600 (69.7) | 1010 (82.7) | 4832 (80.7) | 9365 (88.5) | 3968 (83.6) | |
| Beta blocker, n (%) | 3533 (94.7) | 1125 (92.1) | 5167 (86.3) | 6616 (62.5) | 4558 (96.1) | |
| Statin/ezetimibe, n (%) | — | — | 4131 (70.0) | — | — |
Continuous variables are presented as mean ± standard deviation, categorical variables as N (%).
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; DM, diabetes mellitus; CKD, chronic kidney disease; CHF, congestive heart failure; CVD, cardiovascular disease; n, number; SD, standard deviation; and SGLT-2, sodium–glucose cotransporter 2.
| Characteristics . | CREDENCE . | CANVAS . | DECLARE-TIMI . | EMPA-REG . | VERTIS . | DAPA-CKD . |
|---|---|---|---|---|---|---|
| SGLT-2 inhibitor | Canagliflozin | Canagliflozin | Dapagliflozin | Empagliflozin | Ertugliflozin | Dapagliflozin |
| Year of publication | 2019 | 2017 | 2019 | 2015 | 2020 | 2020 |
| Median follow-up period (years) | 2.6 | 2.4 | 4.2 | 3.1 | 3.0 | 2.4 |
| Patient characteristics | ||||||
| Age, mean (SD) (years) | 63.0 (9.2) | 63.3 (8.3) | 63.9 (6.8) | 63.1 (8.6) | 64.4 (8.1) | 61.8 (12.1) |
| Men, n (%) | 2907 (66.1) | 6509 (64.2) | 10 738 (62.6) | 5016 (71.5) | 5769 (70.0) | 2879 (66.9) |
| Race, n (%) | ||||||
| White | 2931 (66.6) | 7944 (78.3) | 13 653 (79.6) | 5081 (72.4) | 7240 (87.8) | 2290 (53.2) |
| Asian | 877 (19.9) | 1284 (12.7) | 2303 (13.4) | 1517 (21.6) | 498 (6.0) | 1467 (34.1) |
| Black | 224 (5.1) | 336 (3.3) | 603 (3.5) | 357 (5.1) | 235 (2.8) | 191 (4.4) |
| Other/missing | 369 (8.4) | 578 (5.7) | 601 (3.5) | 65 (0.9) | 273 (3.3) | 356 (8.3) |
| Medical history | ||||||
| CVD, n (%) | 2220 (50.4) | 6656 (65.6) | 6974 (40.6) | 7020 (100) | 8246 (100) | 1610 (37.4) |
| CHF, n (%) | 652 (14.8) | 1461 (14.4) | 1724 (10.0) | 706 (10.1) | 1958 (23.7) | 468 (10.9) |
| CKD, n (%) | 2631 (59.8) | 2039 (20.1) | 1265 (7.4) | 1819 (25.9) | 1807 (21.9) | 4304 (100) |
| DM, n (%) | 4401 (100) | 10 142 (100) | 17 160 (100) | 7020 (100) | 8246 (100) | 2906 (67.5) |
| Cardiovascular medications | ||||||
| ACEI or ARB, n (%) | 4395 (99.9) | 8116 (80.0) | 13 950 (81.3) | 5666 (80.7) | 6686 (81.1) | 4224 (98.1) |
| Beta blocker, n (%) | 1770 (40.2) | 5421 (53.5) | 9030 (52.6) | 4554 (64.9) | 5692 (69.0) | — |
| Statin/ezetimibe, n (%) | 3036 (69.0) | 7599 (74.9) | 12868 (75.0) | 5403 (77.0) | 6790 (82.3) | 2794 (64.9) |
| Characteristics | EMPEROR REDUCED | SOLOIST-WHF | EMPEROR-PRESERVED | SCORED | DAPA-HF | |
