-
PDF
- Split View
-
Views
-
Cite
Cite
Juan Tamargo, Keld Per Kjeldsen, Eva Delpón, Anne Grete Semb, Elisabetta Cerbai, Dobromir Dobrev, Gianluigi Savarese, Patrick Sulzgruber, Giuseppe Rosano, Claudio Borghi, Sven Wassmann, Christian Tobias Torp-Pedersen, Stefan Agewall, Heinz Drexel, Iris Baumgartner, Basil Lewis, Claudio Ceconi, Juan Carlos Kaski, Alexander Niessner, Facing the challenge of polypharmacy when prescribing for older people with cardiovascular disease. A review by the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 8, Issue 4, July 2022, Pages 406–419, https://doi.org/10.1093/ehjcvp/pvac005
Close - Share Icon Share
Abstract
Population ageing has resulted in an increasing number of older people living with chronic diseases (multimorbidity) requiring five or more medications daily (polypharmacy). Ageing produces important changes in the cardiovascular system and represents the most potent single cardiovascular risk factor. Cardiovascular diseases (CVDs) constitute the greatest burden for older people, their caregivers, and healthcare systems.
Cardiovascular pharmacotherapy in older people is complex because age-related changes in body composition, organ function, homeostatic mechanisms, and comorbidities modify the pharmacokinetic and pharmacodynamic properties of many commonly used cardiovascular and non-cardiovascular drugs. Additionally, polypharmacy increases the risk of adverse drug reactions and drug interactions, which in turn can lead to increased morbi-mortality and healthcare costs. Unfortunately, evidence of drug efficacy and safety in older people with multimorbidity and polypharmacy is limited because these individuals are frequently underrepresented/excluded from clinical trials. Moreover, clinical guidelines are largely written with a single-disease focus and only occasionally address the issue of coordination of care, when and how to discontinue treatments, if required, or how to prioritize recommendations for patients with multimorbidity and polypharmacy.
This review analyses the main challenges confronting healthcare professionals when prescribing in older people with CVD, multimorbidity, and polypharmacy. Our goal is to provide information that can contribute to improving drug prescribing, efficacy, and safety, as well as drug adherence and clinical outcomes.
Introduction
The progressive rise in life expectancy over the last century has resulted in an unprecedented increase in the number of older people, defined as those aged ≥65 years, and nowadays individuals aged >75 years represent the most rapidly growing population in Western countries. In 2030, the European Union population aged ≥65 years is expected to increase up to 23% and up to 20% in the USA.1 These demographic changes are leading to a progressive increase in the number of older people living with ≥2 chronic conditions simultaneously (multimorbidity) and complex health states (also termed ‘geriatric syndromes’), requiring multiple medications (polypharmacy). Specifically, the advances in prevention and treatment of cardiovascular diseases (CVDs) have led to a decline in cardiovascular morbidity and mortality, so many patients currently survive a heart attack or stroke and suffer from heart failure (HF) of different aetiologies. The main characteristics of older people with CVD are summarized in Table 1.
| 1. Aging is associated with physiological changes in organ body structure and function and in homeostatic mechanisms |
| • This modifies the pharmacodynamic/pharmacokinetic properties of cardiovascular drugs |
| 2. Vulnerability: greater propensity to get sick |
| • Many older people present ≥2 chronic medical or psychiatric conditions simultaneously (multimorbidity) |
| • Produces physical impairment, functional limitation and disability, frailty, impairs the quality of life, and increases sedentary lifestyles |
| • Geriatric syndromes: cognitive impairment and delirium, falls, pressure ulcers, urinary incontinence, functional decline |
| 3. Polypharmacy: older people use multiple medications (prescriptions, over the counter, alternative/herbal medications, vitamins, and supplements) |
| • Higher risk of inappropriate polypharmacy: overuse, underuse, misuse, unnecessary, inappropriate, or harmful drugs |
| • Higher risk of adverse drug reactions and drug–drug and drug–disease interactions |
| 1. Aging is associated with physiological changes in organ body structure and function and in homeostatic mechanisms |
| • This modifies the pharmacodynamic/pharmacokinetic properties of cardiovascular drugs |
| 2. Vulnerability: greater propensity to get sick |
| • Many older people present ≥2 chronic medical or psychiatric conditions simultaneously (multimorbidity) |
| • Produces physical impairment, functional limitation and disability, frailty, impairs the quality of life, and increases sedentary lifestyles |
| • Geriatric syndromes: cognitive impairment and delirium, falls, pressure ulcers, urinary incontinence, functional decline |
| 3. Polypharmacy: older people use multiple medications (prescriptions, over the counter, alternative/herbal medications, vitamins, and supplements) |
| • Higher risk of inappropriate polypharmacy: overuse, underuse, misuse, unnecessary, inappropriate, or harmful drugs |
| • Higher risk of adverse drug reactions and drug–drug and drug–disease interactions |
| 1. Aging is associated with physiological changes in organ body structure and function and in homeostatic mechanisms |
| • This modifies the pharmacodynamic/pharmacokinetic properties of cardiovascular drugs |
| 2. Vulnerability: greater propensity to get sick |
| • Many older people present ≥2 chronic medical or psychiatric conditions simultaneously (multimorbidity) |
| • Produces physical impairment, functional limitation and disability, frailty, impairs the quality of life, and increases sedentary lifestyles |
| • Geriatric syndromes: cognitive impairment and delirium, falls, pressure ulcers, urinary incontinence, functional decline |
| 3. Polypharmacy: older people use multiple medications (prescriptions, over the counter, alternative/herbal medications, vitamins, and supplements) |
| • Higher risk of inappropriate polypharmacy: overuse, underuse, misuse, unnecessary, inappropriate, or harmful drugs |
| • Higher risk of adverse drug reactions and drug–drug and drug–disease interactions |
| 1. Aging is associated with physiological changes in organ body structure and function and in homeostatic mechanisms |
| • This modifies the pharmacodynamic/pharmacokinetic properties of cardiovascular drugs |
| 2. Vulnerability: greater propensity to get sick |
| • Many older people present ≥2 chronic medical or psychiatric conditions simultaneously (multimorbidity) |
| • Produces physical impairment, functional limitation and disability, frailty, impairs the quality of life, and increases sedentary lifestyles |
| • Geriatric syndromes: cognitive impairment and delirium, falls, pressure ulcers, urinary incontinence, functional decline |
| 3. Polypharmacy: older people use multiple medications (prescriptions, over the counter, alternative/herbal medications, vitamins, and supplements) |
| • Higher risk of inappropriate polypharmacy: overuse, underuse, misuse, unnecessary, inappropriate, or harmful drugs |
| • Higher risk of adverse drug reactions and drug–drug and drug–disease interactions |
In this review, we sought to analyse the main challenges that practitioners face when prescribing for older people with CVD, as well as identifying ways of reducing the risk of inappropriate polypharmacy. We also addressed the important issue of patient-centred treatments and the identification of major knowledge gaps to improve cardiovascular therapy in this growing patient population.
Advanced age—a potent cardiovascular risk factor
Ageing produces multiple structural and functional changes in the cardiovascular system that can increase the susceptibility of ageing individuals to develop CVDs, which represent the most prevalent conditions in older people (Supplementary material online, Table S1).2,3 The prevalence of CVD increases from 65–70% in persons aged 60–79 to 79–86% in those aged ≥80 years. Of interest, several cardiovascular syndromes such as isolated systolic hypertension, HF with preserved ejection fraction, and calcific/degenerative aortic stenosis are most prevalent in older people.2–4 Among 46.3 million of elderly Medicare beneficiaries, the prevalence of hypertension, hypercholesterolaemia, ischaemic heart disease (IHD), diabetes, and HF was 61%, 48%, 38%, 28%, and 17%, respectively. Of interest, 27% of patients with hypertension and ∼65% of those with HF had ≥5 concomitant chronic health conditions.5
Because of the high prevalence of CVD in older people, cardiovascular drugs are among the most frequently used drugs in this population. In the National Social Life, Health and Aging Project home medication survey, among the 20 most commonly prescribed drugs in older people were antiplatelet agents (aspirin, clopidogrel), statins (atorvastatin, simvastatin), glucose-lowering agents (metformin), beta-blockers (metoprolol, atenolol), angiotensin-converting enzyme inhibitors (ACEIs: lisinopril), angiotensin receptor blockers (ARBs: valsartan), diuretics (hydrochlorothiazide), calcium channel blockers (amlodipine), and anticoagulants.6,7
Multimorbidity
The majority of older patients with CVD present other non-CVD pathoologies.4,5,7–10 Therefore, old patients should be screened for CV and non-CV comorbidities and, when present, non-CVD should be treated with suitable medications that can improve symptoms and outcomes but do not exacerbate their CVD. Interestingly, disease states in the same patient are interrelated in a sense that disease in one organ facilitates the development and progression of the disease in another organ, which can substantially impact the prognosis and treatment of each condition. Hypertension produces damage in the heart, vessels, kidneys, and central nervous system; diabetes can result in heart disease, stroke, chronic kidney disease (CKD), and nerve and eye damage; patients with CKD exhibit an elevated cardiovascular risk manifesting as IHD, HF, arrhythmias, and sudden cardiac death; increased risk for CVD and accelerated atherosclerosis are reported in almost all rheumatologic conditions; and anticancer-drug-induced cardiotoxicity represents a major cause of morbidity and mortality among cancer survivors. These interrelationships are the basis for new subspecialties (cardio-renal, cardio-rheumatology, cardio-oncology).
Multimorbidity complicates the clinical picture, diagnosis, and decision-making, promotes ‘fragmented”’ care, contributes to a decline in functional status and quality of life (QoL), and increases frailty, healthcare resource utilization (outpatient visits, hospitalizations), and mortality.4,8 Furthermore, it increases drug treatment complexity (polypharmacy) and the risk of adverse drug reactions (ADRs), drug–drug interactions (DDIs), and drug–disease interactions. Indeed, over one-fifth of older people with multimorbidity receive medications that may adversely affect a coexisting condition. Non-steroidal anti-inflammatory drugs (NSAIDs) and some anticancer drugs can worsen HF (i.e. anthracyclines, carfilzomib, cyclophosphamide, docetaxel, sunitinib, and trastuzumab) and hypertension (i.e. vascular endothelial growth factor inhibitors and ponatinib),11 and the administration of beta-blockers in patients with HF, hypertension, or atrial fibrillation worsens chronic obstructive lung disease.12 Thus, effects on coexisting conditions should be considered when prescribing medications to old patients with CVD and multimorbidity.
Therefore, the progressive ageing of the population has naturally resulted in a growing number of older people with CVD and multimorbidity, with CVD representing the greatest burden for the patient, their caregivers, and health systems worldwide.2
Multidisciplinary team approach
Healthcare systems and clinical practice guidelines (CPGs) are mainly oriented towards single-disease rather than multimorbidity.4 However, application of multiple disease-specific CPGs in patients with CVD and multimorbidity without integration may lead to contradictory recommendations and be impractical, or even harmful, and misaligned with patients’ preferences and values.7,8,10 Additionally, these patients are treated simultaneously by several specialists, which can lead to discrepancies in goals of care, drugs prescribed, and overall medical management.10 In these circumstances, a holistic patient care requires a multidisciplinary team for a successful comprehensive geriatric assessment and coordinated management of multimorbidity.13 The coordinated teamwork between the cardiologist and other medical specialists, nurses, pharmacists, social workers, family, and caregivers plays a key role in establishing the goals of cardiovascular pharmacotherapy according to the patient’s preferences and values.7,10 The multidisciplinary team approach assists in decision-making, enables personalized treatment strategies, evaluates the complexity, feasibility, and adherence to treatment, selects drugs and doses to optimize benefits, minimizes harm, and improves QoL and outcomes, and coordinates care across transitions (i.e. between emergency departments, in-/outpatient units, and skilled nursing facilities) when older people are more vulnerable. The multidisciplinary team approach improves the quality of care for patients with chronic CVD.10,13
Goals of care
The main challenge when treating old people with CVD and multimorbidity is to provide optimal care, but older adults may have goals that are different from the outcomes measured in randomized clinical trials (RCTs) performed in younger adults. The main goals of care are to preserve QoL, maintain daily functional capacity (including cognitive and physical function) and independence, control symptoms, and reduce the burden of treatment and hospitalizations, while life extension may be of less interest.7,8 However, prioritizing the goals in this population is challenging as disease-specific CPGs are often not applicable for very elderly patients (age ≥80 years) and even with the same pattern of multimorbidity, older adults are heterogeneous in terms of illness severity, functional status, prognosis, and treatment options.7,8 Therefore, old people with CVD and multimorbidity may benefit from the switch from a disease-specific approach to patient-centred care to ensure that they receive optimal care and drug prescription to maximize efficacy and safety and minimize harms of pharmacotherapy.7,8,10 When the treatment goals are different for patients, family, caregivers, and physicians, collaborative goal setting is useful for personalizing care and adapting it to a patient's goals, values, and resources. Thus, decisions regarding optimal cardiovascular drug treatment in older adults need to be individualized taking into consideration the patient's overall health context, functional status, life expectancy, and personal preferences.
Age-related changes in the pharmacokinetics and pharmacodynamics of cardiovascular drugs
Treatment in older people is complicated by age-associated changes in body composition, organ structure and function, homeostatic mechanisms, and comorbidities that affect the pharmacokinetics (absorption, distribution, metabolism, and excretion) and pharmacodynamics (the relationship between drug concentration at the site of action and drug effect) of many cardiovascular drugs.
Pharmacokinetic changes
Oral (Table 2) drug absorption may be delayed in older individuals, but full drug absorption can be achieved because most drugs are absorbed by passive diffusion.14–16 However, the reduced activity of some gut wall transporters and of first-pass metabolism can modify the bioavailability of selected drugs administered orally. The activation of prodrugs, as in the case of ACEIs and dabigatran, can be initially reduced but this reduction is not clinically relevant during chronic treatment.
Age-related changes in the pharmacokinetic parameters of cardiovascular drugs12–16
| Parameter . | Physiological change . | Consequences . |
|---|---|---|
| Absorption | • ↓ gastric acid production and emptying• ↓ splanchnic blood flow, motility, and absorption surface• ↓ gut wall transporters and first pass metabolism | • Delayed absorption, but no changes in the amount absorbed• Antacids and laxatives can ↓ drug absorption• ↓ the first-pass effect and ↑ the oral bioavailability of diltiazem, opioids, propranolol, simvastatin, and verapamil |
| Distribution | • ↓ cardiac output and tissue perfusion• ↓ extracellular and total body water• ↓ total body and muscle mass• ↑ body fat (18–35% in men, 30–45% in women) | • ↓ Vd and ↑ plasma levels of hydrophilic drugs (digoxin and theophylline)). ↓ the loading dose in the elderly• ↑ Vd and half-life of highly lipophilic drugs: amiodarone, benzodiazepines, dronedarone, lidocaine, opioids, verapamil, and vitamin D3 |
| Plasma protein binding | • ↓ plasma albumin• ↑ α1-acid glycoprotein | • ↑ free drug levels of highly albumin-bound drugs: amiodarone, diltiazem, dronedarone, propafenone, propranolol, verapamil, and warfarin |
| Biotransformation | • ↓ liver mass (20–30%) and hepatic blood flow (20–50%)• ↓ CYP450-mediated phase I reactions• Hepatic diseases (alcoholic liver disease, cirrhosis, carcinoma) are more common in elderly | • ↑ Cmax and t½ of highly metabolized drugs (Supplementary material online, Table S2)• ↓ the dose to minimize the risk of adverse effects in patients with HF or hepatic diseases |
| Excretion | • ↓ renal mass and renal blood flow (30–35%)• ↓ GFR and tubular secretion and reabsorption• ↑ renal diseases that decrease renal function: glomerulosclerosis, interstitial fibrosis, diabetes• ↑ comorbidities that ↓ RBF: HTN, vascular diseases, heart failure, diabetes | • ↑ Cmax and half-life (exposure) of renally excreted drugs (Supplementary material online, Table S2)• Drug accumulation due to reduced renal excretion is the most important cause of ADRs and drug–drug interactions• Monitor the renal function (use the CKD-EPI equation)• ↓ doses of drugs mainly eliminated by the kidneys |
| Parameter . | Physiological change . | Consequences . |
|---|---|---|
| Absorption | • ↓ gastric acid production and emptying• ↓ splanchnic blood flow, motility, and absorption surface• ↓ gut wall transporters and first pass metabolism | • Delayed absorption, but no changes in the amount absorbed• Antacids and laxatives can ↓ drug absorption• ↓ the first-pass effect and ↑ the oral bioavailability of diltiazem, opioids, propranolol, simvastatin, and verapamil |
| Distribution | • ↓ cardiac output and tissue perfusion• ↓ extracellular and total body water• ↓ total body and muscle mass• ↑ body fat (18–35% in men, 30–45% in women) | • ↓ Vd and ↑ plasma levels of hydrophilic drugs (digoxin and theophylline)). ↓ the loading dose in the elderly• ↑ Vd and half-life of highly lipophilic drugs: amiodarone, benzodiazepines, dronedarone, lidocaine, opioids, verapamil, and vitamin D3 |
| Plasma protein binding | • ↓ plasma albumin• ↑ α1-acid glycoprotein | • ↑ free drug levels of highly albumin-bound drugs: amiodarone, diltiazem, dronedarone, propafenone, propranolol, verapamil, and warfarin |
| Biotransformation | • ↓ liver mass (20–30%) and hepatic blood flow (20–50%)• ↓ CYP450-mediated phase I reactions• Hepatic diseases (alcoholic liver disease, cirrhosis, carcinoma) are more common in elderly | • ↑ Cmax and t½ of highly metabolized drugs (Supplementary material online, Table S2)• ↓ the dose to minimize the risk of adverse effects in patients with HF or hepatic diseases |
| Excretion | • ↓ renal mass and renal blood flow (30–35%)• ↓ GFR and tubular secretion and reabsorption• ↑ renal diseases that decrease renal function: glomerulosclerosis, interstitial fibrosis, diabetes• ↑ comorbidities that ↓ RBF: HTN, vascular diseases, heart failure, diabetes | • ↑ Cmax and half-life (exposure) of renally excreted drugs (Supplementary material online, Table S2)• Drug accumulation due to reduced renal excretion is the most important cause of ADRs and drug–drug interactions• Monitor the renal function (use the CKD-EPI equation)• ↓ doses of drugs mainly eliminated by the kidneys |
↑: increase. ↓: decrease.
ADR, adverse drug reaction; CCB, calcium channel blocker; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; Cmax, peak plasma level; GFR, glomerular filtration rate; HCTZ, hydrochlorothiazide; HTN, hypertension; RBF, renal blood flow; t½, half-life; and Vd, volume of distribution.
Age-related changes in the pharmacokinetic parameters of cardiovascular drugs12–16
| Parameter . | Physiological change . | Consequences . |
|---|---|---|
| Absorption | • ↓ gastric acid production and emptying• ↓ splanchnic blood flow, motility, and absorption surface• ↓ gut wall transporters and first pass metabolism | • Delayed absorption, but no changes in the amount absorbed• Antacids and laxatives can ↓ drug absorption• ↓ the first-pass effect and ↑ the oral bioavailability of diltiazem, opioids, propranolol, simvastatin, and verapamil |
| Distribution | • ↓ cardiac output and tissue perfusion• ↓ extracellular and total body water• ↓ total body and muscle mass• ↑ body fat (18–35% in men, 30–45% in women) | • ↓ Vd and ↑ plasma levels of hydrophilic drugs (digoxin and theophylline)). ↓ the loading dose in the elderly• ↑ Vd and half-life of highly lipophilic drugs: amiodarone, benzodiazepines, dronedarone, lidocaine, opioids, verapamil, and vitamin D3 |
| Plasma protein binding | • ↓ plasma albumin• ↑ α1-acid glycoprotein | • ↑ free drug levels of highly albumin-bound drugs: amiodarone, diltiazem, dronedarone, propafenone, propranolol, verapamil, and warfarin |
| Biotransformation | • ↓ liver mass (20–30%) and hepatic blood flow (20–50%)• ↓ CYP450-mediated phase I reactions• Hepatic diseases (alcoholic liver disease, cirrhosis, carcinoma) are more common in elderly | • ↑ Cmax and t½ of highly metabolized drugs (Supplementary material online, Table S2)• ↓ the dose to minimize the risk of adverse effects in patients with HF or hepatic diseases |
| Excretion | • ↓ renal mass and renal blood flow (30–35%)• ↓ GFR and tubular secretion and reabsorption• ↑ renal diseases that decrease renal function: glomerulosclerosis, interstitial fibrosis, diabetes• ↑ comorbidities that ↓ RBF: HTN, vascular diseases, heart failure, diabetes | • ↑ Cmax and half-life (exposure) of renally excreted drugs (Supplementary material online, Table S2)• Drug accumulation due to reduced renal excretion is the most important cause of ADRs and drug–drug interactions• Monitor the renal function (use the CKD-EPI equation)• ↓ doses of drugs mainly eliminated by the kidneys |
| Parameter . | Physiological change . | Consequences . |
|---|---|---|
| Absorption | • ↓ gastric acid production and emptying• ↓ splanchnic blood flow, motility, and absorption surface• ↓ gut wall transporters and first pass metabolism | • Delayed absorption, but no changes in the amount absorbed• Antacids and laxatives can ↓ drug absorption• ↓ the first-pass effect and ↑ the oral bioavailability of diltiazem, opioids, propranolol, simvastatin, and verapamil |
| Distribution | • ↓ cardiac output and tissue perfusion• ↓ extracellular and total body water• ↓ total body and muscle mass• ↑ body fat (18–35% in men, 30–45% in women) | • ↓ Vd and ↑ plasma levels of hydrophilic drugs (digoxin and theophylline)). ↓ the loading dose in the elderly• ↑ Vd and half-life of highly lipophilic drugs: amiodarone, benzodiazepines, dronedarone, lidocaine, opioids, verapamil, and vitamin D3 |
| Plasma protein binding | • ↓ plasma albumin• ↑ α1-acid glycoprotein | • ↑ free drug levels of highly albumin-bound drugs: amiodarone, diltiazem, dronedarone, propafenone, propranolol, verapamil, and warfarin |
| Biotransformation | • ↓ liver mass (20–30%) and hepatic blood flow (20–50%)• ↓ CYP450-mediated phase I reactions• Hepatic diseases (alcoholic liver disease, cirrhosis, carcinoma) are more common in elderly | • ↑ Cmax and t½ of highly metabolized drugs (Supplementary material online, Table S2)• ↓ the dose to minimize the risk of adverse effects in patients with HF or hepatic diseases |
| Excretion | • ↓ renal mass and renal blood flow (30–35%)• ↓ GFR and tubular secretion and reabsorption• ↑ renal diseases that decrease renal function: glomerulosclerosis, interstitial fibrosis, diabetes• ↑ comorbidities that ↓ RBF: HTN, vascular diseases, heart failure, diabetes | • ↑ Cmax and half-life (exposure) of renally excreted drugs (Supplementary material online, Table S2)• Drug accumulation due to reduced renal excretion is the most important cause of ADRs and drug–drug interactions• Monitor the renal function (use the CKD-EPI equation)• ↓ doses of drugs mainly eliminated by the kidneys |
↑: increase. ↓: decrease.
ADR, adverse drug reaction; CCB, calcium channel blocker; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; Cmax, peak plasma level; GFR, glomerular filtration rate; HCTZ, hydrochlorothiazide; HTN, hypertension; RBF, renal blood flow; t½, half-life; and Vd, volume of distribution.
In older people, body fat mass increases, while the total body water mass and lean body mass decrease.15 Thus, the volume of distribution (Vd) and half-life of lipophilic drugs may increase, while the Vd of hydrophilic drugs decreases, leading to a more rapid increase in plasma concentrations. Because plasma albumin levels decrease, the free active fraction of drugs highly bound to albumin available for passive diffusion to their target sites might probably increase. However, changes in plasma protein binding may have limited clinical relevance, because the effect of protein binding on free plasma concentration is rapidly counterbalanced by its effects on clearance.
The biotransformation of some cardiovascular drugs (Supplementary material online, Table S2) occurs mainly in the liver and age-related changes in hepatic function may account for the differences observed in drug metabolism in older people. Hepatic clearance depends on the liver capacity to metabolize a drug (expression/activity of drug-metabolizing enzymes), hepatic blood flow, and plasma protein binding.16 Drugs with high hepatic extraction ratios, such as diltiazem, lidocaine, metoprolol, morphine, nifedipine, propranolol, and verapamil, are rapidly metabolized and their clearance depends primarily on the hepatic blood flow, which decreases with age; thus, dose adjustments may be required to minimize the risk of ADRs.15,16 Conversely, drugs like warfarin, with a low intrinsic clearance, are slowly metabolized and the rate of elimination is mainly dependent on the hepatic metabolizing activity and free drug fraction. Hepatic metabolism via cytochrome P450 (CYP)-mediated phase I reactions (oxidization, reduction, and hydrolysis) leading to active metabolites decreases, while phase II conjugation reactions leading to inactive metabolites are relatively unaffected by age.14,15 Some cardiovascular drugs are metabolized by specific CYP isoforms (mainly CYP3A4, 2D6, and 2C19) and CYP inhibitors/inducers increase/reduce their effects, respectively, leading to important DDIs.
Ageing is associated with a reduction in renal mass, blood flow, estimated glomerular filtration rate (eGFR), and tubular secretion/reabsorption, and an increase in renal diseases impairing renal function. These changes reduce the clearance and increase the exposure and risk of ADRs of renally cleared drugs (Supplementary material online, Table S2). Accurate determination of eGFR is critical to adjust dose requirements of these drugs.17 Equations based on serum creatinine measurement [Cockcroft–Gault, Modification of Diet in Renal Disease (MDRD), and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)] are widely used and the CKD-EPI equation was recommended for estimating eGFR in adults of any age.18 However, because of reduced muscle mass, exercise, and meat intake, in older people serum creatinine levels may be within the reference limits, while renal function is reduced. Thus, these equations can misclassify kidney disease by one stage in >30% of the participants.17 To overcome these problems, equations based on cystatin C alone or in combination with creatinine were developed. CKD-EPICr-cys is more accurate than all creatinine-based equations in older patients across a wide spectrum of eGFR, but it is not recommended, probably because it is not yet generally available.17,19 Unfortunately, there are no specific guideline recommendations based on age, possibly because few studies have compared the different formulas in old and particularly in very elderly frail patients.