| SGLT-2 inhibitor | Empagliflozin | Sotagliflozin | Empagliflozin | Sotagliflozin | Dapagliflozin | |
| Year of publication | 2020 | 2021 | 2021 | 2021 | 2019 | |
| Median follow-up period, (years) | 1.3 | 0.8 | 2.2 | 1.3 | 1.5 | |
| Patient characteristics | ||||||
| Age, mean (SD) (years) | 66.9 (11) | 69.5 | 71.8 (9.3) | 69 | 66.4 (10.9) | |
| Men, n (%) | 2837 (76.1) | 810 (66.2) | 3312 (55.3) | 5830 (55.1) | 3635 (76.6) | |
| Race, n (%) | ||||||
| White | 2629 (70.5) | 1139 (93.2) | 4542 (75.6) | 8749 (82.7) | 3333 (70.3) | |
| Asian | 672 (18.0) | 15 (1.2) | 824 (13.8) | 682 (6.4) | 1116 (23.5) | |
| Black | 257 (6.9) | 50 (4.1) | 258 (4.3) | 364 (3.4) | 226 (4.8) | |
| Other/missing | 172 (4.6) | 18 (1.5) | 364 (6.1) | 789 (7.5) | 69 (1.5) | |
| Medical history | ||||||
| CVD, n (%) | 3730 (100) | 1222 (100) | 5988 (100) | 9381 (88.6) | 4744 (100) | |
| CHF, n (%) | 3730 (100) | 1222 (100) | 5988 (100) | 3283 (31.0) | 4744 (100) | |
| CKD, n (%) | 1799 (48.2) | — | 2988 (49.9) | 10 584 (100) | 1926 (40.6) | |
| DM, n (%) | 1856 (49.8) | 1222 (100) | 2938 (49.1) | 10 584 (100) | 1983 (41.8) | |
| Cardiovascular medications | ||||||
| ACEI or ARB, n (%) | 2600 (69.7) | 1010 (82.7) | 4832 (80.7) | 9365 (88.5) | 3968 (83.6) | |
| Beta blocker, n (%) | 3533 (94.7) | 1125 (92.1) | 5167 (86.3) | 6616 (62.5) | 4558 (96.1) | |
| Statin/ezetimibe, n (%) | — | — | 4131 (70.0) | — | — |
| Characteristics . | CREDENCE . | CANVAS . | DECLARE-TIMI . | EMPA-REG . | VERTIS . | DAPA-CKD . |
|---|---|---|---|---|---|---|
| SGLT-2 inhibitor | Canagliflozin | Canagliflozin | Dapagliflozin | Empagliflozin | Ertugliflozin | Dapagliflozin |
| Year of publication | 2019 | 2017 | 2019 | 2015 | 2020 | 2020 |
| Median follow-up period (years) | 2.6 | 2.4 | 4.2 | 3.1 | 3.0 | 2.4 |
| Patient characteristics | ||||||
| Age, mean (SD) (years) | 63.0 (9.2) | 63.3 (8.3) | 63.9 (6.8) | 63.1 (8.6) | 64.4 (8.1) | 61.8 (12.1) |
| Men, n (%) | 2907 (66.1) | 6509 (64.2) | 10 738 (62.6) | 5016 (71.5) | 5769 (70.0) | 2879 (66.9) |
| Race, n (%) | ||||||
| White | 2931 (66.6) | 7944 (78.3) | 13 653 (79.6) | 5081 (72.4) | 7240 (87.8) | 2290 (53.2) |
| Asian | 877 (19.9) | 1284 (12.7) | 2303 (13.4) | 1517 (21.6) | 498 (6.0) | 1467 (34.1) |
| Black | 224 (5.1) | 336 (3.3) | 603 (3.5) | 357 (5.1) | 235 (2.8) | 191 (4.4) |
| Other/missing | 369 (8.4) | 578 (5.7) | 601 (3.5) | 65 (0.9) | 273 (3.3) | 356 (8.3) |
| Medical history | ||||||
| CVD, n (%) | 2220 (50.4) | 6656 (65.6) | 6974 (40.6) | 7020 (100) | 8246 (100) | 1610 (37.4) |
| CHF, n (%) | 652 (14.8) | 1461 (14.4) | 1724 (10.0) | 706 (10.1) | 1958 (23.7) | 468 (10.9) |
| CKD, n (%) | 2631 (59.8) | 2039 (20.1) | 1265 (7.4) | 1819 (25.9) | 1807 (21.9) | 4304 (100) |
| DM, n (%) | 4401 (100) | 10 142 (100) | 17 160 (100) | 7020 (100) | 8246 (100) | 2906 (67.5) |
| Cardiovascular medications | ||||||
| ACEI or ARB, n (%) | 4395 (99.9) | 8116 (80.0) | 13 950 (81.3) | 5666 (80.7) | 6686 (81.1) | 4224 (98.1) |
| Beta blocker, n (%) | 1770 (40.2) | 5421 (53.5) | 9030 (52.6) | 4554 (64.9) | 5692 (69.0) | — |
| Statin/ezetimibe, n (%) | 3036 (69.0) | 7599 (74.9) | 12868 (75.0) | 5403 (77.0) | 6790 (82.3) | 2794 (64.9) |
| Characteristics | EMPEROR REDUCED | SOLOIST-WHF | EMPEROR-PRESERVED | SCORED | DAPA-HF | |
| SGLT-2 inhibitor | Empagliflozin | Sotagliflozin | Empagliflozin | Sotagliflozin | Dapagliflozin | |
| Year of publication | 2020 | 2021 | 2021 | 2021 | 2019 | |
| Median follow-up period, (years) | 1.3 | 0.8 | 2.2 | 1.3 | 1.5 | |
| Patient characteristics | ||||||
| Age, mean (SD) (years) | 66.9 (11) | 69.5 | 71.8 (9.3) | 69 | 66.4 (10.9) | |
| Men, n (%) | 2837 (76.1) | 810 (66.2) | 3312 (55.3) | 5830 (55.1) | 3635 (76.6) | |
| Race, n (%) | ||||||
| White | 2629 (70.5) | 1139 (93.2) | 4542 (75.6) | 8749 (82.7) | 3333 (70.3) | |
| Asian | 672 (18.0) | 15 (1.2) | 824 (13.8) | 682 (6.4) | 1116 (23.5) | |
| Black | 257 (6.9) | 50 (4.1) | 258 (4.3) | 364 (3.4) | 226 (4.8) | |
| Other/missing | 172 (4.6) | 18 (1.5) | 364 (6.1) | 789 (7.5) | 69 (1.5) | |
| Medical history | ||||||
| CVD, n (%) | 3730 (100) | 1222 (100) | 5988 (100) | 9381 (88.6) | 4744 (100) | |
| CHF, n (%) | 3730 (100) | 1222 (100) | 5988 (100) | 3283 (31.0) | 4744 (100) | |
| CKD, n (%) | 1799 (48.2) | — | 2988 (49.9) | 10 584 (100) | 1926 (40.6) | |
| DM, n (%) | 1856 (49.8) | 1222 (100) | 2938 (49.1) | 10 584 (100) | 1983 (41.8) | |
| Cardiovascular medications | ||||||
| ACEI or ARB, n (%) | 2600 (69.7) | 1010 (82.7) | 4832 (80.7) | 9365 (88.5) | 3968 (83.6) | |
| Beta blocker, n (%) | 3533 (94.7) | 1125 (92.1) | 5167 (86.3) | 6616 (62.5) | 4558 (96.1) | |
| Statin/ezetimibe, n (%) | — | — | 4131 (70.0) | — | — |
Continuous variables are presented as mean ± standard deviation, categorical variables as N (%).
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; DM, diabetes mellitus; CKD, chronic kidney disease; CHF, congestive heart failure; CVD, cardiovascular disease; n, number; SD, standard deviation; and SGLT-2, sodium–glucose cotransporter 2.