Pharmacodynamic changes
Cardiovascular drugs may exert different effects in older compared with younger individuals because ageing produces important changes in cardiovascular structure and function, as discussed previously2,3,12,20 (Supplementary material online, Table S1), and comorbidities can affect the pharmacokinetics and pharmacodynamics (due to changes in receptor number and affinity, signal transduction pathways, cellular responses, and homeostatic compensatory mechanisms) of different agents (Table 3).
| Physiological changes . | Pharmacodynamic effects . |
|---|---|
| Decreased cardiac reserve | • The heart is more susceptible to HF in patients treated with disopyramide or class IV AADs |
| Decreased LV compliance | • Decreased cardiac output with beta-blockers |
| Increased arterial stiffness | • ↑ risk of haemodynamic lability from vasodilator drugs and diuretics |
| Degeneration of sinoatrial and atrioventricular nodal function | • ↑ risk of bradycardia and AV block in patients treated with digoxin or class II and IV AADs |
| Changes in cardiac ion channel expression/activity | • ↑ risk of intracardiac conduction block when treated with class I AADs• Decreased repolarization reserve: ↑ risk of drug-induced proarrhythmia |
| Increased myocardial fibrosis | • Decreased intracardiac conduction velocity and the risk of conduction slowing |
| Decreased baroreceptor sensitivity | • ↑ risk of orthostatic hypotension, instability, and falls with use of antihypertensives, nitrates, and vasodilators |
| Down-regulation of β-adrenoreceptors | • ↓ response to beta-agonists (bronchodilation) and antagonists (antihypertensive effects) |
| Reduced response to diuretics | • ↓ the active transport of the diuretic to its site of action in the lumen tubule |
| Increased sensitivity to hyponatremia | • Due to ageing-related reduction of glomerular filtration rate, drugs, SIAD, or endocrinopathies |
| Increased sensitivity to anticoagulants | • ↑ the risk of bleeding (age explains up to 40% of the variance in warfarin dosing) |
| Increased sensitivity to drugs acting on the central nervous system | • ↓ P-gp activity at the blood–brain barrier leading to an accumulation of their substrates in the brain• ↓ chemoreceptor reflexes: ↑ respiratory depression by opioids• Some CV drugs (amiodarone, digoxin, lidocaine, and metoprolol) can increase neurocognitive impairment in the elderly |
| Physiological changes . | Pharmacodynamic effects . |
|---|---|
| Decreased cardiac reserve | • The heart is more susceptible to HF in patients treated with disopyramide or class IV AADs |
| Decreased LV compliance | • Decreased cardiac output with beta-blockers |
| Increased arterial stiffness | • ↑ risk of haemodynamic lability from vasodilator drugs and diuretics |
| Degeneration of sinoatrial and atrioventricular nodal function | • ↑ risk of bradycardia and AV block in patients treated with digoxin or class II and IV AADs |
| Changes in cardiac ion channel expression/activity | • ↑ risk of intracardiac conduction block when treated with class I AADs• Decreased repolarization reserve: ↑ risk of drug-induced proarrhythmia |
| Increased myocardial fibrosis | • Decreased intracardiac conduction velocity and the risk of conduction slowing |
| Decreased baroreceptor sensitivity | • ↑ risk of orthostatic hypotension, instability, and falls with use of antihypertensives, nitrates, and vasodilators |
| Down-regulation of β-adrenoreceptors | • ↓ response to beta-agonists (bronchodilation) and antagonists (antihypertensive effects) |
| Reduced response to diuretics | • ↓ the active transport of the diuretic to its site of action in the lumen tubule |
| Increased sensitivity to hyponatremia | • Due to ageing-related reduction of glomerular filtration rate, drugs, SIAD, or endocrinopathies |
| Increased sensitivity to anticoagulants | • ↑ the risk of bleeding (age explains up to 40% of the variance in warfarin dosing) |
| Increased sensitivity to drugs acting on the central nervous system | • ↓ P-gp activity at the blood–brain barrier leading to an accumulation of their substrates in the brain• ↓ chemoreceptor reflexes: ↑ respiratory depression by opioids• Some CV drugs (amiodarone, digoxin, lidocaine, and metoprolol) can increase neurocognitive impairment in the elderly |
↑: increase. ↓: decrease.
AAD, antiarrhythmic drugs; AV, atrioventricular; CV, cardiovascular; HF, heart failure; LV, left ventricular; P-gp, P-glycoprotein; and SIAD, syndrome of inappropriate antidiuresis.
| Physiological changes . | Pharmacodynamic effects . |
|---|---|
| Decreased cardiac reserve | • The heart is more susceptible to HF in patients treated with disopyramide or class IV AADs |
| Decreased LV compliance | • Decreased cardiac output with beta-blockers |
| Increased arterial stiffness | • ↑ risk of haemodynamic lability from vasodilator drugs and diuretics |
| Degeneration of sinoatrial and atrioventricular nodal function | • ↑ risk of bradycardia and AV block in patients treated with digoxin or class II and IV AADs |
| Changes in cardiac ion channel expression/activity | • ↑ risk of intracardiac conduction block when treated with class I AADs• Decreased repolarization reserve: ↑ risk of drug-induced proarrhythmia |
| Increased myocardial fibrosis | • Decreased intracardiac conduction velocity and the risk of conduction slowing |
| Decreased baroreceptor sensitivity | • ↑ risk of orthostatic hypotension, instability, and falls with use of antihypertensives, nitrates, and vasodilators |
| Down-regulation of β-adrenoreceptors | • ↓ response to beta-agonists (bronchodilation) and antagonists (antihypertensive effects) |
| Reduced response to diuretics | • ↓ the active transport of the diuretic to its site of action in the lumen tubule |
| Increased sensitivity to hyponatremia | • Due to ageing-related reduction of glomerular filtration rate, drugs, SIAD, or endocrinopathies |
| Increased sensitivity to anticoagulants | • ↑ the risk of bleeding (age explains up to 40% of the variance in warfarin dosing) |
| Increased sensitivity to drugs acting on the central nervous system | • ↓ P-gp activity at the blood–brain barrier leading to an accumulation of their substrates in the brain• ↓ chemoreceptor reflexes: ↑ respiratory depression by opioids• Some CV drugs (amiodarone, digoxin, lidocaine, and metoprolol) can increase neurocognitive impairment in the elderly |
| Physiological changes . | Pharmacodynamic effects . |
|---|---|
| Decreased cardiac reserve | • The heart is more susceptible to HF in patients treated with disopyramide or class IV AADs |
| Decreased LV compliance | • Decreased cardiac output with beta-blockers |
| Increased arterial stiffness | • ↑ risk of haemodynamic lability from vasodilator drugs and diuretics |
| Degeneration of sinoatrial and atrioventricular nodal function | • ↑ risk of bradycardia and AV block in patients treated with digoxin or class II and IV AADs |
| Changes in cardiac ion channel expression/activity | • ↑ risk of intracardiac conduction block when treated with class I AADs• Decreased repolarization reserve: ↑ risk of drug-induced proarrhythmia |
| Increased myocardial fibrosis | • Decreased intracardiac conduction velocity and the risk of conduction slowing |
| Decreased baroreceptor sensitivity | • ↑ risk of orthostatic hypotension, instability, and falls with use of antihypertensives, nitrates, and vasodilators |
| Down-regulation of β-adrenoreceptors | • ↓ response to beta-agonists (bronchodilation) and antagonists (antihypertensive effects) |
| Reduced response to diuretics | • ↓ the active transport of the diuretic to its site of action in the lumen tubule |
| Increased sensitivity to hyponatremia | • Due to ageing-related reduction of glomerular filtration rate, drugs, SIAD, or endocrinopathies |
| Increased sensitivity to anticoagulants | • ↑ the risk of bleeding (age explains up to 40% of the variance in warfarin dosing) |
| Increased sensitivity to drugs acting on the central nervous system | • ↓ P-gp activity at the blood–brain barrier leading to an accumulation of their substrates in the brain• ↓ chemoreceptor reflexes: ↑ respiratory depression by opioids• Some CV drugs (amiodarone, digoxin, lidocaine, and metoprolol) can increase neurocognitive impairment in the elderly |
↑: increase. ↓: decrease.
AAD, antiarrhythmic drugs; AV, atrioventricular; CV, cardiovascular; HF, heart failure; LV, left ventricular; P-gp, P-glycoprotein; and SIAD, syndrome of inappropriate antidiuresis.
Polypharmacy
Polypharmacy, defined as the concurrent use of ≥5 prescribed and non-prescribed medications [over the counter (OTC), vitamins, dietary supplements, herbal preparations], is a growing problem in older people. Multimorbidity, physical and mental health conditions, multiple prescribers, prescribing ‘cascades’, and CPGs are common causes of polypharmacy.21–24 Importantly, inappropriate polypharmacy, i.e. the use of potentially excessive, inappropriate, unnecessary, ineffective, or harmful medications,25 carries important negative consequences in older people that are summarized in Figure 1.
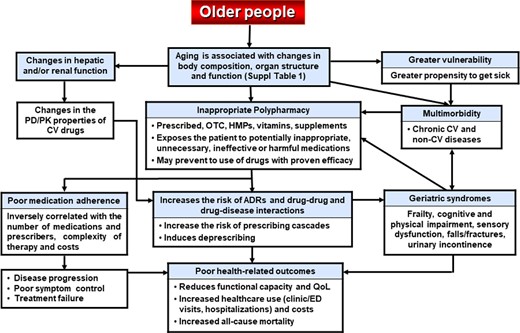
Main characteristics of older people and the consequences of inappropriate polypharmacy. ADR:, adverse drug reaction; CAM, complementary/alternative medicine; CV, cardiovascular; DDI: drug–disease interaction; ED, emergency department; HMP, herbal medicinal product; OTC, over the counter; PD/PK, pharmacodynamics/pharmacokinetics; and QoL, quality of life.
Up to 90% of community-dwelling adults ≥65 years use at least one medication, 30–50% are exposed to polypharmacy, and 10–20% use ≥10 medications (excessive polypharmacy), and most older people will receive polypharmacy during their remaining lifespan.22,24,26 Cardiovascular drugs are the most widely used and the most frequent cause of ADRs in ambulatory older people.6,7,22 The prevalence of polypharmacy increases in nursing home residents, with up to 91%, 74%, and 65% taking more than 5, 9, and 10 medications, respectively.27 Polypharmacy and ADRs increase during hospitalization and correlate with longer hospital stay and mortality.28,29
Almost two-thirds of older people use OTCs (mainly NSAIDs), but only 5% of OTCs used prior to hospitalization appeared in patient charts.6 Additionally, more than 60% of patients with CVD combine complementary/alternative and prescription medications and one-half use dietary supplements potentially interacting with warfarin, amiodarone, or digoxin.6,30 However, patients did not notify the use and because physicians may not routinely ask patients about the use of unconventional medications serious ADRs can be missed and not prevented.
However, because increasing numbers of older people live longer with CVD and the number of available cardiovascular drugs increases, polypharmacy may be just clinically appropriate when all drugs are prescribed in accordance with the best available evidence.25 Therefore, the assumption that polypharmacy is always harmful and indicative of suboptimal care needs to be reconsidered in the clinical context of the conditions for which drugs are prescribed.
Age-related changes in drug pharmacokinetics/pharmacody-namics, multimorbidity, and polypharmacy increase the risk of ADRs that decrease patients’ QoL and drug adherence, and worsen geriatric syndromes and increase morbidity and mortality.14,22–24,28 The risk of ADRs increases with the number of medicines taken, i.e. from 13% in individuals taking 2 medicines, to 58% when taking 5, and ∼100% when taking ≥8 medications.6,23,24 Patients taking drugs for which regular monitoring is recommended (i.e. antiplatelets, antiarrhythmics, digoxin, glucose-lowering drugs, diuretics, ACEIs, ARBs, and warfarin) are at increased risk of ADRs.7,23,24 In a meta-analysis of 42 trials, the prevalence of ADR-related hospitalization among adults ≥60 years of age was 8.7% and cardiovascular drugs associated with admission included beta-blockers, anticoagulants, digoxin, ACEIs, calcium channel blockers, and oral glucose-lowering drugs.29 Thus, monitoring antithrombotic, antihypertensive, and glucose-lowering drugs can reduce drug-related admissions to hospital. Importantly, more than 80% of serious ADRs are type A reactions, i.e. dose dependent, predictable, and potentially avoidable. Thus, as a rule, pharmacological treatment should be started at a low dose that should be gradually titrated upwards, based on the clinical response and ADRs. The most important ADRs in older people treated with cardiovascular drugs are summarized in Table 4.