Outcomes by history of heart failure
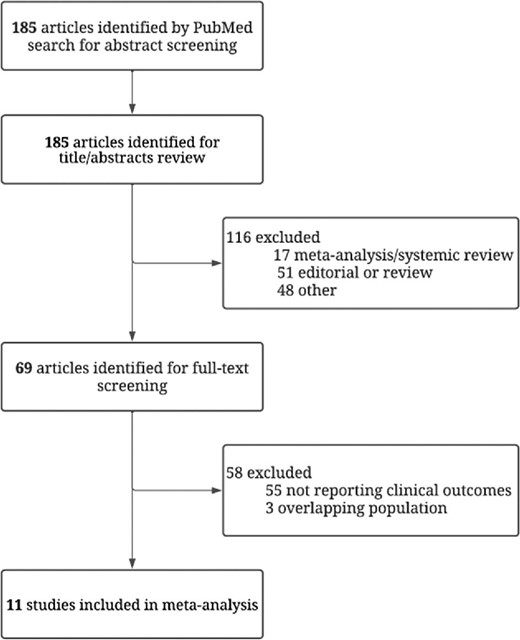
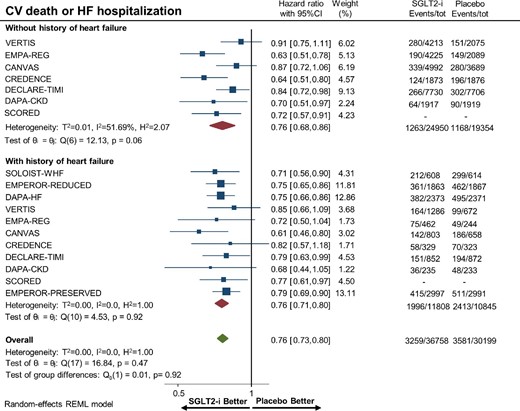
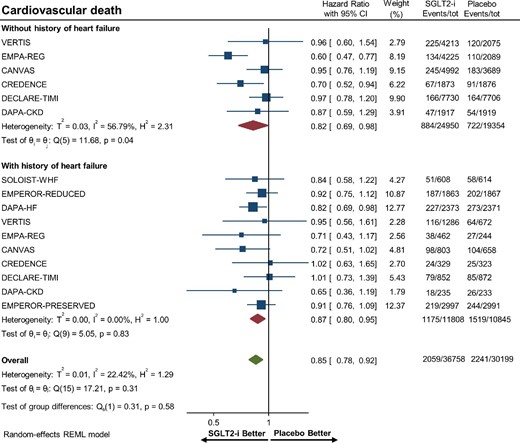
Overall, there were 22 653 (33.8%) patients with heart failure and 44 304 (66.2%) patients without heart failure. SGLT-2 inhibitors significantly reduced the risk of hospitalization for heart failure or CV death in patients with (HR 0.76, 95% CI 0.71–0.80) and without (HR 0.76, 95% CI 0.68–0.86) heart failure (Figure2). Figures3–5 show that patients treated with SGLT-2 inhibitors had a significant reduction in the individual outcomes of all-cause mortality, hospitalization for heart failure, and CV death regardless of the history of heart failure. After metaregression analysis, no significant interaction was observed between the presence of heart failure and effect estimates for the primary composite outcome (Pinteraction = 0.69), CV death (Pinteraction = 0.54), hospitalization for heart failure (Pinteraction = 0.98), and all-cause mortality (Pinteraction = 0.67). A high degree of heterogeneity (I² > 50%) was observed in patients without heart failure for all outcomes except for heart failure hospitalization (I² = 25.6%). In contrast, patients with heart failure had no significant heterogeneity observed for all outcomes.
Forest plot of the primary composite outcome of cardiovascular death or hospitalization for heart failure among patients with and without a history of heart failure treated with sodium–glucose cotransporter 2 inhibitors vs. placebo. HF, heart failure; CI, confidence interval; CV, cardiovascular; and SGLT2-i, sodium–glucose cotransporter 2 inhibitor.
Forest plot of cardiovascular death among patients with and without a history of heart failure treated with sodium–glucose cotransporter 2 inhibitors vs. placebo. CI, confidence interval; SGLT2-i, sodium–glucose cotransporter 2 inhibitor.
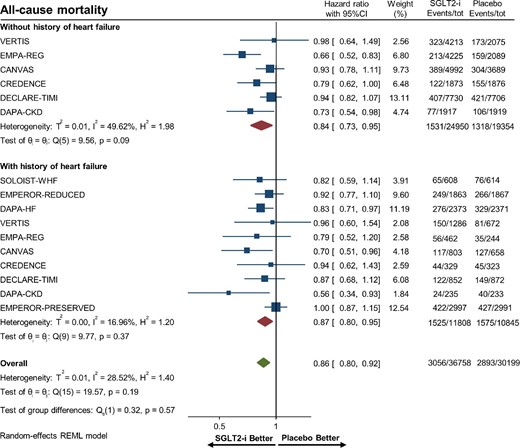
Forest plot of all-cause mortality among patients with and without a history of heart failure treated with sodium–glucose cotransporter 2 inhibitors vs. placebo. CI, confidence interval; SGLT2-i, sodium–glucose cotransporter 2 inhibitor.

Forest plot of hospitalization for heart failure among patients with and without a history of heart failure treated with sodium–glucose cotransporter 2 inhibitors vs. placebo. CI, confidence interval; SGLT2-i, sodium–glucose cotransporter 2 inhibitor.
Outcomes in HFrEF by severity of LVEF reduction
Overall, there were 5310 (9.4%) patients with HFrEF and severely reduced LVEF. SGLT-2 inhibitors significantly reduced the risk of hospitalization for heart failure or CV death in patients with (HR 0.68, 95% CI 0.59–0.78) and without (HR 0.84, 95% CI 0.71–0.99) severely reduced LVEF in HFrEF (Figure6). Metaregression analysis reported no significant interaction between effect estimates and degree of LVEF impairment for the primary composite outcome (Pinteraction = 0.13).
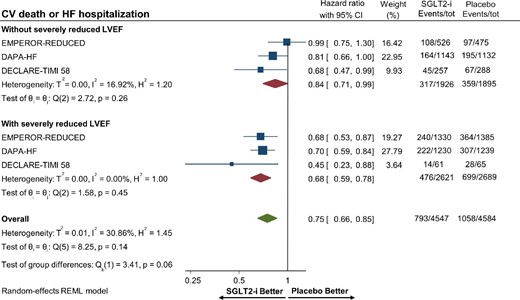
Forest plot displaying the effects of sodium–glucose cotransporter 2 (SGLT-2) inhibitors vs. placebo for the primary composite outcome of cardiovascular death or hospitalization for heart failure among patients with heart failure with reduced ejection with or without severely reduced left ventricular ejection fraction. HF, heart failure; CI, confidence interval; CV, cardiovascular; LVEF, left ventricular ejection fraction; and SGLT2-i, sodium–glucose cotransporter 2 inhibitor.
Outcomes in HFpEF vs. HFrEF
Overall, there were 5936 (8.9%) patients with HFpEF and 10 232 (15.3%) patients with HFrEF. Among patients with HFpEF, SGLT-2 inhibitors were associated with a reduced risk of hospitalization for heart failure or CV death (HR 0.80, 95% CI 0.70–0.92, P = 0.001; Figure7). Patients with HFrEF who were treated with SGLT-2 inhibitors had a significant reduction in the risk of hospitalization for heart failure or CV death (HR 0.76, 95% CI 0.70–0.83) compared with placebo. Metaregression analysis to assess for an interaction between effect estimates and heart failure subtype (HFpEF vs. HFrEF) for the primary composite outcome was not observed to be significant (Pinteraction = 0.65).
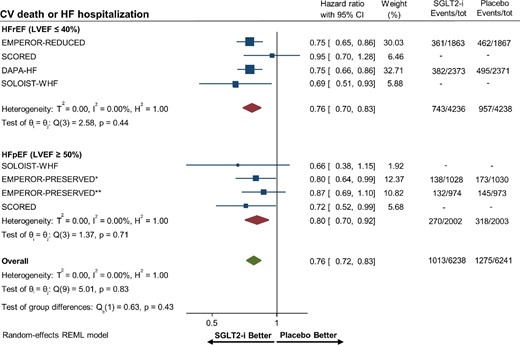
Forest plot of the primary composite outcome of cardiovascular death or hospitalization for heart failure among patients with heart failure with preserved and reduced ejection fraction treated with sodium–glucose cotransporter 2 (SGLT-2) inhibitors vs. placebo. *Heart failure with preserved ejection fraction patients in EMPEROR-PRESERVED with left ventricular ejection fraction ≥50% and <60%. **Heart failure with preserved ejection fraction patients in EMPEROR-PRESERVED with left ventricular ejection fraction ≥60%. HF, heart failure; CI, confidence interval; CV, cardiovascular; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; and SGLT2-i, sodium–glucose cotransporter 2 inhibitor.
Discussion
The main findings in this meta-analysis of SGLT-2 inhibitors among patients with and without a history of heart failure were as follows:
SGLT-2 inhibitors were associated with a reduced risk of all-cause mortality, hospitalization for heart failure, CV death, and the composite outcome of hospitalization for heart failure or CV death irrespective of the history of heart failure.
Among patients with HFrEF, use of SGLT-2 inhibitors reduced the risk of hospitalization for heart failure or CV death in patients with and without severely reduced LVEF.
The significant reduction in the risk of the composite of hospitalization for heart failure or CV death was consistently observed in patients with HFpEF.