The main adverse drugs reactions produced in older people produced by commonly prescribed cardiovascular drugs16
| Drug category . | Main adverse effects . | Monitoring . | Recommendations/cautions . |
|---|---|---|---|
| ACEIs/ARBs | • ↑ the risk of hyperkalaemia, hypotension, falls, dizziness, fatigue, acute kidney injury, and cough (ACEIs) | • Monitor renal function at the beginning of treatment, especially if renal artery stenosis | • Start at low doses; high starting doses can precipitate hypotension or renal insufficiency• PIM in people ≥75 years. |
| Alpha-adrenergic blockers | • Postural hypotensiona, especially in patients treated with diuretics or vasodilators. Dizziness, somnolence, and dry mouth | • Monitor BP. Check standing and recumbent BP | • Not recommended for the treatment of hypertension; alternative agents with better risk/benefit ratio |
| Antiarrhythmic drugs | • Increase the risk of bradycardia and AVB: class II and IV AADs or digoxin• Intracardiac conduction block: class I AADs• HF in patients with poor LV function: class IA, IC, and IV AADs and sotalol• Hypotensiona: amiodarone, class I AADs, sotalol and AADs given i.v.• Anticholinergic effects (dry mouth, constipation, urinary retention): class IA• Cognitive impairment: amiodarone, digoxin, lidocaine, and metoprolol• Fatal hepatotoxicity: dronedarone | • Monitor the ECG and serum K+ and Mg2+ levels• Correct serum K+ levels• Dofetilide: doses individualized according to the QTc interval, renal function, and serum K+ and Mg2+ levels | • Higher risk of proarrhythmia in patients with structural heart disease (not amiodarone)• High risk of TdP: class IA AADs, dofetilide, ibutilide, and sotalol• Avoid with QT-prolonging drugs• Avoid class IA AADs in patients with prostatism• Avoid dofetilide if CrCl <20 mL/min• Avoid dronedarone in patients with permanent AF or severe or recently decompensated HF• Sotalol and propafenone are beta-blockers and can exacerbate bronchospasm |
| Amiodarone | • Gastrointestinal (nausea, emesis, constipation), ocular (corneal deposits, blurred vision, optic neuritis), hyper/hypothyroidism, pulmonary fibrosis, cutaneous (photosensitivity, blue-grey skin), neurological (headache, ataxia, peripheral neuropathy, hepatic (increase in transaminases, hepatitis), renal impairment, and muscle weakness | • Monitor the ECG and BP• Monitor ocular, hepatic, thyroid, and pulmonary function | • Not recommended as first-line therapy for AF, unless in patients with structural heart disease if rhythm control is preferred over rate control• Maintenance should be max 200 mg/day |
| Lidocaine | • Tremor, dysarthria, altered levels of consciousness, nystagmus, and seizures | • Monitor the ECG | • Slower infusion rates should be used in elderly with congestive HF, hepatic impairment, or cardiogenic shock |
| Anticoagulants | • More bleeding complications (gastrointestinal, intracranial) in older adults | • Advise patients about how to recognize bleeding or symptoms and the need to report any unusual bleeding | • Avoid in patients with active bleeding• Avoid combination with antiplatelets, thrombolytics, NSAIDs, SNRIs, or SSRIs• Ensure patient adherence to dosing and monitoring regimen.• Check whether patient is unfit for anticoagulation for cognitive reasons or expired indications (temporary loss of mobility) |
| DOACs | • Renal impairment can ↑ risk of bleeding• Dabigatran and rivaroxaban: ↑ risk of gastrointestinal bleeding compared with warfarin in patients ≥75 years with AF or VTE | • Periodic monitoring of renal (and hepatic) function | • Avoid in patients if CrCl <15 mL/min/1.73 m2 (dabigatran if <30 mL/min/1.73 m2)• Dabigatran and rivaroxaban: with caution in patients ≥75 years with AF or VTE |
| Heparins | • UFH: older people may have higher serum levels and longer aPTT as compared with younger patients | • UFH: monitor aPTT• LMWH: monitor antifactor Xa in renal impairment | • Dose adjustment of UFH may be required• LMWH: reduce the dose or replace by UFH if CrCl <30 mL/min |
| VKAs (warfarin) | • ↑ risk for GI and intracranial bleeding• Multiple drug interactions with other drugs, foods, and supplements | • Patients should receive education about diet and drugs that increase the risk of bleeding | • Reduce the dose in the elderly with periodic monitoring of the INR• PIM in people ≥75 years for uncomplicated DVT for longer than 6 months and uncomplicated PE for longer than 12 months |
| Antiplatelets | • ↑ risk of bleeding | • Advise patients about signs and symptoms of bleeding | • Avoid in patients with active bleeding• Avoid combination of anticoagulants, thrombolytics, NSAIDs, SNRIs, or SSRIs• Avoid prasugrel in patients with history of TIA or stroke (↑ risk of fatal and intracranial bleeding)• Avoid ticagrelor in patients with a history of intracranial haemorrhage• Consider PPI in GI risk of bleeding |
| Aspirin | • Dyspepsia, GI bleeding, peptic ulcer, impaired BP control, nephrotoxicity, and hyperkalaemia. It can worsen renal function in patients with CKD or taking nephrotoxic drugs and can worsen or precipitate HF• Use with caution for short periods of time (stop during intercurrent illness) | • Low doses(<100 mg/day) are recommended | • Doses >160 mg/day increase the risk of bleeding, without evidence for increased efficacy. Possible lack of benefit for primary prevention of CV disease• Patients at increased risk for GI bleeding (≥75 years, peptic ulcer disease, history of GI bleeding, use of anticoagulants, antiplatelets, SSRIs or glucocorticoids) should be treated concomitantly with misoprostol or a PPI |
| Cilostazol | • Tachyarrhythmia, hypotensiona. Can exacerbate angina pectoris or myocardial infarction in patients with IHD | • Monitor platelets and white blood cell counts | • Avoid in HFrEF and LV outflow tract obstruction |
| Dipirydamole | • Orthostatic hypotensiona, dizziness, and elevated hepatic enzymes | • Monitor BP | • Avoid, more effective alternatives available |
| Beta-blockers | • Bradycardia, AVB, confusion, fatigue, bronchospasm, claudication, depression, incontinence, decreased antihypertensive effects. Limit maximum heart rate and exercise performance• Can suppress hypoglycaemic symptoms (tachycardia, tremor) in diabetic patients | • Monitor BP and ECG | • May cause acute cardiac decompensation in patients with HF, intermittent claudication in those with PAD (use carvedilol, nebivolol), and bronchoconstriction in those with asthma/COPD (use with caution β1-cardioselective drugs). Exacerbate the symptoms of depression: use hydrophilic drugs (atenolol and nadolol) |
| Calcium channel blockers | • Greater antihypertensive effects due to a decreased baroreceptor response and age-related increase in drug exposure• Dihydropyridines: peripheral oedema, reflex tachycardia, headache/flushing, hypotensiona, and falls• Non-dihydropyridines: bradycardia, AVB, hypotensiona, constipation, and fallsa | • Monitor BP• Non-dihydropyridines: monitor BP and ECG | • Avoid immediate release nifedipine because of the risk of hypotension and myocardial ischaemia• Avoid in patients with LV dysfunction or HF• Verapamil: PIM in people ≥75 years with chronic constipation; treat constipation |
| Central acting antihypertensive drugs | • They (clonidine, moxonidine, rilmenidine, and guanfacine) may precipitate or exacerbate depression, bradycardia, and orthostatic hypotension* | • Monitor BP | • Not recommended unless intolerance or lack of efficacy of other antihypertensives• Sudden cessation of treatment can produce a withdrawal syndrome |
| Colchicine | • Diarrhoea, nausea, vomiting, abdominal discomfort, and blood dyscrasias | • Monitor renal function | • Increased risk of colchicine toxicity if CrCl <10 mL/min. Reduce the dose |
| Digoxin | • Age reduces its Vd and renal clearance, leading to higher serum levels and risk of adverse effects: nausea, confusion, delirium, ataxia, dizziness, drowsiness, bradycardia, AVB, and tachyarrhythmias | • Monitor ECG and renal function• Monitor serum digoxin levels• Correct hypokalaemia and hypomagnesaemia | • Not recommended as first-line therapy for AF or HF because there are safer/more effective alternatives. No benefit in HFpEF• Maintenance doses <0.125 mg/day for any indication in people ≥75 years without renal impairment. Serum plasma levels >1.0 ng/mL have no additional benefit and may increase toxicity, particularly in women• Risk factors of toxicity: hypokalaemia, hypomagnesaemia, hypercalcaemia, CKD, hypoxia, acidosis, hypothyroidism, and myocardial ischaemia |
| Diuretics: thiazides, loop diuretics | • Hypovolaemia, postural hypotensiona, falls, poor sleep, nocturia, dehydration, electrolyte (hypokalaemia, hyponatraemia) and metabolic (hyperglycaemia, hyperuricaemia) disturbances and pre-renal azotaemia | • Monitor renal function and electrolytes (hypokalaemia)• Advise patients to stop during intercurrent illness | • Thiazides: PIM in elderly with history of gout, diabetes, hyperlipidaemia, or CrCL <30 mL/min• Loop diuretics: reduced diuretic response because of impaired tubular secretion. PIM in people ≥75 years for ankle oedema (without signs of HF) or as first-line therapy of hypertension• Caution in patients with poor mobility, urinary incontinence, AKI, and electrolyte disturbances• Avoid excessive diuresis in elderly patients with HFpEF |
| Glucose-lowering drugs | • Aggressive glycaemic control ↑ the risk of hypoglycaemia, dizziness, confusion, and falls. Establish individual HbA1C targets balancing any benefits vs. hypoglycaemia risk | • Monitor glucose plasma levels | • Avoid (a) metformin if CrCl <30 mL/min (risk of lactic acidosis) and stop with dehydration; (b) long-acting sulfonylureas because of ↑ risk of prolonged hypoglycaemia; (c) sitagliptin, sulfonylureas, and thiazolidinediones (piogliytazone) in patients with HF; and (d) sliding-scale insulin regimens because they increase the risk of hypoglycaemia |
| Iron | • Use low-dose oral iron therapy in vulnerable elderly | • Monitor iron status to avoid iron overload | • Avoid in anaemia not attributed to iron deficiency |
| Mineralocorticoid receptor antagonists | • Hyperkalaemia. Risk factors: CKD (CrCl <30 mL/min), dose >25 mg daily, treatment with ACEI/ARBs, amiloride, triamterene, K+ supplements | • Monitor BP, renal function, and serum K+ levels | • Avoid spironolactone and eplerenone in patients with serum creatinine >2.5 mg/dL, or serum K+ >5.0 (spironolactone) or >5.5 mmol/L (eplerenone) at initiation |
| Nitratesa | • Increased risk of orthostatic hypotensiona in the elderly. Headaches, flushing, rash. Attenuation/loss of the anti-ischaemic effect during continuous nitrate medication (tolerance) | • Monitor BP | • Use the smallest dose for effective relief of angina. Reduced effect of sublingual nitroglycerin may result from use of long-acting nitrates• Avoid in patients with severe anaemia and increased intracranial pressure |
| Potassium | • Risk of hyperkalaemia (particularly when administered i.v.) | • Monitor serum K+ levels | • Increased risk of hyperkalaemia in patients with CKD or in patients treated with ACEI/ARBs, spironolactone, amiloride, triamterene, trimethoprim |
| Proton pump inhibitors | • Increase the risk of Clostridium difficile infection, hypomagnesaemia, and bone loss/fractures | • PIM in old people. Use the minimum dose required to treat symptoms. If used for >12 weeks, the clinical rationale for continued use should support an underlying chronic disease (e.g. GERD) or risk factors (e.g. chronic NSAID use) | |
| QT-prolonging drugs | • The combination of QT-prolonging drugs increases the risk of TdP | • Monitor the ECG (QTc) | • Avoid combination with drugs that prolong the QTc |
| Sacubitril–valsartan | • More symptomatic hypotensiona and angio-oedema but lower increases in the creatinine and K+ levels than valsartan | • Monitor BP, renal function, and serum K+ levels | • Starting dose in patients with CrCl <30 mL/min or moderate hepatic impairment is 24/26 mg twice daily |
| Statins | • Myalgias may decrease physical activity and precipitate falls in oldest old. Sleep problems, confusion, and increase in blood glucose levels and hepatic enzymes | • Check lipid panel and creatinine kinase levels• Perform liver function tests | • Prescribers should balance the benefit/risks in patients ≥80 years• Elderly who stopped taking statins have an increased risk of CVD |
| Peripheral vasodilators | • Increase the risk of orthostatic hypotensiona and falls in the elderly | • Monitor BP | • Rarely effective and indicated long term |
| Drug category . | Main adverse effects . | Monitoring . | Recommendations/cautions . |
|---|---|---|---|
| ACEIs/ARBs | • ↑ the risk of hyperkalaemia, hypotension, falls, dizziness, fatigue, acute kidney injury, and cough (ACEIs) | • Monitor renal function at the beginning of treatment, especially if renal artery stenosis | • Start at low doses; high starting doses can precipitate hypotension or renal insufficiency• PIM in people ≥75 years. |
| Alpha-adrenergic blockers | • Postural hypotensiona, especially in patients treated with diuretics or vasodilators. Dizziness, somnolence, and dry mouth | • Monitor BP. Check standing and recumbent BP | • Not recommended for the treatment of hypertension; alternative agents with better risk/benefit ratio |