We present a comprehensive meta-analysis of SGLT-2 inhibitors using 11 major CV outcome trials published to date. The present meta-analysis expands previously published studies by inclusion of data from the recent DAPA-CKD, SOLOIST-WHF, SCORED, and EMPEROR-PRESERVED trials and adds to the current literature by assessing the benefits of SGLT-2 inhibitors in patients without a history of heart failure but at high CV risk.6,7,12,14,16,18,19 We further analysed the impact of severely reduced LVEF on the treatment effects of SGLT-2 inhibitors among patients with HFrEF. Finally, we evaluated the benefit of SGLT-2 inhibitors in patients with HFpEF according to the well-recognized definition of an LVEF ≥50%. We provide a robust statistical analysis by testing for interaction between treatment effects and (1) history of heart failure, (2) severity of systolic dysfunction in HFrEF, and (3) type of congestive heart failure (HFpEF vs. HFrEF).
SGLT-2 inhibitors are a groundbreaking therapy in the fields of heart failure, atherosclerotic CV disease, and chronic kidney disease. A previous meta-analysis by McGuire et al. showed that SGLT-2 inhibitor use was associated with a reduced risk of major adverse cardiac events especially among patients with established atherosclerotic CV disease.14 Patients with chronic kidney disease (estimated glomerular filtration rate ≤60 mL/min/m2) who received SGLT-2 inhibitors exhibited a lower risk of renal disease progression and development of end-stage renal disease.21 The CV and renal benefits of SGLT-2 inhibitors hold true regardless of type 2 diabetes status suggesting alternative pharmacologic mechanisms other than glucose control.13
SGLT-2 inhibitors block the reabsorption of glucose in the proximal convoluted tubule of the nephron, thereby inducing glucosuria and lowering blood glucose levels.22 The exact mechanism(s) by which SGLT-2 inhibitors improve CV outcomes is unknown but a variety of hypotheses have been proposed. SGLT-2 inhibitors are known to increase diuresis and natriuresis, reduce systolic blood pressure, and promote weight loss, which may explain some of the CV benefits.1,22 Other mechanisms may include (1) reducing unfavourable cardiac remodelling (fibrosis, hypertrophy) with inhibition of cardiac myocyte sodium–hydrogen exchangers; (2) reducing sympathetic nervous system activation; (3) improving the efficiency of myocardial metabolism with increased utilization of ketone bodies; (4) increasing erythropoietin production; and (5) reducing systemic inflammation.23–25 In addition, sotagliflozin is an inhibitor of both SGLT-1 and SGLT-2.7 SGLT-1 is expressed by cardiac myocytes but it is still unclear what effect inhibition of SGLT-1 has on cardiac myocytes.25
The CV benefits of SGLT-2 inhibitors are well established in patients with HFrEF.7–9 Current American College of Cardiology/American Heart Association (ACC/AHA) guidelines still do not recommend the routine use of SGLT-2 inhibitors for the treatment of heart failure or atherosclerotic CV disease in patients without a history of type 2 diabetes.10 Recently, the European Society of Cardiology (ESC) guidelines were updated for the management of heart failure and included SGLT-2 inhibitors as part of goal-directed medical therapy among patients with HFrEF.26 Based on the preliminary results from the EMPLUSE trial, clinicians might soon consider initiation of SGLT-2 inhibitors in patients hospitalized for acute decompensated heart failure, irrespective of type 2 diabetes mellitus history.27,28 Moreover, the EMPULSE trial included a considerable percentage of patients with de novo acute heart failure, suggesting that SGLT-2 inhibitors could be safe and effective in this setting.28
Our meta-analysis suggests that the benefits of SGLT-2 inhibitors are preserved in patients at high CV risk irrespective of heart failure history, as there was no significant interaction observed for all outcomes between patients with and without heart failure. However, this observation on the treatment effects of SGLT-2 inhibitors among patients without heart failure must be interpreted in light of the significant heterogeneity observed in this population, except for the secondary outcome of heart failure hospitalization. Second, only a small proportion of heart failure patients were included in the type 2 diabetes mellitus CV outcome trials. Further studies are warranted to assess whether SGLT-2 inhibitors should be routinely considered for secondary CV disease prevention among patients without heart failure.