| Antiarrhythmic drugs | • Increase the risk of bradycardia and AVB: class II and IV AADs or digoxin• Intracardiac conduction block: class I AADs• HF in patients with poor LV function: class IA, IC, and IV AADs and sotalol• Hypotensiona: amiodarone, class I AADs, sotalol and AADs given i.v.• Anticholinergic effects (dry mouth, constipation, urinary retention): class IA• Cognitive impairment: amiodarone, digoxin, lidocaine, and metoprolol• Fatal hepatotoxicity: dronedarone | • Monitor the ECG and serum K+ and Mg2+ levels• Correct serum K+ levels• Dofetilide: doses individualized according to the QTc interval, renal function, and serum K+ and Mg2+ levels | • Higher risk of proarrhythmia in patients with structural heart disease (not amiodarone)• High risk of TdP: class IA AADs, dofetilide, ibutilide, and sotalol• Avoid with QT-prolonging drugs• Avoid class IA AADs in patients with prostatism• Avoid dofetilide if CrCl <20 mL/min• Avoid dronedarone in patients with permanent AF or severe or recently decompensated HF• Sotalol and propafenone are beta-blockers and can exacerbate bronchospasm |
| Amiodarone | • Gastrointestinal (nausea, emesis, constipation), ocular (corneal deposits, blurred vision, optic neuritis), hyper/hypothyroidism, pulmonary fibrosis, cutaneous (photosensitivity, blue-grey skin), neurological (headache, ataxia, peripheral neuropathy, hepatic (increase in transaminases, hepatitis), renal impairment, and muscle weakness | • Monitor the ECG and BP• Monitor ocular, hepatic, thyroid, and pulmonary function | • Not recommended as first-line therapy for AF, unless in patients with structural heart disease if rhythm control is preferred over rate control• Maintenance should be max 200 mg/day |
| Lidocaine | • Tremor, dysarthria, altered levels of consciousness, nystagmus, and seizures | • Monitor the ECG | • Slower infusion rates should be used in elderly with congestive HF, hepatic impairment, or cardiogenic shock |
| Anticoagulants | • More bleeding complications (gastrointestinal, intracranial) in older adults | • Advise patients about how to recognize bleeding or symptoms and the need to report any unusual bleeding | • Avoid in patients with active bleeding• Avoid combination with antiplatelets, thrombolytics, NSAIDs, SNRIs, or SSRIs• Ensure patient adherence to dosing and monitoring regimen.• Check whether patient is unfit for anticoagulation for cognitive reasons or expired indications (temporary loss of mobility) |
| DOACs | • Renal impairment can ↑ risk of bleeding• Dabigatran and rivaroxaban: ↑ risk of gastrointestinal bleeding compared with warfarin in patients ≥75 years with AF or VTE | • Periodic monitoring of renal (and hepatic) function | • Avoid in patients if CrCl <15 mL/min/1.73 m2 (dabigatran if <30 mL/min/1.73 m2)• Dabigatran and rivaroxaban: with caution in patients ≥75 years with AF or VTE |
| Heparins | • UFH: older people may have higher serum levels and longer aPTT as compared with younger patients | • UFH: monitor aPTT• LMWH: monitor antifactor Xa in renal impairment | • Dose adjustment of UFH may be required• LMWH: reduce the dose or replace by UFH if CrCl <30 mL/min |
| VKAs (warfarin) | • ↑ risk for GI and intracranial bleeding• Multiple drug interactions with other drugs, foods, and supplements | • Patients should receive education about diet and drugs that increase the risk of bleeding | • Reduce the dose in the elderly with periodic monitoring of the INR• PIM in people ≥75 years for uncomplicated DVT for longer than 6 months and uncomplicated PE for longer than 12 months |
| Antiplatelets | • ↑ risk of bleeding | • Advise patients about signs and symptoms of bleeding | • Avoid in patients with active bleeding• Avoid combination of anticoagulants, thrombolytics, NSAIDs, SNRIs, or SSRIs• Avoid prasugrel in patients with history of TIA or stroke (↑ risk of fatal and intracranial bleeding)• Avoid ticagrelor in patients with a history of intracranial haemorrhage• Consider PPI in GI risk of bleeding |
| Aspirin | • Dyspepsia, GI bleeding, peptic ulcer, impaired BP control, nephrotoxicity, and hyperkalaemia. It can worsen renal function in patients with CKD or taking nephrotoxic drugs and can worsen or precipitate HF• Use with caution for short periods of time (stop during intercurrent illness) | • Low doses(<100 mg/day) are recommended | • Doses >160 mg/day increase the risk of bleeding, without evidence for increased efficacy. Possible lack of benefit for primary prevention of CV disease• Patients at increased risk for GI bleeding (≥75 years, peptic ulcer disease, history of GI bleeding, use of anticoagulants, antiplatelets, SSRIs or glucocorticoids) should be treated concomitantly with misoprostol or a PPI |
| Cilostazol | • Tachyarrhythmia, hypotensiona. Can exacerbate angina pectoris or myocardial infarction in patients with IHD | • Monitor platelets and white blood cell counts | • Avoid in HFrEF and LV outflow tract obstruction |
| Dipirydamole | • Orthostatic hypotensiona, dizziness, and elevated hepatic enzymes | • Monitor BP | • Avoid, more effective alternatives available |
| Beta-blockers | • Bradycardia, AVB, confusion, fatigue, bronchospasm, claudication, depression, incontinence, decreased antihypertensive effects. Limit maximum heart rate and exercise performance• Can suppress hypoglycaemic symptoms (tachycardia, tremor) in diabetic patients | • Monitor BP and ECG | • May cause acute cardiac decompensation in patients with HF, intermittent claudication in those with PAD (use carvedilol, nebivolol), and bronchoconstriction in those with asthma/COPD (use with caution β1-cardioselective drugs). Exacerbate the symptoms of depression: use hydrophilic drugs (atenolol and nadolol) |
| Calcium channel blockers | • Greater antihypertensive effects due to a decreased baroreceptor response and age-related increase in drug exposure• Dihydropyridines: peripheral oedema, reflex tachycardia, headache/flushing, hypotensiona, and falls• Non-dihydropyridines: bradycardia, AVB, hypotensiona, constipation, and fallsa | • Monitor BP• Non-dihydropyridines: monitor BP and ECG | • Avoid immediate release nifedipine because of the risk of hypotension and myocardial ischaemia• Avoid in patients with LV dysfunction or HF• Verapamil: PIM in people ≥75 years with chronic constipation; treat constipation |
| Central acting antihypertensive drugs | • They (clonidine, moxonidine, rilmenidine, and guanfacine) may precipitate or exacerbate depression, bradycardia, and orthostatic hypotension* | • Monitor BP | • Not recommended unless intolerance or lack of efficacy of other antihypertensives• Sudden cessation of treatment can produce a withdrawal syndrome |
| Colchicine | • Diarrhoea, nausea, vomiting, abdominal discomfort, and blood dyscrasias | • Monitor renal function | • Increased risk of colchicine toxicity if CrCl <10 mL/min. Reduce the dose |
| Digoxin | • Age reduces its Vd and renal clearance, leading to higher serum levels and risk of adverse effects: nausea, confusion, delirium, ataxia, dizziness, drowsiness, bradycardia, AVB, and tachyarrhythmias | • Monitor ECG and renal function• Monitor serum digoxin levels• Correct hypokalaemia and hypomagnesaemia | • Not recommended as first-line therapy for AF or HF because there are safer/more effective alternatives. No benefit in HFpEF• Maintenance doses <0.125 mg/day for any indication in people ≥75 years without renal impairment. Serum plasma levels >1.0 ng/mL have no additional benefit and may increase toxicity, particularly in women• Risk factors of toxicity: hypokalaemia, hypomagnesaemia, hypercalcaemia, CKD, hypoxia, acidosis, hypothyroidism, and myocardial ischaemia |
| Diuretics: thiazides, loop diuretics | • Hypovolaemia, postural hypotensiona, falls, poor sleep, nocturia, dehydration, electrolyte (hypokalaemia, hyponatraemia) and metabolic (hyperglycaemia, hyperuricaemia) disturbances and pre-renal azotaemia | • Monitor renal function and electrolytes (hypokalaemia)• Advise patients to stop during intercurrent illness | • Thiazides: PIM in elderly with history of gout, diabetes, hyperlipidaemia, or CrCL <30 mL/min• Loop diuretics: reduced diuretic response because of impaired tubular secretion. PIM in people ≥75 years for ankle oedema (without signs of HF) or as first-line therapy of hypertension• Caution in patients with poor mobility, urinary incontinence, AKI, and electrolyte disturbances• Avoid excessive diuresis in elderly patients with HFpEF |
| Glucose-lowering drugs | • Aggressive glycaemic control ↑ the risk of hypoglycaemia, dizziness, confusion, and falls. Establish individual HbA1C targets balancing any benefits vs. hypoglycaemia risk | • Monitor glucose plasma levels | • Avoid (a) metformin if CrCl <30 mL/min (risk of lactic acidosis) and stop with dehydration; (b) long-acting sulfonylureas because of ↑ risk of prolonged hypoglycaemia; (c) sitagliptin, sulfonylureas, and thiazolidinediones (piogliytazone) in patients with HF; and (d) sliding-scale insulin regimens because they increase the risk of hypoglycaemia |
| Iron | • Use low-dose oral iron therapy in vulnerable elderly | • Monitor iron status to avoid iron overload | • Avoid in anaemia not attributed to iron deficiency |
| Mineralocorticoid receptor antagonists | • Hyperkalaemia. Risk factors: CKD (CrCl <30 mL/min), dose >25 mg daily, treatment with ACEI/ARBs, amiloride, triamterene, K+ supplements | • Monitor BP, renal function, and serum K+ levels | • Avoid spironolactone and eplerenone in patients with serum creatinine >2.5 mg/dL, or serum K+ >5.0 (spironolactone) or >5.5 mmol/L (eplerenone) at initiation |
| Nitratesa | • Increased risk of orthostatic hypotensiona in the elderly. Headaches, flushing, rash. Attenuation/loss of the anti-ischaemic effect during continuous nitrate medication (tolerance) | • Monitor BP | • Use the smallest dose for effective relief of angina. Reduced effect of sublingual nitroglycerin may result from use of long-acting nitrates• Avoid in patients with severe anaemia and increased intracranial pressure |
| Potassium | • Risk of hyperkalaemia (particularly when administered i.v.) | • Monitor serum K+ levels | • Increased risk of hyperkalaemia in patients with CKD or in patients treated with ACEI/ARBs, spironolactone, amiloride, triamterene, trimethoprim |
| Proton pump inhibitors | • Increase the risk of Clostridium difficile infection, hypomagnesaemia, and bone loss/fractures | • PIM in old people. Use the minimum dose required to treat symptoms. If used for >12 weeks, the clinical rationale for continued use should support an underlying chronic disease (e.g. GERD) or risk factors (e.g. chronic NSAID use) | |
| QT-prolonging drugs | • The combination of QT-prolonging drugs increases the risk of TdP | • Monitor the ECG (QTc) | • Avoid combination with drugs that prolong the QTc |
| Sacubitril–valsartan | • More symptomatic hypotensiona and angio-oedema but lower increases in the creatinine and K+ levels than valsartan | • Monitor BP, renal function, and serum K+ levels | • Starting dose in patients with CrCl <30 mL/min or moderate hepatic impairment is 24/26 mg twice daily |
| Statins | • Myalgias may decrease physical activity and precipitate falls in oldest old. Sleep problems, confusion, and increase in blood glucose levels and hepatic enzymes | • Check lipid panel and creatinine kinase levels• Perform liver function tests | • Prescribers should balance the benefit/risks in patients ≥80 years• Elderly who stopped taking statins have an increased risk of CVD |
| Peripheral vasodilators | • Increase the risk of orthostatic hypotensiona and falls in the elderly | • Monitor BP | • Rarely effective and indicated long term |
AAD, antiarrhythmic drug; ADR, adverse drug effect; AF, atrial fibrillation; ACEI, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; aPTT, activated partial thromboplastin time; ARB, angiotensin receptor blocker; AVB, atrioventricular block; BP, blood pressure; CKD, chronic kidney disease; CNS, central nervous system; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; CV, cardiovascular; CYP, cytochrome P450; DOAC, direct acting oral anticoagulants; DVT, deep vein thrombosis; GI, gastrointestinal; GERD, gastro-oesophageal reflux disease; HFpEF, heart failure with preserved ejection fraction; IHD, ischaemic heart disease; INR, international normalized ratio; i.v., intravenous; LMWH, low-molecular-weight heparin; LV, left ventricular; NSAID, non-steroidal anti-inflammatory drug; NYHA, New York Heart Association class; PAD, peripheral artery disease; P-gp, P-glycoprotein; PIM, potential inappropriate medication; PK, pharmacokinetics; PPI, proton pump inhibitor; SIADH, syndrome of inappropriate antidiuretic hormone release; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin norepinephrine reuptake inhibitor; TdP, torsades de pointes; TIA, transient ischaemic attack; UFH, unfractionated heparin; Vd, volume of distribution; and VTE, venous thromboembolism.
aPatients need to be educated about postural hypotension.