Mineralocorticoid receptor antagonists such as spironolactone and eplerenone have been shown to reduce all-cause mortality in HFrEF with severe systolic dysfunction (LVEF ≤35%), but whether SGLT-2 inhibitors have a similar effect is unknown.29,30 In our meta-analysis, patients with HFrEF and severely reduced LVEF benefited from SGLT-2 inhibitor use by reducing the risk of the primary composite outcome by 32%. In contrast, HFrEF patients without a severely reduced LVEF only had a borderline significant reduction in the primary composite outcome, although the lack of significant interaction suggests a benefit irrespective of the degree of systolic dysfunction. This finding should be interpreted considering the differences in patient enrolment between the DAPA-HF and EMPEROR-REDUCED trials, with the latter study including sicker patients with higher N-terminal pro-B-type natriuretic peptide concentrations.8,9
Pharmacological therapies to reduce mortality and improve outcomes in patients with HFpEF remain dramatically limited. A recent post hoc analysis of EMPA-REG OUTCOME using a novel validated LVEF predictive model in the subgroup of heart failure patients showed that empagliflozin consistently improved CV outcomes in patients with HFpEF, HFrEF, and no history of heart failure.31 To date, no meta-analysis has assessed for interaction between the heart failure subtype (HFrEF vs. HFpEF) and treatment effects of SGLT-2 inhibitors. Our results showed a significant 20% risk reduction in hospitalization for heart failure or CV death in patients with HFpEF. In addition, this reduced risk was consistently observed irrespective of the heart failure subtype (HFpEF vs. HFrEF). This signal of benefit must be interpreted in light of the limited power of the subgroup analysis in evaluating HFpEF patients and of the lack of data on individual outcomes stratified by HFpEF status for most of the included studies. The recent EMPEROR-PRESERVED trial showed that empagliflozin improved CV outcomes in patients with HFpEF; however, no all-cause mortality reduction was observed.18 Of note, the study included patients with a LVEF ≥40%.18 More specific clinical trials including only HFpEF patients with an LVEF ≥50% are needed to shed light on the role of SGLT-2 inhibitors in this unique patient population.
Study limitations
The present meta-analysis has several important limitations. First, despite the large sample size (n = 66 957), individual outcomes and subgroup analyses may be underpowered. Second, the weight of the type 2 diabetes mellitus CV outcome trials was low given the limited number of heart failure patients enrolled in these studies. Third, variations in follow-up duration, enrolment criteria, demographic characteristics, and different SGLT-2 inhibitor subtypes may have contributed to the observed heterogeneity across studies, particularly in the group of patients without heart failure. Fourth, relevant information on the degree of LVEF reduction and presence of HFpEF was not available for all trails. Fifth, regional differences in the diagnostic criteria for heart failure may have led to some degree of selection bias. Lastly, although the definition for the primary composite endpoint was very similar between trials, slight variations may exist, such as in SOLOIST-WHF (total heart failure hospitalization or CV death).7
Conclusions
SGLT-2 inhibitors significantly improved CV outcomes and reduced all-cause mortality in patients with and without a history of heart failure. Patients with HFrEF had improved CV outcomes with SGLT-2 inhibitors irrespective of LVEF reduction. SGLT-2 inhibitors reduced the risk of adverse cardiovascular outcomes among patients with HFpEF and irrespective of the type of heart failure (HFpEF vs. HFrEF).
Funding
None declared.
Conflict of interest: G.D.D. has received consulting fees from GE HealthCare, Janssen Pharmaceuticals, Inc., and Medtronic, Inc.; has <1% equity with Claret Medical and Elixir Medical; has delivered industry-sponsored lectures for The Medicines Company; and is on the scientific advisory board for AstraZeneca. R.M. has received institutional research funding from AstraZeneca, Bayer, Beth Israel Deaconess, Bristol Myers Squibb/Sanofi, CSL Behring, Eli Lilly/Daiichi Sankyo, Medtronic, Novartis, and OrbusNeich; is a consultant for Boston Scientific, Abbott Vascular, Medscape, Siemens Medical Solutions, Regeneron Pharmaceuticals Inc. (no fees), Roivant Sciences, Inc., and Sanofi; is an institution consultant (payment to institution) with Abbott Vascular and Spectranetics/Phillips/Volcano Corporation; is on the executive committee for Janssen Pharmaceuticals and BMS; receives institutional (payment to institution) advisory board funding from Bristol Myers Squibb and Novartis; has received DSMB membership funding (payment to institution) from Watermark Research; and has <1% equity with Claret Medical and Elixir Medical. All other authors report no disclosures relevant to this paper.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Notes
PROSPERO registration: https://www.crd.york.ac.uk/prospero/CRD42020219082
References
Author notes
These authors contributed equally to this work.