The main adverse drugs reactions produced in older people produced by commonly prescribed cardiovascular drugs16
| Drug category . | Main adverse effects . | Monitoring . | Recommendations/cautions . |
|---|---|---|---|
| ACEIs/ARBs | • ↑ the risk of hyperkalaemia, hypotension, falls, dizziness, fatigue, acute kidney injury, and cough (ACEIs) | • Monitor renal function at the beginning of treatment, especially if renal artery stenosis | • Start at low doses; high starting doses can precipitate hypotension or renal insufficiency• PIM in people ≥75 years. |
| Alpha-adrenergic blockers | • Postural hypotensiona, especially in patients treated with diuretics or vasodilators. Dizziness, somnolence, and dry mouth | • Monitor BP. Check standing and recumbent BP | • Not recommended for the treatment of hypertension; alternative agents with better risk/benefit ratio |
| Antiarrhythmic drugs | • Increase the risk of bradycardia and AVB: class II and IV AADs or digoxin• Intracardiac conduction block: class I AADs• HF in patients with poor LV function: class IA, IC, and IV AADs and sotalol• Hypotensiona: amiodarone, class I AADs, sotalol and AADs given i.v.• Anticholinergic effects (dry mouth, constipation, urinary retention): class IA• Cognitive impairment: amiodarone, digoxin, lidocaine, and metoprolol• Fatal hepatotoxicity: dronedarone | • Monitor the ECG and serum K+ and Mg2+ levels• Correct serum K+ levels• Dofetilide: doses individualized according to the QTc interval, renal function, and serum K+ and Mg2+ levels | • Higher risk of proarrhythmia in patients with structural heart disease (not amiodarone)• High risk of TdP: class IA AADs, dofetilide, ibutilide, and sotalol• Avoid with QT-prolonging drugs• Avoid class IA AADs in patients with prostatism• Avoid dofetilide if CrCl <20 mL/min• Avoid dronedarone in patients with permanent AF or severe or recently decompensated HF• Sotalol and propafenone are beta-blockers and can exacerbate bronchospasm |
| Amiodarone | • Gastrointestinal (nausea, emesis, constipation), ocular (corneal deposits, blurred vision, optic neuritis), hyper/hypothyroidism, pulmonary fibrosis, cutaneous (photosensitivity, blue-grey skin), neurological (headache, ataxia, peripheral neuropathy, hepatic (increase in transaminases, hepatitis), renal impairment, and muscle weakness | • Monitor the ECG and BP• Monitor ocular, hepatic, thyroid, and pulmonary function | • Not recommended as first-line therapy for AF, unless in patients with structural heart disease if rhythm control is preferred over rate control• Maintenance should be max 200 mg/day |
| Lidocaine | • Tremor, dysarthria, altered levels of consciousness, nystagmus, and seizures | • Monitor the ECG | • Slower infusion rates should be used in elderly with congestive HF, hepatic impairment, or cardiogenic shock |
| Anticoagulants | • More bleeding complications (gastrointestinal, intracranial) in older adults | • Advise patients about how to recognize bleeding or symptoms and the need to report any unusual bleeding | • Avoid in patients with active bleeding• Avoid combination with antiplatelets, thrombolytics, NSAIDs, SNRIs, or SSRIs• Ensure patient adherence to dosing and monitoring regimen.• Check whether patient is unfit for anticoagulation for cognitive reasons or expired indications (temporary loss of mobility) |
| DOACs | • Renal impairment can ↑ risk of bleeding• Dabigatran and rivaroxaban: ↑ risk of gastrointestinal bleeding compared with warfarin in patients ≥75 years with AF or VTE | • Periodic monitoring of renal (and hepatic) function | • Avoid in patients if CrCl <15 mL/min/1.73 m2 (dabigatran if <30 mL/min/1.73 m2)• Dabigatran and rivaroxaban: with caution in patients ≥75 years with AF or VTE |
| Heparins | • UFH: older people may have higher serum levels and longer aPTT as compared with younger patients | • UFH: monitor aPTT• LMWH: monitor antifactor Xa in renal impairment | • Dose adjustment of UFH may be required• LMWH: reduce the dose or replace by UFH if CrCl <30 mL/min |
| VKAs (warfarin) | • ↑ risk for GI and intracranial bleeding• Multiple drug interactions with other drugs, foods, and supplements | • Patients should receive education about diet and drugs that increase the risk of bleeding | • Reduce the dose in the elderly with periodic monitoring of the INR• PIM in people ≥75 years for uncomplicated DVT for longer than 6 months and uncomplicated PE for longer than 12 months |
| Antiplatelets | • ↑ risk of bleeding | • Advise patients about signs and symptoms of bleeding | • Avoid in patients with active bleeding• Avoid combination of anticoagulants, thrombolytics, NSAIDs, SNRIs, or SSRIs• Avoid prasugrel in patients with history of TIA or stroke (↑ risk of fatal and intracranial bleeding)• Avoid ticagrelor in patients with a history of intracranial haemorrhage• Consider PPI in GI risk of bleeding |
| Aspirin | • Dyspepsia, GI bleeding, peptic ulcer, impaired BP control, nephrotoxicity, and hyperkalaemia. It can worsen renal function in patients with CKD or taking nephrotoxic drugs and can worsen or precipitate HF• Use with caution for short periods of time (stop during intercurrent illness) | • Low doses(<100 mg/day) are recommended | • Doses >160 mg/day increase the risk of bleeding, without evidence for increased efficacy. Possible lack of benefit for primary prevention of CV disease• Patients at increased risk for GI bleeding (≥75 years, peptic ulcer disease, history of GI bleeding, use of anticoagulants, antiplatelets, SSRIs or glucocorticoids) should be treated concomitantly with misoprostol or a PPI |
| Cilostazol | • Tachyarrhythmia, hypotensiona. Can exacerbate angina pectoris or myocardial infarction in patients with IHD | • Monitor platelets and white blood cell counts | • Avoid in HFrEF and LV outflow tract obstruction |
| Dipirydamole | • Orthostatic hypotensiona, dizziness, and elevated hepatic enzymes | • Monitor BP | • Avoid, more effective alternatives available |
| Beta-blockers | • Bradycardia, AVB, confusion, fatigue, bronchospasm, claudication, depression, incontinence, decreased antihypertensive effects. Limit maximum heart rate and exercise performance• Can suppress hypoglycaemic symptoms (tachycardia, tremor) in diabetic patients | • Monitor BP and ECG | • May cause acute cardiac decompensation in patients with HF, intermittent claudication in those with PAD (use carvedilol, nebivolol), and bronchoconstriction in those with asthma/COPD (use with caution β1-cardioselective drugs). Exacerbate the symptoms of depression: use hydrophilic drugs (atenolol and nadolol) |
| Calcium channel blockers | • Greater antihypertensive effects due to a decreased baroreceptor response and age-related increase in drug exposure• Dihydropyridines: peripheral oedema, reflex tachycardia, headache/flushing, hypotensiona, and falls• Non-dihydropyridines: bradycardia, AVB, hypotensiona, constipation, and fallsa | • Monitor BP• Non-dihydropyridines: monitor BP and ECG | • Avoid immediate release nifedipine because of the risk of hypotension and myocardial ischaemia• Avoid in patients with LV dysfunction or HF• Verapamil: PIM in people ≥75 years with chronic constipation; treat constipation |
| Central acting antihypertensive drugs | • They (clonidine, moxonidine, rilmenidine, and guanfacine) may precipitate or exacerbate depression, bradycardia, and orthostatic hypotension* | • Monitor BP | • Not recommended unless intolerance or lack of efficacy of other antihypertensives• Sudden cessation of treatment can produce a withdrawal syndrome |
| Colchicine | • Diarrhoea, nausea, vomiting, abdominal discomfort, and blood dyscrasias | • Monitor renal function | • Increased risk of colchicine toxicity if CrCl <10 mL/min. Reduce the dose |
| Digoxin | • Age reduces its Vd and renal clearance, leading to higher serum levels and risk of adverse effects: nausea, confusion, delirium, ataxia, dizziness, drowsiness, bradycardia, AVB, and tachyarrhythmias | • Monitor ECG and renal function• Monitor serum digoxin levels• Correct hypokalaemia and hypomagnesaemia | • Not recommended as first-line therapy for AF or HF because there are safer/more effective alternatives. No benefit in HFpEF• Maintenance doses <0.125 mg/day for any indication in people ≥75 years without renal impairment. Serum plasma levels >1.0 ng/mL have no additional benefit and may increase toxicity, particularly in women• Risk factors of toxicity: hypokalaemia, hypomagnesaemia, hypercalcaemia, CKD, hypoxia, acidosis, hypothyroidism, and myocardial ischaemia |
| Diuretics: thiazides, loop diuretics | • Hypovolaemia, postural hypotensiona, falls, poor sleep, nocturia, dehydration, electrolyte (hypokalaemia, hyponatraemia) and metabolic (hyperglycaemia, hyperuricaemia) disturbances and pre-renal azotaemia | • Monitor renal function and electrolytes (hypokalaemia)• Advise patients to stop during intercurrent illness | • Thiazides: PIM in elderly with history of gout, diabetes, hyperlipidaemia, or CrCL <30 mL/min• Loop diuretics: reduced diuretic response because of impaired tubular secretion. PIM in people ≥75 years for ankle oedema (without signs of HF) or as first-line therapy of hypertension• Caution in patients with poor mobility, urinary incontinence, AKI, and electrolyte disturbances• Avoid excessive diuresis in elderly patients with HFpEF |
| Glucose-lowering drugs | • Aggressive glycaemic control ↑ the risk of hypoglycaemia, dizziness, confusion, and falls. Establish individual HbA1C targets balancing any benefits vs. hypoglycaemia risk | • Monitor glucose plasma levels | • Avoid (a) metformin if CrCl <30 mL/min (risk of lactic acidosis) and stop with dehydration; (b) long-acting sulfonylureas because of ↑ risk of prolonged hypoglycaemia; (c) sitagliptin, sulfonylureas, and thiazolidinediones (piogliytazone) in patients with HF; and (d) sliding-scale insulin regimens because they increase the risk of hypoglycaemia |
| Iron | • Use low-dose oral iron therapy in vulnerable elderly | • Monitor iron status to avoid iron overload | • Avoid in anaemia not attributed to iron deficiency |
| Mineralocorticoid receptor antagonists | • Hyperkalaemia. Risk factors: CKD (CrCl <30 mL/min), dose >25 mg daily, treatment with ACEI/ARBs, amiloride, triamterene, K+ supplements | • Monitor BP, renal function, and serum K+ levels | • Avoid spironolactone and eplerenone in patients with serum creatinine >2.5 mg/dL, or serum K+ >5.0 (spironolactone) or >5.5 mmol/L (eplerenone) at initiation |
| Nitratesa | • Increased risk of orthostatic hypotensiona in the elderly. Headaches, flushing, rash. Attenuation/loss of the anti-ischaemic effect during continuous nitrate medication (tolerance) | • Monitor BP | • Use the smallest dose for effective relief of angina. Reduced effect of sublingual nitroglycerin may result from use of long-acting nitrates• Avoid in patients with severe anaemia and increased intracranial pressure |
| Potassium | • Risk of hyperkalaemia (particularly when administered i.v.) | • Monitor serum K+ levels | • Increased risk of hyperkalaemia in patients with CKD or in patients treated with ACEI/ARBs, spironolactone, amiloride, triamterene, trimethoprim |
| Proton pump inhibitors | • Increase the risk of Clostridium difficile infection, hypomagnesaemia, and bone loss/fractures | • PIM in old people. Use the minimum dose required to treat symptoms. If used for >12 weeks, the clinical rationale for continued use should support an underlying chronic disease (e.g. GERD) or risk factors (e.g. chronic NSAID use) | |
| QT-prolonging drugs | • The combination of QT-prolonging drugs increases the risk of TdP | • Monitor the ECG (QTc) | • Avoid combination with drugs that prolong the QTc |
| Sacubitril–valsartan | • More symptomatic hypotensiona and angio-oedema but lower increases in the creatinine and K+ levels than valsartan | • Monitor BP, renal function, and serum K+ levels | • Starting dose in patients with CrCl <30 mL/min or moderate hepatic impairment is 24/26 mg twice daily |
| Statins | • Myalgias may decrease physical activity and precipitate falls in oldest old. Sleep problems, confusion, and increase in blood glucose levels and hepatic enzymes | • Check lipid panel and creatinine kinase levels• Perform liver function tests | • Prescribers should balance the benefit/risks in patients ≥80 years• Elderly who stopped taking statins have an increased risk of CVD |
| Peripheral vasodilators | • Increase the risk of orthostatic hypotensiona and falls in the elderly | • Monitor BP | • Rarely effective and indicated long term |
| Drug category . | Main adverse effects . | Monitoring . | Recommendations/cautions . |
|---|---|---|---|
| ACEIs/ARBs | • ↑ the risk of hyperkalaemia, hypotension, falls, dizziness, fatigue, acute kidney injury, and cough (ACEIs) | • Monitor renal function at the beginning of treatment, especially if renal artery stenosis | • Start at low doses; high starting doses can precipitate hypotension or renal insufficiency• PIM in people ≥75 years. |
| Alpha-adrenergic blockers | • Postural hypotensiona, especially in patients treated with diuretics or vasodilators. Dizziness, somnolence, and dry mouth | • Monitor BP. Check standing and recumbent BP | • Not recommended for the treatment of hypertension; alternative agents with better risk/benefit ratio |
| Antiarrhythmic drugs | • Increase the risk of bradycardia and AVB: class II and IV AADs or digoxin• Intracardiac conduction block: class I AADs• HF in patients with poor LV function: class IA, IC, and IV AADs and sotalol• Hypotensiona: amiodarone, class I AADs, sotalol and AADs given i.v.• Anticholinergic effects (dry mouth, constipation, urinary retention): class IA• Cognitive impairment: amiodarone, digoxin, lidocaine, and metoprolol• Fatal hepatotoxicity: dronedarone | • Monitor the ECG and serum K+ and Mg2+ levels• Correct serum K+ levels• Dofetilide: doses individualized according to the QTc interval, renal function, and serum K+ and Mg2+ levels | • Higher risk of proarrhythmia in patients with structural heart disease (not amiodarone)• High risk of TdP: class IA AADs, dofetilide, ibutilide, and sotalol• Avoid with QT-prolonging drugs• Avoid class IA AADs in patients with prostatism• Avoid dofetilide if CrCl <20 mL/min• Avoid dronedarone in patients with permanent AF or severe or recently decompensated HF• Sotalol and propafenone are beta-blockers and can exacerbate bronchospasm |
| Amiodarone | • Gastrointestinal (nausea, emesis, constipation), ocular (corneal deposits, blurred vision, optic neuritis), hyper/hypothyroidism, pulmonary fibrosis, cutaneous (photosensitivity, blue-grey skin), neurological (headache, ataxia, peripheral neuropathy, hepatic (increase in transaminases, hepatitis), renal impairment, and muscle weakness | • Monitor the ECG and BP• Monitor ocular, hepatic, thyroid, and pulmonary function | • Not recommended as first-line therapy for AF, unless in patients with structural heart disease if rhythm control is preferred over rate control• Maintenance should be max 200 mg/day |
| Lidocaine | • Tremor, dysarthria, altered levels of consciousness, nystagmus, and seizures | • Monitor the ECG | • Slower infusion rates should be used in elderly with congestive HF, hepatic impairment, or cardiogenic shock |
| Anticoagulants | • More bleeding complications (gastrointestinal, intracranial) in older adults | • Advise patients about how to recognize bleeding or symptoms and the need to report any unusual bleeding | • Avoid in patients with active bleeding• Avoid combination with antiplatelets, thrombolytics, NSAIDs, SNRIs, or SSRIs• Ensure patient adherence to dosing and monitoring regimen.• Check whether patient is unfit for anticoagulation for cognitive reasons or expired indications (temporary loss of mobility) |
| DOACs | • Renal impairment can ↑ risk of bleeding• Dabigatran and rivaroxaban: ↑ risk of gastrointestinal bleeding compared with warfarin in patients ≥75 years with AF or VTE | • Periodic monitoring of renal (and hepatic) function | • Avoid in patients if CrCl <15 mL/min/1.73 m2 (dabigatran if <30 mL/min/1.73 m2)• Dabigatran and rivaroxaban: with caution in patients ≥75 years with AF or VTE |
| Heparins | • UFH: older people may have higher serum levels and longer aPTT as compared with younger patients | • UFH: monitor aPTT• LMWH: monitor antifactor Xa in renal impairment | • Dose adjustment of UFH may be required• LMWH: reduce the dose or replace by UFH if CrCl <30 mL/min |
| VKAs (warfarin) | • ↑ risk for GI and intracranial bleeding• Multiple drug interactions with other drugs, foods, and supplements | • Patients should receive education about diet and drugs that increase the risk of bleeding | • Reduce the dose in the elderly with periodic monitoring of the INR• PIM in people ≥75 years for uncomplicated DVT for longer than 6 months and uncomplicated PE for longer than 12 months |
| Antiplatelets | • ↑ risk of bleeding | • Advise patients about signs and symptoms of bleeding | • Avoid in patients with active bleeding• Avoid combination of anticoagulants, thrombolytics, NSAIDs, SNRIs, or SSRIs• Avoid prasugrel in patients with history of TIA or stroke (↑ risk of fatal and intracranial bleeding)• Avoid ticagrelor in patients with a history of intracranial haemorrhage• Consider PPI in GI risk of bleeding |
| Aspirin | • Dyspepsia, GI bleeding, peptic ulcer, impaired BP control, nephrotoxicity, and hyperkalaemia. It can worsen renal function in patients with CKD or taking nephrotoxic drugs and can worsen or precipitate HF• Use with caution for short periods of time (stop during intercurrent illness) | • Low doses(<100 mg/day) are recommended | • Doses >160 mg/day increase the risk of bleeding, without evidence for increased efficacy. Possible lack of benefit for primary prevention of CV disease• Patients at increased risk for GI bleeding (≥75 years, peptic ulcer disease, history of GI bleeding, use of anticoagulants, antiplatelets, SSRIs or glucocorticoids) should be treated concomitantly with misoprostol or a PPI |
| Cilostazol | • Tachyarrhythmia, hypotensiona. Can exacerbate angina pectoris or myocardial infarction in patients with IHD | • Monitor platelets and white blood cell counts | • Avoid in HFrEF and LV outflow tract obstruction |
| Dipirydamole | • Orthostatic hypotensiona, dizziness, and elevated hepatic enzymes | • Monitor BP | • Avoid, more effective alternatives available |
| Beta-blockers | • Bradycardia, AVB, confusion, fatigue, bronchospasm, claudication, depression, incontinence, decreased antihypertensive effects. Limit maximum heart rate and exercise performance• Can suppress hypoglycaemic symptoms (tachycardia, tremor) in diabetic patients | • Monitor BP and ECG | • May cause acute cardiac decompensation in patients with HF, intermittent claudication in those with PAD (use carvedilol, nebivolol), and bronchoconstriction in those with asthma/COPD (use with caution β1-cardioselective drugs). Exacerbate the symptoms of depression: use hydrophilic drugs (atenolol and nadolol) |
| Calcium channel blockers | • Greater antihypertensive effects due to a decreased baroreceptor response and age-related increase in drug exposure• Dihydropyridines: peripheral oedema, reflex tachycardia, headache/flushing, hypotensiona, and falls• Non-dihydropyridines: bradycardia, AVB, hypotensiona, constipation, and fallsa | • Monitor BP• Non-dihydropyridines: monitor BP and ECG | • Avoid immediate release nifedipine because of the risk of hypotension and myocardial ischaemia• Avoid in patients with LV dysfunction or HF• Verapamil: PIM in people ≥75 years with chronic constipation; treat constipation |
| Central acting antihypertensive drugs | • They (clonidine, moxonidine, rilmenidine, and guanfacine) may precipitate or exacerbate depression, bradycardia, and orthostatic hypotension* | • Monitor BP | • Not recommended unless intolerance or lack of efficacy of other antihypertensives• Sudden cessation of treatment can produce a withdrawal syndrome |
| Colchicine | • Diarrhoea, nausea, vomiting, abdominal discomfort, and blood dyscrasias | • Monitor renal function | • Increased risk of colchicine toxicity if CrCl <10 mL/min. Reduce the dose |
| Digoxin | • Age reduces its Vd and renal clearance, leading to higher serum levels and risk of adverse effects: nausea, confusion, delirium, ataxia, dizziness, drowsiness, bradycardia, AVB, and tachyarrhythmias | • Monitor ECG and renal function• Monitor serum digoxin levels• Correct hypokalaemia and hypomagnesaemia | • Not recommended as first-line therapy for AF or HF because there are safer/more effective alternatives. No benefit in HFpEF• Maintenance doses <0.125 mg/day for any indication in people ≥75 years without renal impairment. Serum plasma levels >1.0 ng/mL have no additional benefit and may increase toxicity, particularly in women• Risk factors of toxicity: hypokalaemia, hypomagnesaemia, hypercalcaemia, CKD, hypoxia, acidosis, hypothyroidism, and myocardial ischaemia |
| Diuretics: thiazides, loop diuretics | • Hypovolaemia, postural hypotensiona, falls, poor sleep, nocturia, dehydration, electrolyte (hypokalaemia, hyponatraemia) and metabolic (hyperglycaemia, hyperuricaemia) disturbances and pre-renal azotaemia | • Monitor renal function and electrolytes (hypokalaemia)• Advise patients to stop during intercurrent illness | • Thiazides: PIM in elderly with history of gout, diabetes, hyperlipidaemia, or CrCL <30 mL/min• Loop diuretics: reduced diuretic response because of impaired tubular secretion. PIM in people ≥75 years for ankle oedema (without signs of HF) or as first-line therapy of hypertension• Caution in patients with poor mobility, urinary incontinence, AKI, and electrolyte disturbances• Avoid excessive diuresis in elderly patients with HFpEF |
| Glucose-lowering drugs | • Aggressive glycaemic control ↑ the risk of hypoglycaemia, dizziness, confusion, and falls. Establish individual HbA1C targets balancing any benefits vs. hypoglycaemia risk | • Monitor glucose plasma levels | • Avoid (a) metformin if CrCl <30 mL/min (risk of lactic acidosis) and stop with dehydration; (b) long-acting sulfonylureas because of ↑ risk of prolonged hypoglycaemia; (c) sitagliptin, sulfonylureas, and thiazolidinediones (piogliytazone) in patients with HF; and (d) sliding-scale insulin regimens because they increase the risk of hypoglycaemia |
| Iron | • Use low-dose oral iron therapy in vulnerable elderly | • Monitor iron status to avoid iron overload | • Avoid in anaemia not attributed to iron deficiency |
| Mineralocorticoid receptor antagonists | • Hyperkalaemia. Risk factors: CKD (CrCl <30 mL/min), dose >25 mg daily, treatment with ACEI/ARBs, amiloride, triamterene, K+ supplements | • Monitor BP, renal function, and serum K+ levels | • Avoid spironolactone and eplerenone in patients with serum creatinine >2.5 mg/dL, or serum K+ >5.0 (spironolactone) or >5.5 mmol/L (eplerenone) at initiation |
| Nitratesa | • Increased risk of orthostatic hypotensiona in the elderly. Headaches, flushing, rash. Attenuation/loss of the anti-ischaemic effect during continuous nitrate medication (tolerance) | • Monitor BP | • Use the smallest dose for effective relief of angina. Reduced effect of sublingual nitroglycerin may result from use of long-acting nitrates• Avoid in patients with severe anaemia and increased intracranial pressure |
| Potassium | • Risk of hyperkalaemia (particularly when administered i.v.) | • Monitor serum K+ levels | • Increased risk of hyperkalaemia in patients with CKD or in patients treated with ACEI/ARBs, spironolactone, amiloride, triamterene, trimethoprim |
| Proton pump inhibitors | • Increase the risk of Clostridium difficile infection, hypomagnesaemia, and bone loss/fractures | • PIM in old people. Use the minimum dose required to treat symptoms. If used for >12 weeks, the clinical rationale for continued use should support an underlying chronic disease (e.g. GERD) or risk factors (e.g. chronic NSAID use) | |
| QT-prolonging drugs | • The combination of QT-prolonging drugs increases the risk of TdP | • Monitor the ECG (QTc) | • Avoid combination with drugs that prolong the QTc |
| Sacubitril–valsartan | • More symptomatic hypotensiona and angio-oedema but lower increases in the creatinine and K+ levels than valsartan | • Monitor BP, renal function, and serum K+ levels | • Starting dose in patients with CrCl <30 mL/min or moderate hepatic impairment is 24/26 mg twice daily |
| Statins | • Myalgias may decrease physical activity and precipitate falls in oldest old. Sleep problems, confusion, and increase in blood glucose levels and hepatic enzymes | • Check lipid panel and creatinine kinase levels• Perform liver function tests | • Prescribers should balance the benefit/risks in patients ≥80 years• Elderly who stopped taking statins have an increased risk of CVD |
| Peripheral vasodilators | • Increase the risk of orthostatic hypotensiona and falls in the elderly | • Monitor BP | • Rarely effective and indicated long term |
AAD, antiarrhythmic drug; ADR, adverse drug effect; AF, atrial fibrillation; ACEI, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; aPTT, activated partial thromboplastin time; ARB, angiotensin receptor blocker; AVB, atrioventricular block; BP, blood pressure; CKD, chronic kidney disease; CNS, central nervous system; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; CV, cardiovascular; CYP, cytochrome P450; DOAC, direct acting oral anticoagulants; DVT, deep vein thrombosis; GI, gastrointestinal; GERD, gastro-oesophageal reflux disease; HFpEF, heart failure with preserved ejection fraction; IHD, ischaemic heart disease; INR, international normalized ratio; i.v., intravenous; LMWH, low-molecular-weight heparin; LV, left ventricular; NSAID, non-steroidal anti-inflammatory drug; NYHA, New York Heart Association class; PAD, peripheral artery disease; P-gp, P-glycoprotein; PIM, potential inappropriate medication; PK, pharmacokinetics; PPI, proton pump inhibitor; SIADH, syndrome of inappropriate antidiuretic hormone release; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin norepinephrine reuptake inhibitor; TdP, torsades de pointes; TIA, transient ischaemic attack; UFH, unfractionated heparin; Vd, volume of distribution; and VTE, venous thromboembolism.
aPatients need to be educated about postural hypotension.
The term prescribing ‘cascades’ refers to the sequence of events that take place when an ADR is misinterpreted as a new medical condition, leading to the administration of additional, potentially avoidable, medications contributing further to polypharmacy (e.g. OTC NSAIDs used to treat pain or a common cold or statin-induced myalgia increasing blood pressure, leading to the prescription of antihypertensive agents).31 Thus, before adding a new drug, prescribers should keep in mind that any new symptom in older people should be considered a possible ADR until proven otherwise.
Polypharmacy also increases the risk of pharmacokinetic (modifications in drug exposure) or pharmacodynamic (modifications in response leading to additive, synergistic, or antagonistic effects) DDIs and drug–disease interactions.22,24,28,32 The incidence of DDIs increases with the number of medications (10.9% when 2–4 drugs are used and 80.8% if ≥10 drugs are used). In a meta-analysis of 31 trials, the median DDI prevalence rates for hospital admissions and hospital visits were 22.2% and 8.9%, respectively.33 The most common potential serious DDIs and drug–disease interactions with cardiovascular drugs are shown in the Supplementary material online, Tables S3 and S4, respectively. Thus, before prescribing a new drug in older people, it is necessary to review the medications already prescribed and consider the possible interactions that the new drug might produce.
Inappropriate polypharmacy
Improving prescribing for older people is an essential part of medical care and a priority for all healthcare systems. Inappropriate polypharmacy is a common practice and includes the prescription of medications when there is no evidence-based indication or the indication has expired; it fails to achieve the therapeutic goals and causes inacceptable ADRs when safer and/or more effective drugs are available or the patient is not willing or able to take the medicines as intended.25,34
The problem of prescribing potentially inappropriate medications
Optimizing cardiovascular drug therapy avoiding the use of potentially inappropriate medications (PIMs) can improve clinical outcomes and reduce ADRs. Inappropriate treatments can lead to overprescribing (more drugs than necessary), misprescribing (drug use can be improved by changing the dose and frequency), and underprescribing (not prescribing all the agents that are needed or using lower doses than required).
Approximately 14–27% of older people in the general population, 29–45% of nursing home residents, and 44–85% of hospitalized patients are prescribed at least one PIM.35,36 Most (59%) of the PIMs at hospital discharge were first prescribed in the intensive care unit and 20% in the wards.37 Prescription of PIMs is particularly frequent in individuals with multimorbidity, disability, polypharmacy, poor functional or mental status, renal impairment, and when multiple prescribers are involved, and is a common cause of preventable ADRs, frailty, falls, cognitive impairment, hospitalizations, and wasteful utilization of resources.25,35,36 Common PIMs include NSAIDs, class I and III antiarrhythmics, calcium channel blockers, sulfonylureas, and antithrombotic agents.34,35,37
Several tools can help to identify PIM and/or potential prescription omissions in older people, including Beers, STOPP/START (Screening Tool of Older People's Prescriptions/Screening Tool to Alert to Right Treatment) criteria, EURO-FORTA (Fit fOR The Aged) list, and the Medication Appropriateness Index.34,35,38 The ACOVE-3 (Assessing Care of Vulnerable Elders-3) and GPGPA (Good Palliative-Geriatric Practice Algorithm) tools are useful in determining the need for medication continuation in vulnerable older adults who are closer to the end of life. However, no one validated tool assesses all aspects of PIMs or has been shown to be superior in improving patient-related outcomes and decreasing polypharmacy risks, and it remains unclear whether they reduce hospital admissions.23,35,38 A simple and effective approach to systematically identify PIMs is to match each of the patient's conditions with their medications.
Deprescribing
This is the process of withdrawing drugs in an attempt to reduce polypharmacy and ADRs, and improve outcomes, taking into account multimorbidity, care goals, and the patient’s values and preferences.23,39 The finding that it is feasible to reduce the medication burden in older people without significant ADRs and apparently improving QoL should encourage physicians to consider deprescribing as an integral component of good prescribing practice.7,39 However, discontinuation of some cardiovascular drugs (i.e. beta-blockers, clonidine, digoxin, antiplatelets and statins) could be associated with adverse withdrawal effects and therefore deprescribing requires careful planning. The lack of data on the appropriate duration of cardiovascular medications, including time to benefit or harm, and on their effectiveness in older adults with multimorbidity is a major barrier for deprescribing in patients with CVD.
Prescribing all beneficial medications that are needed—the problem of over- and underprescribing in clinical practice
Older people often do not receive all the necessary cardiovascular medications recommended by international guidelines or are prescribed inadequate doses or duration. Drug underuse has been seen for aspirin and beta-blockers post myocardial infarction, ACEIs in HF, or anticoagulants in individuals with atrial fibrillation.8,10,40 Reasons for underprescribing include limited evidence of clinical benefit in older people, fear of ADRs, cost issues, and inadequate attempts to reduce polypharmacy or increase adherence to other medications and/or prioritize benefits of active over preventative therapies.40
Drug adherence—a key issue
Approximately 30–75% of older people do not take the medications they are supposed to be taking, 50% of prescriptions are known to be incorrectly taken by the patient, and 33–69% of drug-related hospital admissions can be attributed to patients not taking the medications as prescribed.26 Non-adherence increases with polypharmacy, multimorbidity, physical or cognitive impairment, poor patient education, and treatment cost and complexity,26,41 and is associated with poor QoL and increases hospitalizations, mortality, and medical costs.7,8 Thus, assessment of adherence should be a routine part of care. Because barriers to medication adherence are multifactorial, no single strategy is optimal for all patients and a combination of strategies should always be used to improve drug adherence.41 Simplification of complex treatments using long-acting formulations, medications that can treat several conditions simultaneously (i.e. beta-blockers in patients with hypertension, angina, HF, and atrial fibrillation), and clearly written/oral instructions have been shown to be simple strategies to improve adherence.41
Regular monitoring of drug efficacy and safety is critical to prevent ADRs and improve QoL and clinical outcomes. However, up to two-thirds of patients receiving cardiovascular drugs that require laboratory-based monitoring (i.e. renin–angiotensin–aldosterone system inhibitors, digoxin, glucose-lowering drugs, and warfarin) are not regularly monitored.21
The importance of periodic systematic medication reviews
Careful planning of drug regimens to meet the complex needs of older people appears to help in optimizing medicine use and improve clinical outcomes.18,42,43 Structured periodic reviews of all medications, matching each medication to the patient's comorbidities and goals of care, are of importance, as shown in Figure 2. A careful medication review has been shown to be particularly important in patients with hepatic and/or renal impairment, in those who develop new symptoms, ADRs, or DDIs, and at times of transitioning from hospital to home or long-term care facilities.21,44,45 Transition of care is also commonly associated with medication changes that cause confusion and ADRs and this needs to be taken into account. At hospital discharge, 41% of patients received 5–8 medications, 37% received ≥9, and 44% received at least 1 PIM (mainly due to lack of indication).45 A review of all medications at this time appears to allow the identification and avoidance of PIMs and can prevent potential ADRs.
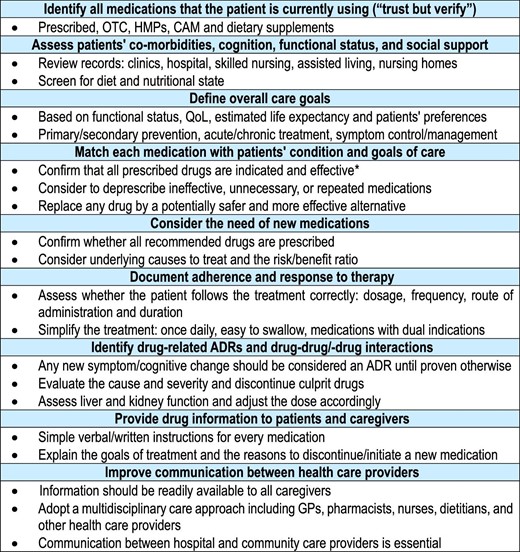
Main steps when prescribing in older people with cardiovascular diseases and polypharmacy. *Use the Beers, STOPP/START (Screening Tool of Older People's Prescriptions/Screening Tool to Alert to Right Treatment) criteria and the Medication Appropriateness Index. ADR, adverse drug reaction; CAM, complementary/alternative medicine; GP, general practitioner; HMP, herbal medicinal product; OTC, over the counter; and QoL, quality of life.
Prescribing in older people—a clinical challenge
Drug prescription in older people with CVD and multimorbidity or frailty is a complex process and represents the main challenge facing healthcare systems worldwide.8,9 Importantly, these patients are frequently underrepresented in/excluded from RCTs and when recruited, they differ significantly from real-world older patients.8 Furthermore, RCTs mainly focus on the reduction of ‘hard’ clinical outcomes (myocardial infarction, stroke, revascularizations, admissions, mortality) and pay less attention to symptom relief, preservation of physical and cognitive function, and QoL, which might be of greater concern among older people than prolonging survival and offer limited information regarding ARDs that may limit QoL and DDIs. Thus, the benefit–risk balance of drugs in the prevention/treatment of CVD in older people with multimorbidity is limited and based on the extrapolation of results from studies performed in younger people. Indeed, ∼25% of new drug approvals lack dosing recommendations for older people46 and only 45% of newly developed drugs in the period 2010–18 included reports on efficacy/safety in older people.8
CPGs incorporate scientific evidence derived from RCTs in health decision-making through the formulation of recommendations that have enhanced day-to-day cardiology patient management. However, they are mainly configured for individual diseases rather than for patients with multimorbidity. This disease-specific approach assumes that benefits and risks of cardiovascular drugs remain constant over time and rarely considers the time to benefit and time to harm of therapy, when or how to deprescribe, or how to prioritize recommendations for patients with multimorbidity and polypharmacy.8,47 Dumbreck et al.48 analysed 12 CPGs and found that potentially serious DDIs were common: 133 and 111 for drugs recommended for the treatment of type 2 diabetes and HF, respectively, and 19% involved one of the two to four drugs recommended as first-line treatment. This is not a surprise because treatment of one comorbidity may have a negative impact on another comorbidity (see Supplementary material online, Table S4), polypharmacy increases the risk of ADRs and DDIs, and the attempt to reach recommended targets may lead to ADRs; i.e., blood pressure lowering increases instability and falls in older people.
The current literature suggests that despite the relatively limited information available, a successful practical approach in older people with CVD is to take therapeutic decisions based not on chronological age alone but on a comprehensive individual geriatric risk assessment, taking in consideration health habits, cardiovascular risk factors, multimorbidity, physical/cognitive status, life expectancy, time to benefit or harm, and goals of care.7,8,23,43,49,50 Then, clinicians must prioritize which long-term medications for the prevention/treatment of CVD are most likely to produce benefit and least likely to harm the patient, and use their best clinical judgment in their attempts to adhere to prescribing guidelines.21,25,38 This patient-centred care approach allows a more comprehensive assessment of the individual's health status (personalized pharmacotherapy).7–9 The general steps/actions that should be taken into consideration when prescribing in older people with CVD and multimorbidity are summarized in Figure 3.
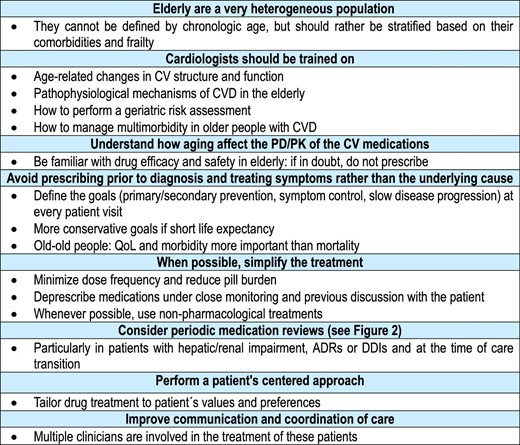
The rational use of cardiovascular drugs in older patients with multimorbidity. ADR, adverse drug reaction; CAM, complementary/alternative medicine; CV, cardiovascular; CVD, cardiovascular disease; HMP, herbal medicinal product; OTC, over the counter; PD/PK, pharmacodynamics/pharmacokinetics; QoL, quality of life; and RCT, randomized clinical trial.
Optimal prescribing in older people with CVD and limited life expectancy remains an unmet need due to lack of evidence-based data. Many patients with limited life expectancy, multimorbidity, functional impairments, and frailty can start or continue to receive some recommended drugs for secondary prevention and treatment of chronic diseases until death, increasing the likelihood of ADRs and potentially adding morbidity to the last phase of life.50 Because this may not be the best way to optimize care, the concept of time to benefit (or to harm) of cardiovascular drugs with respect to symptoms, QoL, morbidity, and mortality must also be incorporated into the therapeutic decisions.7,50 CPGs rarely mention the time to benefit or harm of therapy, but recommend preventive interventions in older adults when the estimated life expectancy is greater than the time to benefit of the drug. In older patients with a short life expectancy or with advanced diseases (cancer, dementia) in which the goals of care are just palliative, treatment of CVD until death and/or use of secondary prevention medications that take several years to provide benefits may no longer be beneficial or appropriate, particularly when they can produce ADRs early in treatment (e.g. statins: myalgia; glucose-lowering drugs: hypoglycaemia).49,50 In these patients, alternative goals of care include the preservation of functional independence and QoL and the alleviation of distressing symptoms (e.g. pain, dyspnea, oedema, anxiety, and depressed mood), although some forms of prophylaxis could be appropriate if consistent with the goals of care. CPGs are needed to inform decision-making around deprescribing long-term medications in patients with limited life expectancy. Therefore, when reviewing the need for existing or new medications, we must keep in mind the remaining life expectancy, time to benefit, and goals of care for the individual elderly patient.
Conclusions
Appropriate prescription of safe and effective pharmacotherapy in older people with CVD and multimorbidity remains one of the greatest challenges in geriatric medicine. Ageing produces cumulative changes in cardiovascular structure and function, increasing the risk of developing CVD. Additionally, age-related changes in body composition and pharmacokinetics/pharmacodynamics can modify drug exposure and responsiveness to cardiovascular drugs in older adults as compared with younger patients. Thus, dose adjustments are required to minimize the risk of ADRs, and certain cardiovascular drugs should be administered with caution, avoided, or closely monitored when prescribed in older people. In accordance with disease-specific CPGs, older patients are treated with polypharmacy for primary/secondary prevention, symptom control, slow disease progression, and improving outcomes. Nevertheless, better clinical evidence is needed regarding the efficacy and safety of cardiovascular drugs in older people with CVD and multimorbidity. There is an urgent need to develop appropriate and specific CPGs for this growing population based on RCTs (or consensus, until trial data become available) that discuss how the most common comorbidities impact the applicability of guideline recommendations and prioritize the treatments that optimize benefits, improve physical and psychosocial function, QoL, and outcomes, and minimize harm (ARDs and DDIs) in this population. Focus on the comprehensive assessment of the risk and complexity of prescribing cardiovascular drugs is important to ensure that older people with CVD and multimorbidity receive the most effective and safest cardiovascular pharmacotherapy.
Funding
None declared.
Conflict of interest: KPK, GS, GR, SA and BSL are all Editors of The European Heart Journal—Cardiovascular Pharmacotherapy and were not involved in the peer review process or publication decision.
References
Author notes
Joint senior authors.



